Translate this page into:
Antiretroviral therapy in Indian setting: When & what to start with, when & what to switch to?
Reprint requests: Dr N. Kumarasamy, Chief Medical Officer, YRG CARE Medical Centre, Voluntary Health Services, Chennai 600 113, India. e-mail: kumarasamy@yrgcare.org
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
With the rapid scale up of antiretroviral therapy, there is a dramatic decline in HIV related morbidity and mortality in both developed and developing countries. Several new safe antiretroviral, and newer class of drugs and monitoring assays are developed recently. As a result the treatment guideline for the management of HIV disease continue to change. This review focuses on evolving science on Indian policy - antiretroviral therapy initiation, which drugs to start with, when to change the initial regimen and what to change.
Keywords
Antiretroviral drugs
antiretroviral therapy
ART
CD4
HIV
IRIS
viral load
Highly active antiretroviral therapy (HAART) is the cornerstone of management of patients with HIV infection. Initiation of widespread use antiretroviral therapy marked declines in the incidence of most AIDS defining conditions and mortality both in the developed and developing world12. Suppression of HIV replication is an important component to prevent HIV associated morbidity and mortality as well as in improving the quality of life in patients with HIV infection. Adequate suppression requires strict adherence of antiretroviral therapy. This has been facilitated by the co-formulation of antiretroviral drugs and the development of once daily regimens. This review focuses on issues like when to initiate antiretroviral therapy, what drugs to start with, when to change the initial regimen and what to change to in Indian settings.

When to initiate ART? (Table I)
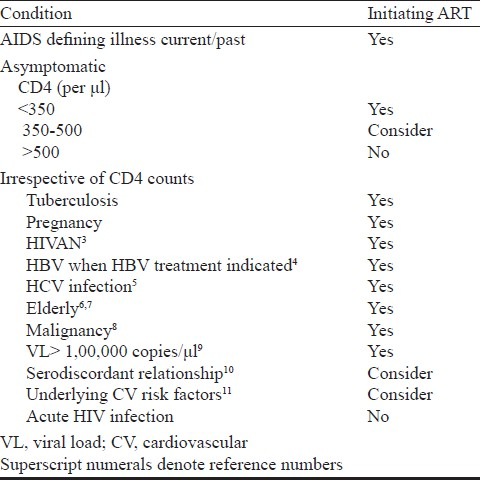
Strong evidence based on randomized controlled trials exists for initiating ART amongst asymptomatic patients with CD4<350/mm31213. Large observational studies have demonstrated the effectiveness of ART amongst patients with CD4<500mm3 in reducing mortality and clinical events including non-AIDS defining events1415. There is limited evidence about the prevalence of non-AIDS defining events from resource limited settings (RLS). Studies have also consistently demonstrated the usefulness of ART in preventing sexual transmission of HIV and this can be considered for the decision of when to initiate ART1617. The benefit of initiating ART in the setting of acute HIV infection is limited and consequently this has to be done only in the context of clinical trial18–20.
Assessing patient readiness prior to initiating ART
Although no readiness measure has accurately predicted adherence, it is essential to prepare a patient prior to initiating ART50–52. Issues that need to be discussed include conceptual understanding of treatment and its benefits, the importance of high level lifelong adherence to drugs and the consequences of sub-optimal adherence (more expensive second line regimens, progression of clinical disease) .Only after ensuring that patient has understood the consequences of initiating and being on ART, should treatment be initiated.
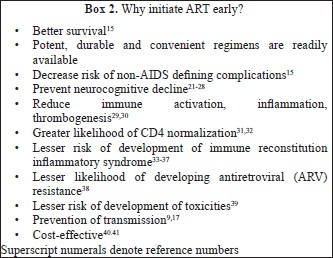
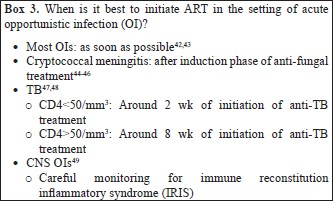
What to start with? (Table II)
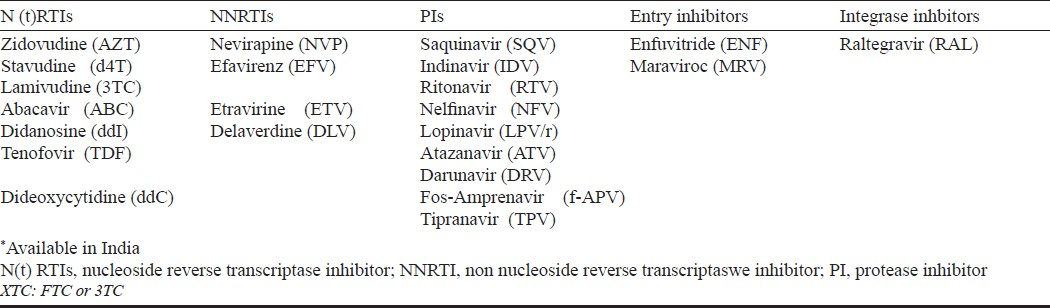
An non nucleoside reverse transcriptase inhibitor (NNRTI) based regimen is preferred over protease inhibitors (PI/r) based regimens considering similar potency, convenience, lesser expense and lower prevalence of primary resistance in the population53–55. Efavirenz (EFV) is preferred over nevirapine (NVP) when concomitant use of rifampicin is indicated, patients preference for once daily (lower pill burden) regimen and if pre-therapy CD4 count is >250/mm3 and >400/mm3 in women and men respectively56–60. Nevirapine is preferred over EFV in women planning pregnancy and those with underlying severe psychiatric illness.
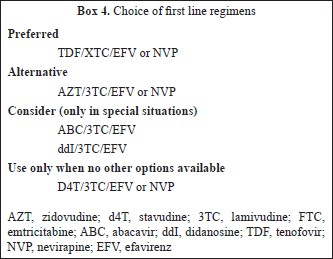
Tenofovir/Emtricitabine or Lamivudine is the preferred backbone because it has similar virologic response as compared to zidovudine/lamivudine (AZT/3TC) but has been associated with lower toxicity particularly in women61–64. Further advantages associated with TDF/XTC include low pill burden (one pill once a day when combined with EFV), better sequencing options after failure of first line regimen, concomitant treatment of underlying undiagnosed HBV infection, and it has been proven to be cost-effective in an analysis in India65–67. Tenofovir use has been associated with renal toxicity (although clinical effect is modest) and bone toxicity, and further research to characterize the incidence and risk factors for these need to be carried out in India68–72.
