Translate this page into:
Antioxidant & anticancer activities of isatin (1H-indole-2,3-dione), isolated from the flowers of Couroupita guianensis Aubl
Reprint requests: Dr M. Premanathan, Department of Biotechnology, Mepco Schlenk Engineering College, Mepco Nagar, Sivakasi 608 502, Tamil Nadu, India e-mail: ma.premanathan@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Derivatives of isatin are known to have cytotoxicity against human carcinoma cell lines. This compound therefore, has a potential to be used as a chemotherapeutic agent against cancer. This study was done to investigate the antioxidant and anticancer activities of isatin, extracted from flower of a folklore medicinal plant Couroupita guianensis against human promylocytic leukemia (HL60) cells.
Methods:
Active fractions demonstrating anticancer and antioxidant activities were isolated from the extracts of shade-dried flowers of C. guianensis by bioassay guided fractionation. The free radical scavenging activity was determined using lipid peroxidation assay. Cytotoxicity against human promylocytic leukemia HL60 cells was determined by MTT assay. Apoptotic activity was analyzed by DNA fragmentation and flowcytometry.
Results:
Isatin isolated from the active fraction showed antioxidant activity with the EC50 value of 72.80 μg/ml. It also exhibited cytotoxicity against human promylocytic leukemia HL60 cells in dose-dependant manner with the CC50 value of 2.94 μg/ml. The isatin-treated cells underwent apoptosis and DNA fragmentation. Apoptosis was confirmed by the FACS analysis using FITC-annexin V markers.
Interpretation & conclusions:
Isatin showed antioxidant activity and was cytotoxic to the HL60 cells due to induction of apoptosis. The isatin can be further evaluated to be used as a prophylactic agent to prevent the free radical-induced cancer and as a chemotherapeutic agent to kill the cancer cells.
Keywords
Anticancer
antioxidant
apoptosis
Couroupita guianensis
free radicals
isatin
Isatin (1H-indole-2,3-dione) is an endogenous compound, identified in humans and possesses a wide range of biological activities such as anxiogenic, sedative, anticonvulsant activities and acts as a potent antagonist on atrial natriuretic peptide receptors in vitro12. Derivatives of isatin have been reported to have cytotoxicity against human carcinoma cell lines derived from breast, prostrate3, human acute lymphoblastic leukemia (MOLT-4)45, colon67, and lung8. Isatin was first isolated from the fruits of the Cannon ball tree Couroupita guianensis9. This plant species has been used in folklore medicines1011. However, it is not scientifically validated. We report here the potential of isatin isolated from the flowers of C. guianensis for antioxidant and anticancer activities against human promyelocytic leukemia (HL60) cells.
Material & Methods
Couroupita guianensis Aubl. (Lecythidaceae) is an evergreen tree, native to tropical northern South America, southern Caribbean and also India. Its flowers are orange, scarlet and pink in colour, and form large bunches. Floral parts of C. guianensis were collected from Kattalagar kovil with elevation of 100.07 m above sea level, Madurai, Tamil Nadu, India (9° 28’ N and 77 ° 48’ E). The voucher specimen was identified and deposited in the herbarium of the Centre of Advanced Study in Marine Biology, Parangipettai, Tamil Nadu. The samples were washed, air-dried and powdered.
Extraction and purification: Extraction and purification of the floral parts were carried out as described earlier9. Dried, powdered (250 g) floral parts were extracted with CHCl3 using soxhlet extractor and concentrated in a rotary vacuum evaporator. The residue thus obtained was dissolved in CH2 Cl2 and passed through a column of silica gel to remove the colouring material. The resulting solution was concentrated and chromatographed on silica gel column (5 × 50 cm, 60-120 mesh) using CH2Cl2 containing slowly increasing amounts of methanol as eluent (flow rate: 3 ml/min). Fractions of 250 ml were collected and monitored by HPLC (Shimadzu, Japan) using shimpack ODS-18 column as stationary phase (4.5 × 120 mm), eluted with methanol-water 80:20 at a flow rate of 1 ml/min. The samples were analysed using a UV detector at a wave length of 295 nm. Elution of column chromatography afforded four major fractions. Each fraction was concentrated using a rotary evaporator and the residue was tested for its biological activity. The third fraction which showed promising activity was analyzed for structural elucidation by infra red (IR), ultra violet (UV), 1H nuclear magnetic resonance (NMR), 13C NMR and gas chromatography-mass spectral technique (GC-MS) spectral techniques.
