Translate this page into:
Apo E genotyping from blood stored on filter paper
Reprint requests: Dr R. Quraishi, National Drug Dependence Treatment Centre, Department of Psychiatry, Room No.4080, 4th Floor, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110 029, India e-mail: rizwanaquraishi@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Dried blood spotted on to filter paper has been found suitable for a large number of studies. In tropical countries with varying temperature conditions the use of dried blood needs to be validated. We carried out this study to assess the use of blood spotted filter paper as a transport system to study genotyping of Apo E gene.
Methods:
Fifty five patients visiting Cardiothoracic Neuroscience Centre (CNC) OPD at the All India Institute of Medical Sciences (AIIMS), New Delhi, and referred for lipid investigations to Cardiac Biochemistry Laboratory were selected at random. Blood was spotted on to Whatman 3 MM filter paper, dried and stored at room temperature. Genomic DNA was extracted and genotyping was carried out at the end of 0, 3 and 12 months. The study was further validated using samples collected on to filter paper from four centres and stored for eight years at room temperature. The temperature and humidity conditions of the centre varied widely.
Results:
Fifty five samples collected on to filter paper showed exact match of the genotyping when compared to fresh blood. In dried blood samples collected and stored for 1 yr at room temperature DNA extraction and apo E genotyping was done successfully.
Interpretation & conclusions:
The present results showed the feasibility of using dried blood samples on filter paper for apo E genotyping in tropical temperature. The findings need to be validated on a large sample before being recommended for use.
In tropical countries the field temperature varies from 0 to 45°C. Collection of blood samples from field is difficult and practically beset with many problems. Inadequate facilities add to the need to transport samples to a well equipped laboratory. Transportation to a distant laboratory often involves problems of spillage, leakage, cross contamination, and shipment in cold which adds to the cost.
Dried blood (DB) spotted on filter paper has several advantages over liquid blood samples for genetic screening/diagnosis12. It is in frequent use for collection and storage for HIV3, genomic DNA4 and pathogen DNA5. The stability of blood stored in the form of dried blood spots on filter paper under the hot and humid conditions in tropical countries needs to be elucidated before it can be put into routine use for field studies.
Apolipoprotein E (apo E) polymorphism is one of the well recognized genetic factors associated with Alzheimer's disease, coronary heart disease and cerebrovascular disorders67. To access cardiac risk profile among families, Apo E genotyping would be of importance8. In this study, we evaluated dried blood samples collected on filter paper for apo E genotyping study and the effect of storage, temperature and humidity.
Material & Methods
The study was conducted at All India Institute of Medical s0 ciences (AIIMS), New Delhi, during 2005 to 2007. Fifty five patients visiting Cardiothoracic Neuroscience Centre (CNC, AIIMS) Out Patient Department (OPD) referred to Cardiac Biochemistry Laboratory for lipid investigations were selected at random. The estimated sample size was found to be 36 subjects with an alpha value of 5 per cent and acceptable absolute error of 0.8. Blood (10 ml) was collected by venipuncture into tubes with anticoagulant. Ethical clearance for the conduct of the study was obtained from institutional ethics committee. Blood spots were prepared by pipetting 200 μl (~1.5 inch circle) of the blood onto the Whatman 3 MM filter paper (Whatman International Ltd, England) kept on a nonabsorbent surface (thermacol) and left at room temperature for drying at 20-30°C. After drying, the filter discs were kept in sealed plastic bags to protect them from dust and moisture, and stored at room temperature (20-30°C).
Genomic DNA was extracted from blood using standard protocol of phenol chloroform extraction and DNA precipitation using ethanol9. The dried blood DNA extraction was performed by cutting the filter spotted blood. It was suspended in sodium Tris buffer (STE), protease K and sodium dodecyl sulphate (SDS). The sample was incubated at 50°C for 2 h. After protein denaturation, the samples were extracted twice with phenol and chloroform. DNA precipitation was done overnight using ethanol10. The DNA concentration and purity were measured by determining the absorbance at 260/280 nm wavelength ratio using a spectrophotometer9. Apo E genotyping was carried out using one stage PCR11. The amplified product was digested with restriction enzyme Hha I12. The digested products were resolved on a 10 per cent polyacrylamide gel and the bands were visualized by treating with ethidium bromide.
To assess the stability, the blood spots were processed for DNA extraction, quantification and genotyping study at the end of 3 and 12 months. Because of insufficient sample volume, only 36 of the 55 dried blood samples were stored for further study. The effect of temperature and humidity was assessed by using dried blood samples collected from four centres (Bangalore, Hyderabad, Lucknow and Delhi) in a multicentric study initiated in 1998. The dried blood samples were collected as described and transported to AIIMS at 4°C. After storage for 8 yr at room temperature (25-28°C). DNA was isolated from 40 samples (10 from each centre). The genotyping study for apo E gene was carried out.