AZT/3TC is preferred in women who plan pregnancy/or are pregnant but has been associated with higher short-term haematological and long term morphologic and metabolic toxicities73–79. Stavudine (d4T) should be avoided because of long term toxicity concerns that are often irreversible80–82.
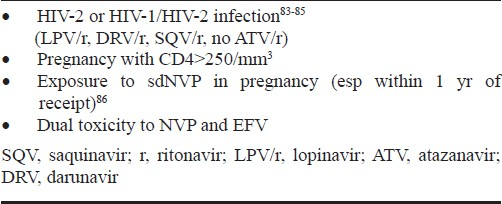
Boosted protease inhibitors need to be used as the third drug in first line regimen along with nucleoside reverse transinptases (NRTs) backbone in certain clinical situations (Table III).
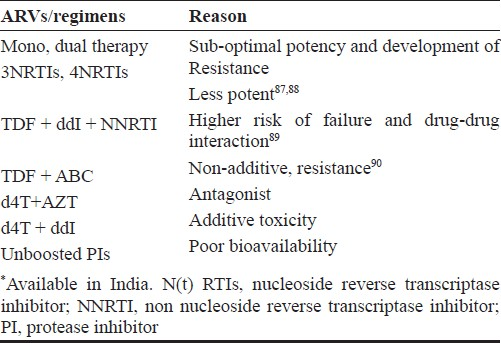
Certain antiretroviral combinations are less potent or can interact with other medications (Table IV). Various laboratory assays are performed before initiating therapy to choose appropriate drug regimen and on follow up to identify early toxicities and efficacy of the regimens. Table V describes various assays to be performed on patients initiating ART.
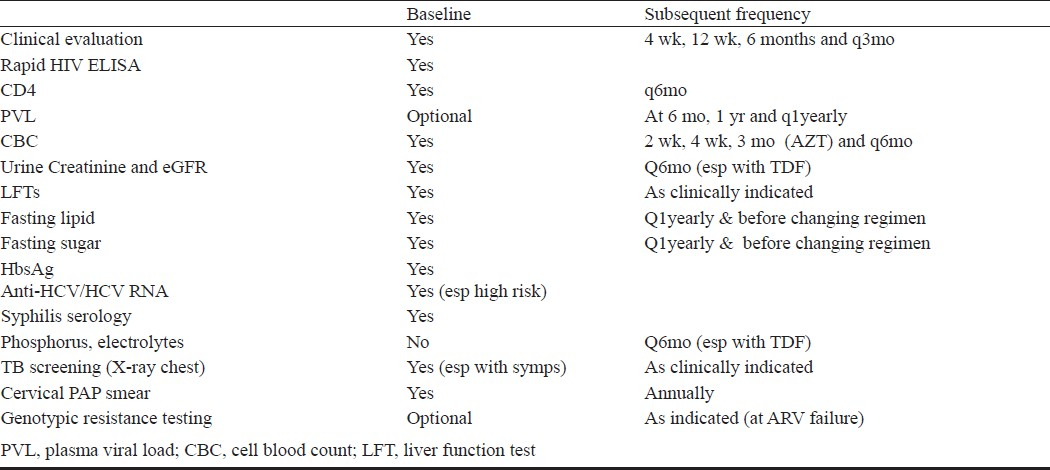
The utility of virologic monitoring has been debated. Trials have shown no advantage in using viral loads to monitor treatment response (especially disease progression and mortality) as compared to immunological and clinical monitoring91–93. Additionally, using CD4 criteria for failure to identify virologic failure has poor positive predictive value and low sensitivity94. However, not monitoring virologically is associated with identification of failure late as the gap between virologic and immunologic failure can be many years. This leads to exposure of the virus to a failing regimen amplifying resistance and further cross resistance can compromise future regimens95–102134. Hence after achieving virologic suppression, viral load may be monitored at least once a year. In patients in whom virologic monitoring can be done more frequently (e.g. every 3-6 months), CD4 counts may be monitored on an yearly basis after achieving good immunologic response.
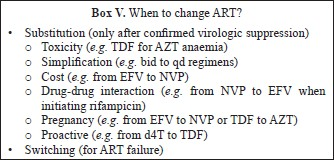
Complications in the use of ART
Immune reconstitution inflammatory syndrome (IRIS)
Occurring in a sub-population of HIV infected individuals after initiation of ART, IRIS is associated with inflammatory response to clinical or sub-clinical pathogens or non-pathogenic antigens103. Definitions for diagnosis of TB and cryptococcal IRIS have been proposed, however for most other no clear definitions exist104105. Two types have been described: paradoxical IRIS is the worsening of well controlled underlying infection while unmasking IRIS is the occurrence of new manifestations in a patient apparently well prior to initiation of ART35106–113. The major risk factor for development of IRIS is a low pre-therapy CD4 count (usually<50/mm3)35114–117. Differential diagnosis includes anti-microbial resistance, ARV toxicity or progression of underlying OI. No clear strategies exist for management of IRIS, however, 4 wk of steroids treatment (1.5 mg/kg/day for 2 wk followed by 0.75 mg/kg/day for 2 wk) has been found to be effective for treatment of mild to moderate paradoxical TB IRIS.
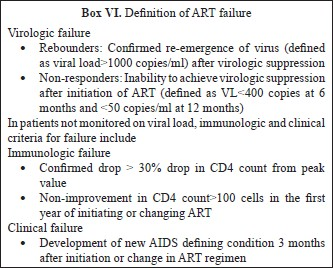
ARV toxicities
A range of toxicities is associated with use of ART6869 Table VI. Some of these toxicities are acute and also certain medications can cause chronic toxicities (Tables VII & VIII). While some are mild and self-limited, some can be fatal and irreversible. It is important to forewarn the patients about the same and a discussion on these issues should be part of the discussion prior to initiating ART.