IR spectra, GC-MS and NMR analysis were made at CSIR-Central Electro Chemical Research Institute, Karaikudi, Tamil Nadu using a Bruker Optik GmbH, make TENSOR 27 spectrometer (Germany), Agilent GC-MS (USA) and Bruker 400 MHz NMR spectrometer (Switzerland), respectively.
Biological activities and FACS analysis were done at Mepco Schlenk Engineering College, Sivakasi, Tamil Nadu.
Free radical scavenging activity: The free radical scavenging activity was determined using lipid peroxidation assay1213. Briefly, lipid peroxidation was induced in liposome prepared from egg lecithin by adding 5 μl of 400 mM FeCl3 and 5 μl of 200 mM L-ascorbic acid. To this, different concentrations of the test compound were added. The control was prepared without the treatment of compound. The samples were incubated at 37°C for 60 min. The reaction was inhibited by adding 1 ml of stopping solution which contains 0.25 N HCl, 1.5 per cent trichloroacetic acid, 0.375 per cent thiobarbituric acid. These reaction mixtures were kept in boiling water bath for 15 min, cooled and centrifuged. The absorbance of the resulting solution was measured at 532 nm. The activity was calculated by using the formula: 50% inhibition (EC50) = [(control OD - sample OD) / control OD] × 100.
Cytotoxicity assay: HL60 cells obtained from the National Centre for Cell Science, Pune, India were grown in RPMI1640 medium supplemented with 2 mg/ml sodium bicarbonate, 4.5 mg/ml glucose, 100 μg/ml streptomycin sulphate, 40 μg/ml gentamycin, 100 U/ml penicillin as well as 10 per cent heat inactivated foetal calf serum. An environment of humidified air containing 5 per cent CO2 was maintained at 37°C. Cytotoxicity was determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay14. Briefly, the cells were suspended at 3 × 105 cells/ml. The cells were placed in 96 well microtitre plate (200 μl/well) and incubated at 37°C in a CO2 incubator in the presence of the test compound. After 5 days, cell viability was measured by MTT assay14, from which 50 per cent cytotoxic concentration (CC50) was calculated15.
DNA fragmentation study: HL60 cells were incubated with appropriate concentration of the test compound with their CC50 value. After 48 h DNA was extracted using DNA isolation kit (Genei, Bangalore, India), evaluated on 0.8 per cent agarose gel using ethidium bromide and DNA pattern was documented by gel documentation system (Vilber Lourmet, France)13.
Apoptosis detection by Annexin V marker: HL60 cells were incubated with appropriate concentration of the test compound with their CC50 value. After 24 h, apoptosis induction was analyzed using the apoptotic, necrotic, and healthy cell quantification kit (Biotium inc., USA) following the manufacturer's protocol for flow cytometry (FACS caliber, BD Biosciences, USA) assay1314.
Statistical analysis: Data were analysed using Biostat, Analyst Soft Inc. software using one-way (ANOVA) for statistical significance of the model with post hoc comparisons to test for statistically significant effects.
Results & Discussion
The chemical fractions extracted from flower of the Cannonball tree exhibited promising biological activity only in the third fraction and hence this fraction was analyzed by Fourier Transform Infra Red (FTIR), UV, 1H NMR, 13C NMR, GC-MS and CHN Elemental analysis techniques for its structural elucidation.