Results
On day of collection (day 0), the dried blood samples of 55 individuals were processed for genomic DNA isolation. The concentration of DNA from dried blood was compared with whole blood DNA by reading absorbance at 260/280 nm. The mean (± SD) DNA concentrations in blood and their dried blood samples were 123.4±43.4 and 138.4±46.0 ng/μl with r = 0.57, P=0.00 (Paired t test). The results showed efficient extraction from blood samples dried on filter paper.
After DNA extraction the amplification for apo E gene was carried out (Fig. 1). After PCR, the samples were subjected to restriction digestion for genotyping. All the 55 samples showed exact match of number and size of alleles for Apo E in DNA extracted from both fresh and dried blood samples on the day of collection (Fig. 2).
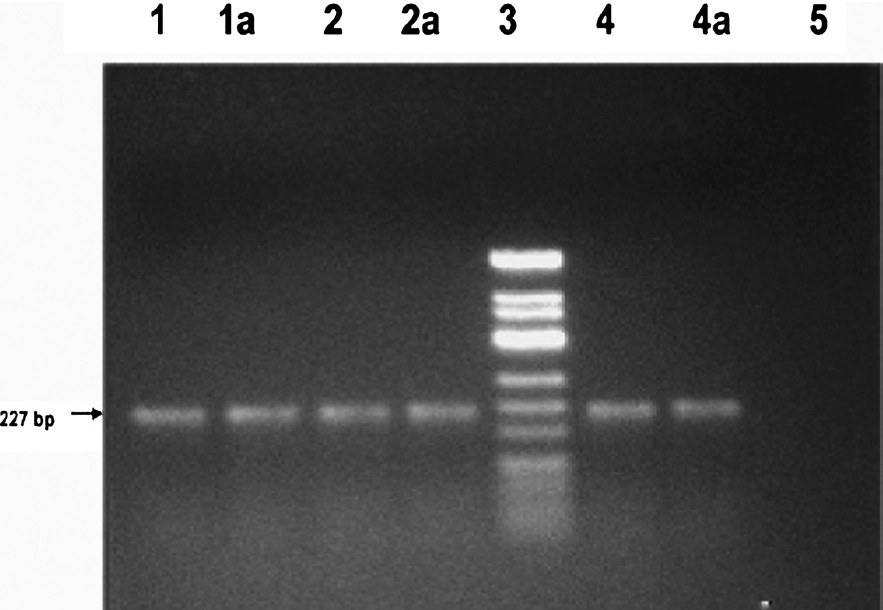
- Gel photograph of Apo E amplified gene. Agarose gel (2%) showing a 227 bp amplified product of Apo E gene. Lane 1, 2 & 4 amplified gene from direct blood extracted DNA samples; Lane 1a, 2a & 4a amplified gene from respective dried blood extracted DNA samples; Lane 3 ΦX174DNA/HinfI marker; Lane 5 - ve control (without genomic DNA).
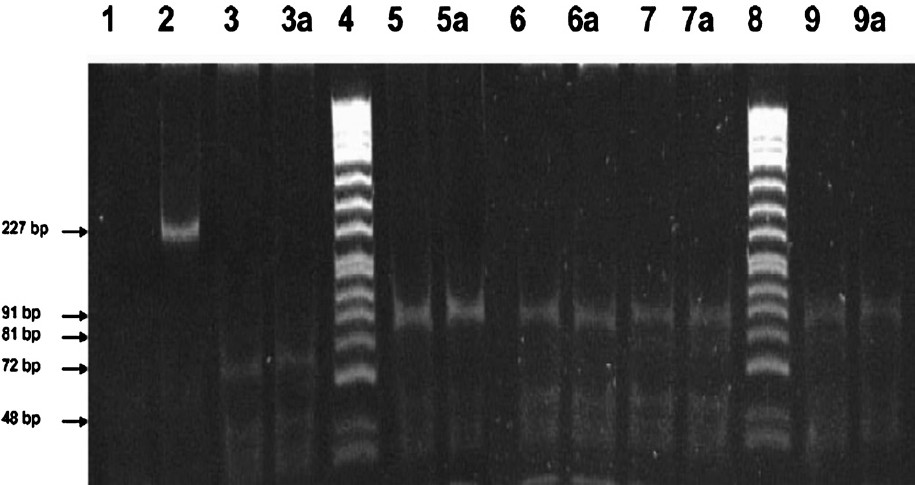
- Apo E polymorphism in corresponding blood with dried blood on the day of collection (n=55). Polyacrylamide gel (10%) photograph of HhaI digested amplified Apo E gene. Lane 1 - ve control (without genomic DNA); Lane 2 Undigested amplified 227bp apo E gene; Lane 3 and 3a E4/E4 genotype blood and dried blood sample; Lane 4 ΦX174DNA/HinfI marker; Lane 5 and 5a E3/E3 genotype blood and dried blood sample; Lane 6 and 6a E3/E3 genotype blood and dried blood sample; Lane 7 and 7a E2/E3 genotype blood and dried blood sample; Lane 8 ΦX174DNA/HinfI marker; Lane 9 and 9a E2/E2 genotype blood and dried blood sample.