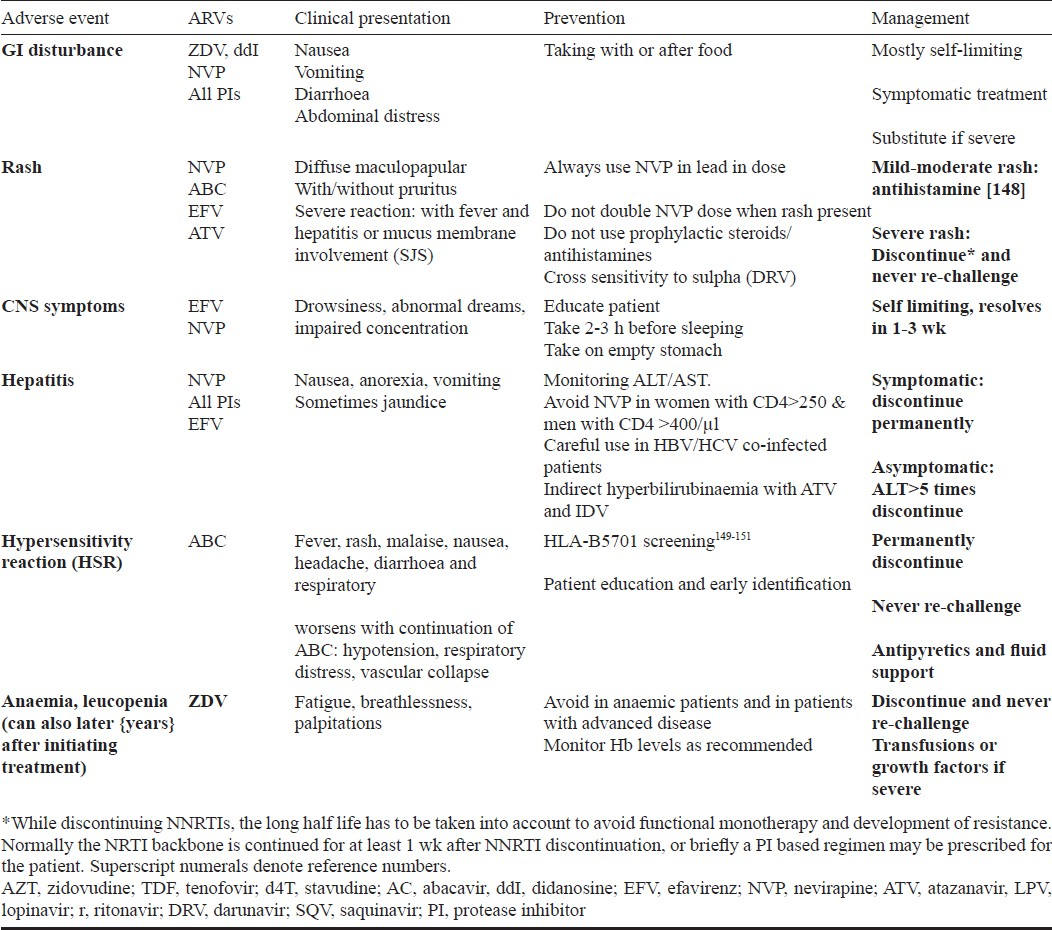
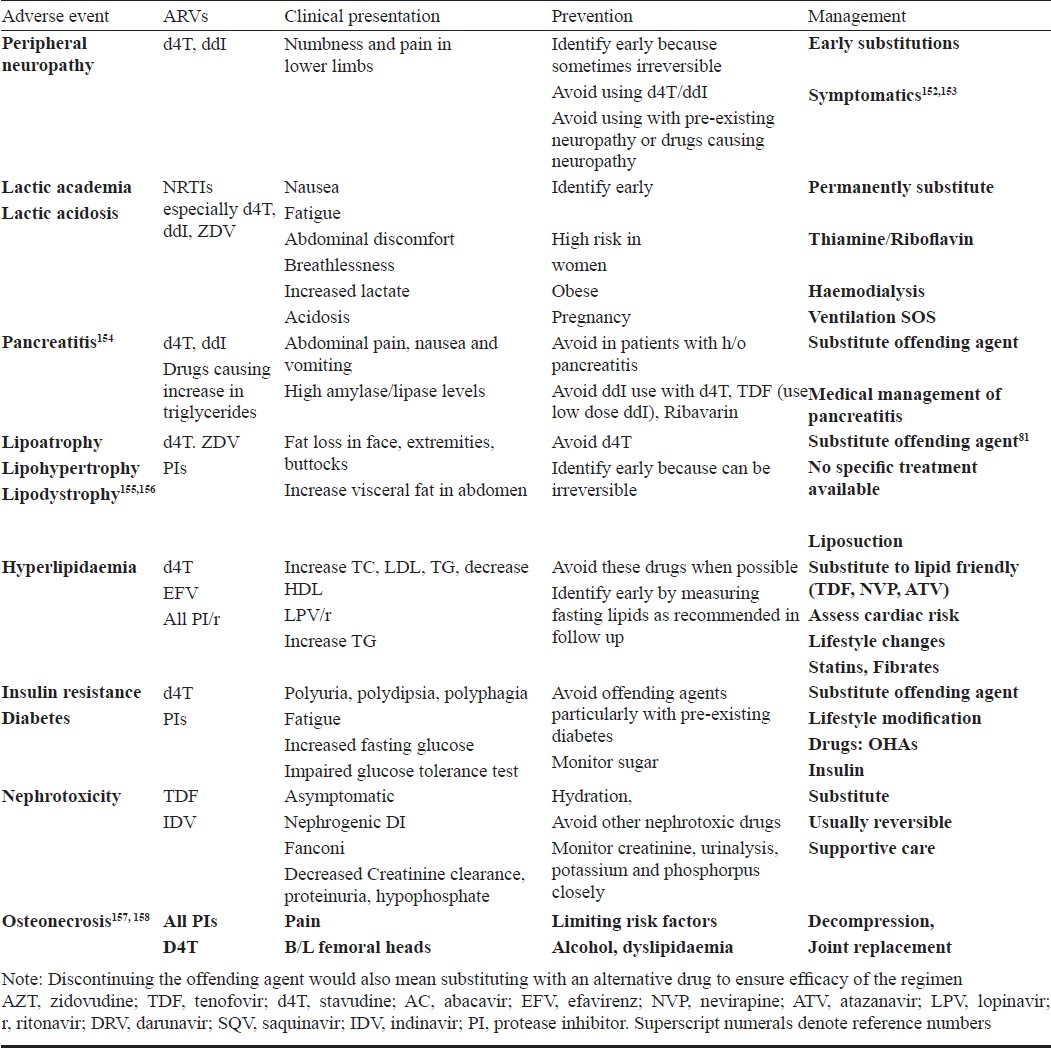
Immunologic and/or clinical failure is an indication to determine viral load (targeted viral load) to identify disconnect or true failure. The disadvantage of immunologic/clinical monitoring is late identification of failure causing increased accumulation of drug resistant mutations that can compromise efficacy of future regimens100134. Patients on failing regimen should be switched to secondline regimens (Table IX). Antiretroviral resistance testing should be used to guide the second line regimens if the patient has access.