FTIR spectrum (Fig. 1) showed stretching bands at 1730 and 1616/cm representing the presence of carbonyl and secondary amino groups, respectively. A peak at a wave number of 3187/cm confirmed the existence of amino functional group. Carbon-hydrogen stretching in aromatic was observed at 3100-3000/ cm. The UV spectrum of the active fraction showed the absorption at a wavelength of 240 and 280 nm in methanol medium. In the 1H NMR spectrum (Bruker FT-NMR, 400 MHz) four aromatic protons were observed at δ 7-8. 13C NMR spectrum indicated eight different carbon atoms. 13C NMR (100 MHz, Acetone-d6): δ 112, 118, 123, 125, 138, 151, 159, 184. Elemental analysis showed: C, 65.26; H, 3.40; N, 9.52. C8H5NO2 requires: C, 65.31; H, 3.45; N, 9.48.
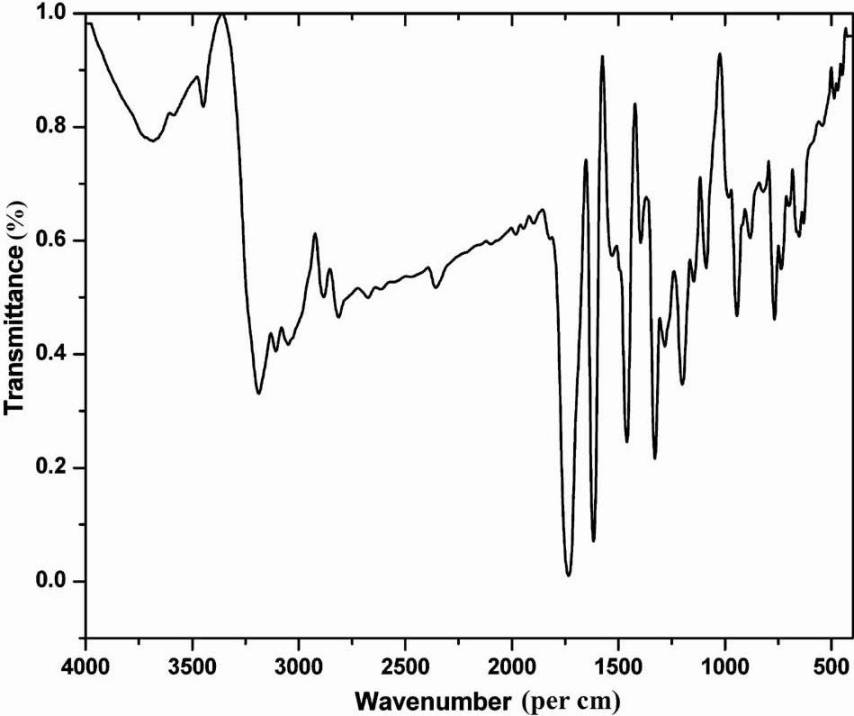
- FTIR spectrum of purified compound (Fraction 3).
The GC-MS was operated at the oven temperature of 250°C with Quadrupole detector. The spectrum of the isolated compound indicated the presence of molecular ion (m/z = 147[M+]). The purified compound was confirmed to be isatin (1H-indole-2,3-dione) and its yield was 3 mg (melting point: 203°C) from 250 g dry powder of flower.
The antioxidant activity of the purified compound was measured by the inhibition of lipid peroxidation. Isatin inhibited the ultrasound induced lipid peroxidation in liposome prepared from egg lecithin. It showed the radical scavenging activity with EC50 of 72.80 μg/ml (Fig. 2). Ascorbic acid was used as positive control with EC50 of 754.14 μm.

- Cytotoxicity and radical modulation activity of isatin. Values are mean ± SD of three separate experiments. Statistical analysis was performed by ANOVA (P<0.05).
The cytotoxicity of isatin was studied in the human promyelocytic leukemia HL60 cells. The 50 per cent cytotoxicity (CC50) values were determined after five days of exposure. Isatin showed cytotoxicity in dose-dependent at manner with CC50 of 2.94 μg/ml (Fig. 2); 5-Fluorouracil was used as positive control with CC50 of 0.07 μm.