The dried blood filter discs stored at room temperature at the end of 3 and 12 months were processed for DNA extraction, PCR amplification and RFLP of Apo E. The DNA extracted at the end of 12 months was compared with day 0 extracted DNA. Of the 36 stored dried blood samples, extraction, amplification and genotyping were achieved successfully in 33 samples.
Of the from 40 samples of dried blood stored for 8 yr at room temperature DNA was isolated and PCR for apo E gene was carried out. Amplification was achieved in 34 of the 40 dried blood samples.
Discussion
Apo E is an established risk factor associated with Alzheimer's disease, cerebrovascular and coronary heart disease13. Apo E genotyping is clinically useful in subjects who have a predisposition to these diseases. Storage and transportation of blood samples collected from field is a difficult task. It is, therefore, essential to establish the stability of dried blood samples so collected for genetic analysis.
Various methods have been described for DNA extraction from dried blood1415. The results obtained in the present study compared well with the method described by Rubin et al10. The benefit of storage of blood on filter paper is that inhibitors of PCR such as protein, haemoglobin, and iron seem to become increasingly resistant to elution, whereas fixation of nucleic acid to the matrix of filter paper seems to aid stability and does not impair its elution16. This could be the reason for higher recovery of genetic material from filter paper matrix as compared to fresh blood.
Chaisomchit et al4 observed the stability of genomic DNA on filter paper with regard to paired box gene 8 (PAXB) and beta goblin gene, for as long as 11 yr at ambient tropical conditions. However, the quality for amplification of larger DNA fragments decreased after 10 years of storage. Amplification was achieved in seven out of ten samples stored for over 10 years, and five out of ten samples stored for over 11 years4. Our study showed amplification in 36 out of 40 samples stored for 8 year.
The present study shows that dried blood stored up to eight years, collected from temperature conditions varying between 20˚C to 45˚C with humidity of 16 to 80 per cent can be used for genotyping of Apo E gene. One of the limitations of the study was small number of samples in which stability was studied due to constraint of time. The study, therefore, needs to be validated with a large sample size before the practical application in the field is recommended.
Acknowledgment
Authors acknowledge the funding agency Indian Council of Medical Research (ICMR), New Delhi, and the participating centres New Delhi (Institute: All India Institute of Medical Sciences, PI: Dr D. Prabhakaran), Hyderabad (Institute: Osmania Medical College and associated hospitals, PI: Dr P. Krishnam Raju), Lucknow (Institute: K.G. Medical University, PI: Dr V.K. Puri) and Bangalore (Institute: St. John's Academy of Health Sciences, PI: Dr Prem Pais) for their kind support for the study.
References
- A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338-43.
- [Google Scholar]
- The use of the dried blood spot sample in epidemiological studies. J Clin Pathol. 1999;52:633-9.
- [Google Scholar]
- Simple, sensitive and specific detection of human immunodeficiency virus type 1 subtype B DNA in dried blood samples for diagnosis in infants in the field. J Clin Microbiol. 2001;39:29-33.
- [Google Scholar]
- Stability of genomic DNA in dried blood spots on filter paper. Southeast Asian J Trop Med Public Health. 2005;36:270-3.
- [Google Scholar]
- Filter paper for preservation, storage, and distribution of insect and pathogen DNA samples. J Med Entomol. 2005;42:709-11.
- [Google Scholar]
- Apolipoprotein E polymorphism and cardiovascular diseases: a HUGE review. Am J Epidemiol. 2002;155:487-95.
- [Google Scholar]
- Apolipoprotein E polymorphism and coronary heart disease. J Assoc Physicians India. 2003;51:784-8.
- [Google Scholar]
- Molecular cloning: A laboratory manual (2nd ed). Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989.
- Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545-8.
- [Google Scholar]
- Apolipoprotein E polymorphism in cerebrovascular & coronary heart diseases. Indian J Med Res. 2010;132:363-78.
- [Google Scholar]
- Improved procedure for eluting DNA from dried blood spots. Clin Chem. 1996;42:1115-6.
- [Google Scholar]
- A simple method for extraction and purification of genomic DNA from dried blood spots on filter paper. Southeast Asian J Trop Med Public Health. 2003;34:641-5.
- [Google Scholar]
- The effect of storage on Guthrie cards: implications for deoxyribonucleic acid amplification. Ann Clin Lab Sci. 1996;26:458-69.
- [Google Scholar]