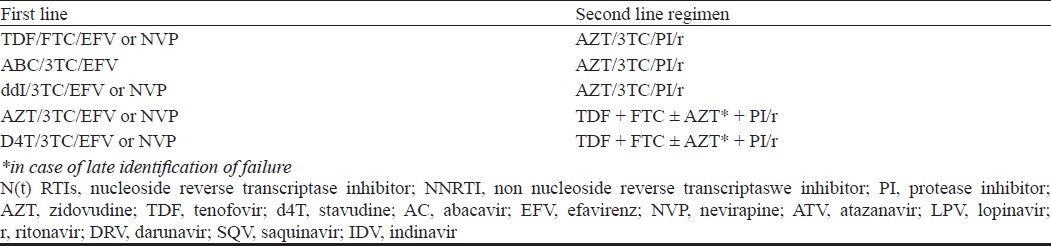
Preferred choice of PI/r in second line includes ATV/r, DRV/r159, and alternative is LPV/r.
The essential principle of constructing an effective second/third line regimen is to combine at least two or preferably three fully active drugs. These drugs should ideally include one from a new class (e.g. PI/r if NNRTI based first line regimen) or those drugs from the same class of drugs with the least likelihood of resistance as determined by genotypic resistance testing (GRT). Choosing an active drug using GRT has better outcomes than based on expert opinion alone160–163. Genotypic resistance testing has to be performed when the patient is on or within 2 wk of discontinuation of a failing regimen.
An expert consultation is advisable. Early identification of second line regimen failure is critical (e.g. virologic failure) to preserve effective ARV options. CCR5 inhibitors, second generation NNRTIs and entry inhibitors are available in resource rich settings to construct salvage regimens164–167.
Clinical investigators, industry, federal government, patient advocates and clinical trial networks are involved in the process of drug development and clinical trials. As a result, new HIV therapies and new therapeutic strategies are continually emerging and makes this disease as yet another chronic manageable disease.
References
- Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853-60.
- [Google Scholar]
- The changing natural history of HIV disease before and after the introduction of generic antiretroviral therapy in Southern India. Clin Infect Dis. 2005;41:1525-8.
- [Google Scholar]
- Renal disease: the effects of HIV and antiretroviral therapy and the implications for early antiretroviral therapy initiation. Curr Opin HIV AIDS. 2009;4:167-70.
- [Google Scholar]
- British HIV Association guidelines for the management of coinfection with HIV-1 and hepatitis B or C virus 2010. HIV Med. 2010;11:1-30.
- [Google Scholar]
- HIV-HCV co-infected patients with low CD4+ cell nadirs are at risk for faster fibrosis progression and portal hypertension. J Viral Hepat. 2010;17:400-9.
- [Google Scholar]
- Successful immunologic and virologic outcomes in elderly HIV-infected patients. J Acquir Immune Defic Syndr. 2010;54:332-3.
- [Google Scholar]
- Epidemiology of HIV and response to antiretroviral therapy in the middle aged and elderly. Aging health. 2008;4:615-27.
- [Google Scholar]
- Role of uncontrolled HIV RNA level and immunodeficiency in the occurrence of malignancy in HIV-infected patients during the combination antiretroviral therapy era: Agence Nationale de Recherche sur le Sida (ANRS) CO3 Aquitaine Cohort. Clin Infect Dis. 2009;49:1109-16.
- [Google Scholar]
- Impact of baseline viral load and adherence on survival of HIV-infected adults with baseline CD4 cell counts > or = 200 cells/microl. AIDS. 2006;20:1117-23.
- [Google Scholar]
- HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
- [Google Scholar]
- HIV and the body: a review of multidisciplinary management. HIV Med. 2010;11(Suppl 2):1-8.
- [Google Scholar]
- Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257-65.
- [Google Scholar]
- Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133-44.
- [Google Scholar]
- When to start antiretroviral therapy? NA-ACCORD stimulates the debate. AIDS Read. 2009;19:49-50.
- [Google Scholar]
- Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815-26.
- [Google Scholar]
- Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397-404.
- [Google Scholar]
- Diagnosis and initial management of acute HIV infection. Am Fam Physician. 2010;81:1239-44.
- [Google Scholar]
- Antiretroviral therapy in acute and recent HIV infection: a prospective multicenter stratified trial of intentionally interrupted treatment. AIDS. 2009;23:1987-95.
- [Google Scholar]
- Early initiation of highly active antiretroviral therapy fails to reverse immunovirological abnormalities in gut-associated lymphoid tissue induced by acute HIV infection. Antivir Ther. 2009;14:321-30.
- [Google Scholar]
- HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087-96.
- [Google Scholar]
- Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol. 2010;16:101-14.