Studies were performed to determine if isatin-induced cytotoxicity occurred via an apoptotic pathway. The DNA profile of the cells treated with the purified compound along with control cells was analyzed. In control cells, the DNA was not fragmented whereas the cells treated with isatin showed the apoptosis and DNA fragmentation (data not showm). FACS analysis was carried out to confirm the apoptotic activity (Fig. 3).
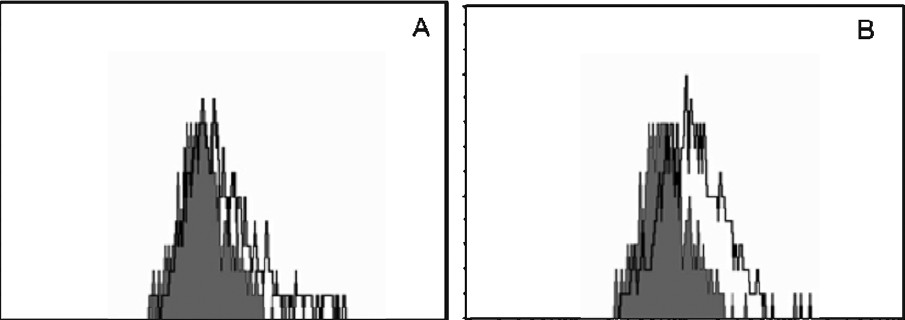
- FACS analysis for apoptosis induction. HL60 cells were treated with isatin and stained with FITC-Annexin V. Shaded histograms represent cellular fluorescence of the control cells and open histograms represent the cellular fluorescence resulting from the specific binding of FITC-Annexin V to apoptotic cells. (A) Cell control, (B) isatin treated.
There are a few reports on the isolation of isatin from the genus Isatis16, in fruits of the cannon ball tree, C. guianensis Aubl9 and in secretions from the parotid gland of the Bufo frog17. Various substituted isatins have also been identified in plants1819, fungi520 and marine molluscs21.
Isatin inhibited the ultrasonic radiation induced lipid peroxidation in liposomal membrane with EC50 of 72.80 μg/ml. Increase in lipid peroxidation denotes cytotoxicity and hepatocellular dysfunction in mice22. In the present study isatin showed antioxidant activity with EC50 value of 72.80 μg/ml and cytotoxicity in HL60 cells with CC50 of 2.94 μg/ml. We have earlier shown that compounds which exhibit high cytotoxicity have less antioxidant activity13.
To interpret the data regarding the mode of cell death after exposure to isatin, it is recommended that a combination of at least three criteria of cell death be evaluated: cell morphology, DNA fragmentation, annexin V binding, and/or caspase activation23. Hence, in the present study isatin induced cytotoxicity was determined through an apoptotic pathway. Isatin started the apoptosis process with fragmentation of DNA (data not shown). Cleavage of DNA at the internucleosomal linker sites yielding DNA fragments is regarded as a biochemical hallmark of apoptosis24. Apoptosis induced by isatin was confirmed by flow cytometry to further elucidate the extent and causes of apoptosis.
In conclusion, isatin was isolated from the floral parts of cannon ball tree and it exhibited antioxidant activity and cytotoxicity against HL60 cells. Isatin can be further evaluated to be used as prophylactic agent to prevent the free radicals induced cancer and as chemotherapeutic agent to kill the cancer cells.
Acknowledgment
The authors thank the Management, Principal of the Mepco Schlenk Engineering College (M.P) and Ayya Nadar Janaki Ammal College (V.T & T.S) Sivakasi, Tamil Nadu, India for providing the necessary facilities to carry out this work. FACS analysis was done with the help of C. Gowri Priya at TIFAC-Center of Relevance and Excellence in Diabetic Retinopathy, Aravind Medical Research Foundation, Aravind Eye Hospital, Madurai, Tamil Nadu, India.
References
- The endogenous oxindoles 5-hydroxyoxindole and isatin are antiproliferative and proapoptotic. Biochem Biophys Res Commun. 2000;276:379-84.
- [Google Scholar]
- N-phenethyl and N-naphthylmethyl isatins and analogues as in vitro cytotoxic agents. Bioorg Med Chem. 2008;16:3118-24.
- [Google Scholar]
- The threesubstituted indolinone cyclin-dependent kinase 2 inhibitor 3-[1-(3H-imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydroindol-2-one (SU9516) kills human leukemia cells via downregulation of Mcl-1 through a transcriptional mechanism. Mol Pharmacol. 2006;70:645-55.
- [Google Scholar]
- Seven new prenylated indole diketopiperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron. 2008;64:7986-91.
- [Google Scholar]
- A novel cdk2-nselective inhibitor, SU9516, induces apoptosis in colon carcinoma cells. Cancer Res. 2001;61:6170-7.
- [Google Scholar]
- SU14813: A novel multiple receptor tyrosine kinase inhibitor with potent antiangiogenic and antitumor activity. Mol Cancer Ther. 2006;5:1774-82.
- [Google Scholar]
- Design, synthesis, antitumor evaluations and molecular modeling studies of novel 3,5-substituted indolin-2-one derivatives. Acta Pharmacol Sin. 2007;28:140-52.
- [Google Scholar]
- The structure and properties of some indolic constituents in Couroupita guianensis aubl. Tetrahedron. 1985;4:12879-81.
- [Google Scholar]
- An ICS. In: Indian medicinal plants (2nd ed). Allahabad, India: Lalit Mohan Basu; 1981.
- [Google Scholar]
- Glossary of Indian medicinal plants (2nd ed). New Delhi: Publication and Information Directorate, Council of Scientific & Industrial Research; 1956.
- The induction of lipid peroxidation in liposomal membrane by ultrasound and the role of hydroxyl radicals. Radiat Res. 1990;124:7-14.
- [Google Scholar]
- Synthesis, anticancer and antioxidant activities of isoflavanone and 2,3-diarylchromanones. Eur J Med Chem. 2010;45:2447-52.
- [Google Scholar]
- In vitro anti human immunodeficiency virus activity of mangrove plants. Indian J Med Res. 1996;103:278-81.
- [Google Scholar]
- TLC-UV-spectrophotometric and TLC-scanning determination of isatin in leaf of Isatis. Zhongcaoyao. 1986;17:8-11.
- [Google Scholar]
- Application of thin-layer chromatography in quality control of Chinese medicinal preparations. II. Qualitative analysis of some Chinese medicinal preparations of Chansu. Yaowu Fenxi Zazhi. 1982;2:288-91.
- [Google Scholar]
- Potential carcinogens.13. The melosatins - a novel class of alkaloids from Melochia-tomentosa. Tetrahedron. 1980;36:2441-7.
- [Google Scholar]
- Picratidine, a new indole alkaloid from Picralima nitida seeds. J Nat Prod. 1990;53:975-7.
- [Google Scholar]
- Isolation and structure elucidation of 6-(3’- methyl-buten-2’-yl)isatin, an unusual metabolite from Streptomyces- albus. J Antibiot. 1986;39:162-3.
- [Google Scholar]
- Indole derivatives from the egg masses of Muricid molluscs. Molecules. 2001;6:70-8.
- [Google Scholar]
- Effect of Homeopathic drug, Chelidolium, in amelioration of p-DAB induced hepatocarcinogenesis in mice. Complement Altern Med. 2002;2:1-9.
- [Google Scholar]
- Current protocols in toxicology. New York: John Wiley & Sons, Inc; 2005.
- A biochemical hallmark of apoptosis: internucleosomal degradation of the genome. Cancer Metastasis Rev. 1992;11:105-19.
- [Google Scholar]