- [Google Scholar]
- Risk factors for HIV-associated neurocognitive disorders (HAND) in sub-Saharan Africa: the case of Yaounde-Cameroon. J Neurol Sci. 2009;285:149-53.
- [Google Scholar]
- Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses. 2008;24:1301-7.
- [Google Scholar]
- Developing neuroprotective strategies for treatment of HIV-associated neurocognitive dysfunction. Futur HIV Ther. 2008;2:271-80.
- [Google Scholar]
- The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915-21.
- [Google Scholar]
- Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174-82.
- [Google Scholar]
- Neurocognitive consequences of HIV in southern India: a preliminary study of clade C virus. J Int Neuropsychol Soc. 2006;12:424-30.
- [Google Scholar]
- Rapid suppression of HIV-RNA is associated with improved control of immune activation in Mozambican adults initiating antiretroviral therapy with low CD4 counts. AIDS Res Hum Retroviruses. 2011;27:705-11.
- [Google Scholar]
- Baseline viral load and immune activation determine the extent of reconstitution of innate immune effectors in HIV-1-infected subjects undergoing antiretroviral treatment. J Immunol. 2007;179:2642-50.
- [Google Scholar]
- Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370:407-13.
- [Google Scholar]
- Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009;48:350-61.
- [Google Scholar]
- Risk factors for immune reconstitution inflammatory syndrome under combination antiretroviral therapy can be aetiology-specific. Int J STD AIDS. 2010;21:573-9.
- [Google Scholar]
- The clinical pattern, prevalence, and factors associated with immune reconstitution inflammatory syndrome in Ugandan children. AIDS. 2010;24:2009-17.
- [Google Scholar]
- Risk factors for ‘unmasking immune reconstitution inflammatory syndrome’ presentation of tuberculosis following combination antiretroviral therapy initiation in HIV-infected patients. AIDS. 2010;24:1519-25.
- [Google Scholar]
- Immune reconstitution inflammatory syndrome after initiation of highly active anti-retroviral therapy in HIV/AIDS. Indian J Dermatol Venereol Leprol. 2010;76:301-4.
- [Google Scholar]
- Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251-61.
- [Google Scholar]
- Initiation of HAART at higher CD4 cell counts is associated with a lower frequency of antiretroviral drug resistance mutations at virologic failure. J Acquir Immune Defic Syndr. 2009;51:450-3.
- [Google Scholar]
- Initiation of antiretroviral therapy at CD4 cell counts >/=350 cells/mm3 does not increase incidence or risk of peripheral neuropathy, anemia, or renal insufficiency. J Acquir Immune Defic Syndr. 2008;47:27-35.
- [Google Scholar]
- Clinical impact and cost-effectiveness of antiretroviral therapy in India: starting criteria and second-line therapy. AIDS. 2007;21(Suppl 4):S117-8.
- [Google Scholar]
- Cost-effectiveness analysis of strategies to combat HIV/AIDS in developing countries. BMJ. 2005;331:1431-7.
- [Google Scholar]
- Early antiretroviral therapy for patients with acute aids-related opportunistic infections: a cost-effectiveness analysis of ACTG A5164. HIV Clin Trials. 2010;11:248-59.
- [Google Scholar]
- Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4:e5575.
- [Google Scholar]
- Should antiretroviral therapy be delayed for 10 weeks for patients treated with fluconazole for cryptococcal meningitis? Clin Infect Dis. 2010;51:986-7.
- [Google Scholar]
- Concerns regarding a randomized study of the timing of antiretroviral therapy in zimbabweans with AIDS and acute cryptococcal meningitis. Clin Infect Dis. 2010;51:984-5.
- [Google Scholar]
- Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-saharan Africa. Clin Infect Dis. 2010;50:1532-8.
- [Google Scholar]
- Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482-91.
- [Google Scholar]
- Optimal timing of ART during TB therapy: Findings from the SAPiT trial Conf Retroviruses and opportunsitic infections 2011 (Abstr no 39LB) 2011
- [Google Scholar]
- Optimum time to start antiretroviral therapy during HIV-associated opportunistic infections. Curr Opin Infect Dis. 2011;24:34-42.
- [Google Scholar]
- Readiness: the state of the science (or the lack thereof) Curr HIV /AIDS Rep. 2010;7:245-52.
- [Google Scholar]
- Patient readiness to adhere to HAART. J Int Assoc Physicians AIDS Care (Chic). 2009;8:364-6.
- [Google Scholar]
- Patients’ readiness to start highly active antiretroviral treatment for HIV. BMJ. 2005;331:772-5.
- [Google Scholar]
- Prospective, randomized, open label trial of Efavirenz vs Lopinavir/Ritonavir in HIV+ treatment-naive subjects with CD4+<200 cell/mm3 in Mexico. J Acquir Immune Defic Syndr. 2010;53:582-8.
- [Google Scholar]
- First-line antiretroviral therapy with efavirenz or lopinavir/ritonavir plus two nucleoside analogues: the SUSKA study, a non-randomized comparison from the VACH cohort. J Antimicrob Chemother. 2008;61:1348-58.
- [Google Scholar]
- A comparison of the long-term durability of nevirapine, efavirenz and lopinavir in routine clinical practice in Europe: a EuroSIDA study. HIV Med. 2010;12:259-68.
- [Google Scholar]
- Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530-9.
- [Google Scholar]
- A randomized trial comparing plasma drug concentrations and efficacies between 2 nonnucleoside reverse-transcriptase inhibitor-based regimens in HIV-infected patients receiving rifampicin: the N2R Study. Clin Infect Dis. 2009;48:1752-9.
- [Google Scholar]
- Once daily dosing improves adherence to antiretroviral therapy. AIDS Behav. 2011;15:1397-409.
- [Google Scholar]
- Comparison of once-daily versus twice-daily combination antiretroviral therapy in treatment-naive patients: results of AIDS clinical trials group (ACTG) A5073, a 48-week randomized controlled trial. Clin Infect Dis. 2010;50:1041-52.
- [Google Scholar]
- Risk of discontinuation of nevirapine due to toxicities in antiretroviral-naive and -experienced HIV-infected patients with high and low CD4+ T-cell counts. Antivir Ther. 2007;12:325-33.
- [Google Scholar]
- Efficacy and safety of EFV with either co-formulated 3TC/ZDV or FTC/TDF for initial treatmetn of HIV-1 infected men and women in diverse multinational settings: ACTG PEARLS study conference on retorviruses and opportunistic infections 2011 (abstr no 149LB) 2011
- [Google Scholar]
- Cost-effectiveness analysis of emtricitabine/tenofovir versus lamivudine/zidovudine, in combination with efavirenz, in antiretroviral-naive, HIV-1-infected patients. Clin Ther. 2008;30:372-81.
- [Google Scholar]
- Tenofovir DF, emtricitabine, and efavirenz vs.zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251-60.
- [Google Scholar]
- Tenofovir or zidovudine in three-drug combination therapy with one nucleoside reverse transcriptase inhibitor and one non-nucleoside reverse transcriptase inhibitor for initial treatment of HIV infection in antiretroviral-naive individuals. Cochrane Database Syst Rev. 2010;10:CD008740.
- [Google Scholar]
- Drug resistance after failure of initial antiretroviral therapy in resource-limited countries. Clin Infect Dis. 2007;44:453-5.
- [Google Scholar]
- Cost-effectiveness of tenofovir as first-line antiretroviral therapy in India. Clin Infect Dis. 2010;50:416-25.
- [Google Scholar]
- Development of HIV-1 drug resistance through 144 weeks in antiretroviral-naive subjects on emtricitabine, tenofovir disoproxil fumarate, and efavirenz compared with lamivudine/zidovudine and efavirenz in study GS-01-934. J Acquir Immune Defic Syndr. 2009;52:209-21.
- [Google Scholar]
- Spectrum of adverse events after generic HAART in s0 outhern Indian HIV-infected patients. AIDS Patient Care STDs. 2008;22:337-44.
- [Google Scholar]
- Reasons for modification of generic HAART regimens among patients in Southern India. J Acquir Immune Defic Syndr. 2006;41:53-8.
- [Google Scholar]
- Renal function with use of a tenofovir-containing initial antiretroviral regimen. AIDS. 2009;23:1971-5.
- [Google Scholar]
- HIV-1 infection and antiretroviral therapies: risk factors for osteoporosis and bone fracture. Curr Opin Endocrinol Diabetes Obes. 2010;17:523-9.
- [Google Scholar]
- Emerging mutations at virological failure of HAART combinations containing tenofovir and lamivudine or emtricitabine. AIDS. 2010;24:1013-8.
- [Google Scholar]
- High incidence of zidovudine induced anaemia in HIV infected patients in eastern India. Indian J Med Res. 2010;132:386-9.
- [Google Scholar]
- Durability of initial antiretroviral therapy in a resource-constrained setting and the potential need for z0 idovudine weight-based dosing. J Acquir Immune Defic Syndr. 2010;53:215-21.
- [Google Scholar]
- Durability of initial antiretroviral therapy in a resource constrained setting and the potential need for zidovudine weight-based dosing. J Acquir Immune Defic Syndr. 2010;53:215-21.
- [Google Scholar]
- Zidovudine/lamivudine for HIV-1 infection contributes to limb fat loss. PLoS One. 2009;4:e5647.
- [Google Scholar]
- Mechanisms of zidovudine-induced mitochondrial toxicity and myopathy. Pharmacology. 2008;82:83-8.
- [Google Scholar]
- Evolution of bone mineral density in AIDS patients on treatment with zidovudine/lamivudine plus abacavir or lopinavir/ritonavir. HIV Med. 2008;9:89-95.
- [Google Scholar]
- Stavudine toxicity in women is the main reason for treatment change in a 3-year prospective cohort of adult patients started on first-line antiretroviral treatment in Uganda. J Acquir Immune Defic Syndr. 2011;56:59-63.
- [Google Scholar]
- Improvements in subcutaneous fat, lipid profile, and parameters of mitochondrial toxicity in patients with peripheral lipoatrophy when stavudine is switched to tenofovir (LIPOTEST study) HIV Clin Trials. 2008;9:407-17.
- [Google Scholar]
- Sex differences in antiretroviral therapy toxicity: lactic acidosis, stavudine, and women. Clin Infect Dis. 2007;45:261-2.
- [Google Scholar]
- Virological response to highly active antiretroviral therapy in patients infected with human immunodeficiency virus type 2 (HIV-2) and in patients dually infected with HIV-1 and HIV-2 in the Gambia and emergence of drug-resistant variants. J Clin Microbiol. 2009;47:2200-8.
- [Google Scholar]
- Virologic and immunologic responses to antiretroviral therapy among HIV-1 and HIV-2 dually infected patients: case reports from Abidjan, Cote d’Ivoire. J Clin Virol. 2009;45:72-5.
- [Google Scholar]
- Good response to lopinavir/ritonavir-containing antiretroviral regimens in antiretroviral-naive HIV-2-infected patients. AIDS. 2009;23:1171-3.
- [Google Scholar]
- Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010;363:1499-509.
- [Google Scholar]
- Quadruple nucleoside therapy with zidovudine, lamivudine, abacavir and tenofovir in the treatment of HIV. Antivir Ther. 2007;12:695-703.
- [Google Scholar]
- Evolution of resistance mutations during low-level viral replication in HIV-1-infected patients treated with zidovudine/lamivudine/abacavir as a first-line regimen. Antivir Ther. 2007;12:25-30.
- [Google Scholar]
- Suboptimal CD4 gains in HIV-infected patients receiving didanosine plus tenofovir. J Antimicrob Chemother. 2006;57:806-9.
- [Google Scholar]
- Early virologic failure and rescue therapy of tenofovir, abacavir, and lamivudine for initial treatment of HIV-1 infection: TONUS study. HIV Clin Trials. 2005;6:291-301.
- [Google Scholar]
- PHPT-3: A randomized clinical trial comparing CD4 vs Viral load ART monitoring/switching startegies in Thailand conference on retroviruses and opportuntistic infections 2011 (abstr no 44) 2011
- [Google Scholar]
- Monitoring of HIV viral load, CD4 cell count, and clinical monitoring versus clinical monitoring alone for antiretroviral therapy in rural hospitals in Cameroon: Startall ANRS 12110/ESTHER trial, a randomized non-inferiority trial Conf retroviruses and opportunistic infections 2011 (abstr no 45LB) . 2011;11:825-33.
- [Google Scholar]
- Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010;375:123-31.
- [Google Scholar]
- Performance of immunologic responses in predicting viral load suppression: implications for monitoring patients in resource-limited settings. J Acquir Immune Defic Syndr. 2006;43:436-9.
- [Google Scholar]
- Evolution of drug resistance after virological failure of a first-line highly active antiretroviral therapy regimen in Uganda. Antivir Ther. 2009;14:293-7.
- [Google Scholar]
- Emergence of drug resistance in HIV type 1-infected patients after receipt of first-line highly active antiretroviral therapy: a systematic review of clinical trials. Clin Infect Dis. 2008;47:712-22.
- [Google Scholar]
- Genotypic resistance profiles in antiretroviral-naive HIV-1 infections before and after initiation of first-line HAART: impact of polymorphism on resistance to therapy. Int J Antimicrob Agents. 2008;31:277-81.
- [Google Scholar]
- The effect of HIV-1 resistance mutations after first-line virological failure on the possibility to sequence antiretroviral drugs in second-line regimens. Antivir Ther. 2006;11:923-9.
- [Google Scholar]
- Genotypic HIV type-1 drug resistance among patients with immunological failure to first-line antiretroviral therapy in south India. Antivir Ther. 2009;14:1005-9.
- [Google Scholar]
- Monitoring antiretroviral therapy in resource-limited settings: balancing clinical care, technology, and human resources. Curr HIV /AIDS Rep. 2010;7:168-74.
- [Google Scholar]
- Laboratory monitoring to guide switching antiretroviral therapy in resource-limited settings: clinical benefits and cost-effectiveness. J Acquir Immune Defic Syndr. 2010;54:258-68.
- [Google Scholar]
- Monitoring HIV antiretroviral therapy in resource-limited settings: time to avoid costly outcomes. Clin Infect Dis. 2009;49:463-5.
- [Google Scholar]
- Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335-41.
- [Google Scholar]
- Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516-23.
- [Google Scholar]
- Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10:791-802.
- [Google Scholar]
- Unmasking of PML by HAART: unusual clinical features and the role of IRIS. J Neuroimmunol. 2010;219:100-4.
- [Google Scholar]
- Unmasking tuberculosis-associated immune reconstitution inflammatory syndrome in HIV-1 infection after antiretroviral therapy. Asian Pac J Allergy Immunol. 2010;28:206-9.
- [Google Scholar]
- Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clin Chest Med. 2009;30:797-810.
- [Google Scholar]
- Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177:680-5.
- [Google Scholar]
- Unmasking leprosy: an unusual immune reconstitution inflammatory syndrome in a patient infected with human immunodeficiency virus. Am J Trop Med Hyg. 2010;83:13-4.
- [Google Scholar]
- Worsening and unmasking of tuberculosis in HIV-1 infected patients after initiating highly active anti-retroviral therapy in Uganda. Afr Health Sci. 2008;8:190-5.
- [Google Scholar]
- Clinical case definition and manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2009;23:2467-71.
- [Google Scholar]
- Paradoxical immune reconstitution inflammatory syndrome associated with previous Cryptococcus neoformans infection in an HIV-positive patient requiring neurosurgical intervention. New Microbiol. 2009;32:209-12.
- [Google Scholar]
- Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19:399-406.
- [Google Scholar]
- Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. 2006;42:418-27.
- [Google Scholar]
- Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601-10.
- [Google Scholar]
- Risk factor analyses for immune reconstitution inflammatory syndrome in a randomized study of early vs.deferred ART during an opportunistic infection. PLoS One. 2010;5:e11416.
- [Google Scholar]
- Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24:2381-90.
- [Google Scholar]
- Renal function in patients with preexisting renal disease receiving tenofovir-containing highly active antiretroviral therapy in the HIV outpatient study. AIDS Patient Care STDs. 2009;23:589-92.
- [Google Scholar]
- Renal function in Tenofovir-exposed and Tenofovir-unexposed patients receiving highly active antiretroviral therapy in the HIV Outpatient Study. J Int Assoc Physicians AIDS Care (Chic). 2007;6:178-87.
- [Google Scholar]
- The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin Pharmacol Ther. 2008;83:265-72.
- [Google Scholar]
- The 3-year renal safety of a tenofovir disoproxil fumarate vs.a thymidine analogue-containing regimen in antiretroviral-naive patients. AIDS. 2008;22:2155-63.
- [Google Scholar]
- Changes in the renal function after tenofovir-containing antiretroviral therapy initiation in a Senegalese cohort (ANRS 1215) AIDS Res Hum Retroviruses. 2010;26:1221-7.
- [Google Scholar]
- The development of hypophosphataemic osteomalacia with myopathy in two patients with HIV infection receiving tenofovir therapy. HIV Med. 2005;6:341-6.
- [Google Scholar]
- Tenofovir-associated severe bone pain: I cannot walk! J Int Assoc Physicians AIDS Care (Chic). 2010;9:328-34.
- [Google Scholar]
- Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS. 2008;22:152-4.
- [Google Scholar]
- Haematological changes in adults receiving a zidovudine-containing HAART regimen in combination with cotrimoxazole in Cote d’Ivoire. Antivir Ther. 2005;10:615-24.
- [Google Scholar]
- Prior antiretroviral therapy experience protects against zidovudine-related anaemia. HIV Med. 2007;8:465-71.
- [Google Scholar]
- Zidovudine induced pure red cell aplasia: a case report. Niger J Med. 2009;18:332-3.
- [Google Scholar]
- The relationship between nucleoside analogue treatment duration, insulin resistance, and fasting arterialized lactate level in patients with HIV infection. Clin Infect Dis. 2005;41:1335-40.
- [Google Scholar]
- Antiretroviral therapy using zidovudine, lamivudine, and efavirenz in South Africa: tolerability and clinical events. AIDS. 2008;22:67-74.
- [Google Scholar]
- Hepatotoxicity in patients prescribed efavirenz or nevirapine. Eur J Med Res. 2008;13:343-8.
- [Google Scholar]
- Efavirenz replacement by immediate full-dose nevirapine is safe in HIV-1-infected patients in Cambodia. HIV Med. 2008;9:514-8.
- [Google Scholar]
- Safety of switching to nevirapine-based highly active antiretroviral therapy at elevated CD4 cell counts in a resource-constrained setting. J Acquir Immune Defic Syndr. 2007;45:598-600.
- [Google Scholar]
- Incidence and risk factors of nevirapine-associated skin rashes among HIV-infected patients with CD4 cell counts <250 cells/microL. Int J STD AIDS. 2007;18:782-6.
- [Google Scholar]
- Risk factors for nevirapine-associated rash among HIV-infected patients with low CD4 cell counts in resource-limited settings. Curr HIV Res. 2008;6:65-9.
- [Google Scholar]
- Incidence and risk factors of nevirapine-associated severe hepatitis among HIV-infected patients with CD4 cell counts less than 250 cells/microL. J Med Assoc Thai. 2008;91:159-65.
- [Google Scholar]
- Hyperbilirubinemia during atazanavir treatment in 2,404 patients in the Italian atazanavir expanded access program and MASTER Cohorts. Infection. 2009;37:244-9.
- [Google Scholar]
- Use of bilirubin as a marker of adherence to atazanavir-based antiretroviral therapy. AIDS. 2005;19:1700-2.
- [Google Scholar]
- Use of serum bilirubin levels as surrogate marker of early virological response to atazanavir-based antiretroviral therapy. AIDS Res Hum Retroviruses. 2011;27:1043-5.
- [Google Scholar]
- A prospective evaluation of the effect of atazanavir on the QTc interval and QTc dispersion in HIV-positive patients. HIV Med. 2006;7:317-22.
- [Google Scholar]
- Case presentation: nephrolithiasis in a patient treated with atazanavir. J Assoc Nurses AIDS Care. 2008;19:225-7.
- [Google Scholar]
- Lipid disorders in antiretroviral-naive patients treated with lopinavir/ritonavir-based HAART: frequency, characterization and risk factors. J Antimicrob Chemother. 2005;55:800-4.
- [Google Scholar]
- Impact of steady-state lopinavir plasma levels on plasma lipids and body composition after 24 weeks of lopinavir/ritonavir-containing therapy free of thymidine analogues. J Antimicrob Chemother. 2007;60:824-30.
- [Google Scholar]
- Efficacy and safety of replacing lopinavir with atazanavir in HIV-infected patients with undetectable plasma viraemia: final results of the SLOAT trial. J Antimicrob Chemother. 2008;61:200-5.
- [Google Scholar]
- Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22:1389-97.
- [Google Scholar]
- [Darunavir in treatment-naive patients. The ARTEMIS study] Enferm Infecc Microbiol Clin. 2008;26(Suppl 10):10-3.
- [Google Scholar]
- [Rashes in HIV-infected patients undergoing therapy with nevirapine or efavirenz] Hautarzt. 2005;56:847-53.
- [Google Scholar]
- HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568-79.
- [Google Scholar]
- HLA-B*5701 testing to predict abacavir hypersensitivity. PLoS Curr. 2010;2:RRN1203.
- [Google Scholar]
- HLA-B*5701 screening for susceptibility to abacavir hypersensitivity. J Antimicrob Chemother. 2007;59:591-3.
- [Google Scholar]
- Acetyl-L-carnitine in HIV-associated antiretroviral toxic neuropathy. CNS Drugs. 2007;21(Suppl 1):25-30.
- [Google Scholar]
- Metabolic and hepatobiliary side effects of antiretroviral therapy (ART) Emerg Med Clin North Am. 2010;28:409-19.
- [Google Scholar]
- Long-term complications of antiretroviral therapy: lipoatrophy. Int J Clin Pract. 2007;61:999-1014.
- [Google Scholar]
- High prevalence of lipoatrophy among patients on stavudine-containing first-line antiretroviral therapy regimens in Rwanda. Trans R Soc Trop Med Hyg. 2007;101:793-8.
- [Google Scholar]
- Osteonecrosis in human immunodeficiency virus (HIV)-infected patients: a multicentric case-control study. J Bone Miner Metab 2011
- [Google Scholar]
- Risk factors for osteonecrosis in HIV-infected patients: impact of treatment with combination antiretroviral therapy. AIDS. 2006;20:1627-35.
- [Google Scholar]
- Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet. 2007;370:49-58.
- [Google Scholar]
- The long-term benefits of genotypic resistance testing in patients with extensive prior antiretroviral therapy: a model-based approach. HIV Med. 2007;8:439-50.
- [Google Scholar]
- Utility of repeat genotypic resistance testing and clinical response in patients with three class resistance and virologic treatment failure. AIDS Patient Care STDs. 2007;21:544-50.
- [Google Scholar]
- Cost-effectiveness of genotypic antiretroviral resistance testing in HIV-infected patients with treatment failure. PLoS One. 2007;2:e173.
- [Google Scholar]
- A randomized controlled trial of the clinical utility of genotypic resistance testing in patients with limited prior exposure to antiretroviral drugs. HIV Clin Trials. 2005;6:183-6.
- [Google Scholar]
- Long-term efficacy and safety of the HIV integrase inhibitor raltegravir in patients with limited treatment options in a Phase II study. J Acquir Immune Defic Syndr. 2010;53:456-63.
- [Google Scholar]
- Long-term efficacy and safety of Raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 Phase III trials. Clin Infect Dis. 2010;50:605-12.
- [Google Scholar]
- Implementation of raltegravir in routine clinical practice: selection criteria for choosing this drug, virologic response rates, and characteristics of failures. J Acquir Immune Defic Syndr. 2010;53:464-71.
- [Google Scholar]
- High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis. 2009;49:1441-9.
- [Google Scholar]






