Translate this page into:
Antibiotic resistance pattern among common bacterial uropathogens with a special reference to ciprofloxacin resistant Escherichia coli
Reprint requests: Dr Subhash Chandra Parija, Professor & Head, Department of Microbiology, Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry 605 006, India e-mail: subhashparija@yahoo.co.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The resistance of bacteria causing urinary tract infection (UTI) to commonly prescribed antibiotics is increasing both in developing as well as in developed countries. Resistance has emerged even to more potent antimicrobial agents. The present study was undertaken to report the current antibiotic resistance pattern among common bacterial uropathogens isolated in a tertiary care hospital in south India, with a special reference to ciprofloxacin.
Methods:
A total of 19,050 consecutive urine samples were cultured and pathogens isolated were identified by standard methods. Antibiotic susceptibility was done by Kirby Bauer disk diffusion method. The clinical and demographic profile of the patients was noted.
Results:
Of the 19,050 samples, 62 per cent were sterile, 26.01 per cent showed significant growth, 2.3 per cent showed insignificant growth and 9.6 per cent were found contaminated. Significant association (P<0.001) of prior use of antibiotics in males, UTI in adults, gynaecological surgery in females, obstructive uropathy in males and complicated UTI in females with the occurrence of UTI with ciprofloxacin resistant Escherichia coli was noted. Significant association was noted in females with prior antibiotics, with prior urological surgery and in males with prior complicated UTI. There was no significant association with diabetes mellitus with the occurrence of UTI with ciprofloxacin resistant E. coli. Fluoroquinolone resistance was found to increase with age.
Interpretations & conclusions:
Ciprofloxacin resistance has emerged due to its frequent use. This resistance was seen more in the in-patients, elderly males and females. Also the resistance to other antibiotics was also high. Increasing antibiotic resistance trends indicate that it is imperative to rationalize the use of antimicrobials in the community and also use these conservatively.
Keywords
Ciprofloxacin
Escherichia coli
minimum inhibitory concentration
urinary tract infection
Urinary tract infections (UTIs) are amongst the most common infections encountered in clinical practice1. The commonest bacterial agent involved in causation of UTIs is Escherichia coli, being the principal pathogen both in the community as well as in the hospital23.
The treatment of UTIs varies according to the age of the patient, sex, underlying disease, infecting agent and whether there is lower or upper urinary tract involvement. Trimethoprim/sulphamethoxazole is the recommended drug for the treatment of UTIs in settings where the prevalence of resistance is <10-20 per cent and ciprofloxacin is recommended where this resistance is >20 per cent, according to the Infectious Diseases Society of America (IDSA) guidelines45. The other agents used in the treatment of UTI include fluoroquinolones, cephalosporins and other β-lactams with or without β-lactamase inhibitors, nitrofurantoin45. Recently, several studies have revealed increasing trends of resistance to many antimicrobials including the fluoroquinolones6–8. The increase in bacterial resistance to fluoroquinolone is multifactorial9–13. With the increasing trend of antibiotic-resistance in E. coli, the management of urinary tract infections is likely to become complicated with limited therapeutic options.
The present study was undertaken to assess the current antibiotic resistance pattern in the common uropathogens isolated in a tertiary care hospital in south India with a special emphasis on ciprofloxacin. Since Escherichia coli is the predominant pathogen, the study was focused on it. Further, risk assessment was also performed to determine the factors responsible for the emergence of ciprofloxacin resistance in E. coli.
Material & Methods
Study site: The study was carried out in the department of Microbiology, Jawaharlal Institute of Postgraduate Institute Medical Education & Research (JIPMER), Puducherry, India, during March 2008 to April 2009. This was an analysis of data generated from the records of consecutive urine samples received in the laboratory during the study period. Only the initial sample of an individual received was included to avoid duplication. Analysis of the data was carried out focussing on the age, gender, whether admitted or not, whether received prior antibiotic therapy, any surgical or gynaecological intervention performed in the recent past, and any history of urinary tract infection in the past. The antibiotic susceptibility data of all isolates were also reviewed and analyzed.
Samples received included mid-stream clean catch urine, suprapubic aspirate, urine collected from Foley's catheter and from the nephrostomy tube under sterile precautions, in patients who had undergone percutaneous nephrostomy. Samples were processed and isolates were identified as per standard methods14. All urine samples were inoculated onto cysteine lactose electrolyte deficient (CLED) medium (Himedia, Mumbai, India) using a calibrated loop (volume-0.005 ml) and were incubated for 18-24 h at 37°C.Wet mount preparations were also made from all urine samples to look for pus cells and epithelial cells. Depending upon the number of the colonies grown on the CLED medium, the interpretations of urine culture were made as insignificant (<50 colonies), doubtful significance (>50 - <500 colonies) and significant (≥500 colonies) with due clinical correlation as per recommendations1415. The antibiotic susceptibility testing of the isolated bacteria was carried out by the Kirby Bauer method1516.
Determination of minimum inhibitory concentration (MIC): The MIC testing was performed as per guidelines1516. The MIC interpretive standards for the susceptibility categories were as per the recommended breakpoints by the Clinical Laboratory Standards Institute (CLSI) for ciprofloxacin16. Ciprofloxacin hydrochloride was obtained from Hi-media (Mumbai, India). The antibiotic was dissolved in sterile distilled water as per the recommendations. The antibiotic was used immediately after reconstitution. The different concentrations of the drug analyzed were 0.5 to 256 μg/ml1516. ATCC E. coli 25922 was inoculated on each plate as the growth control. The growth control was read first followed by the MICs of the test strains1516.
Statistical analysis: The chi-square test or Fisher's exact test was used to compare different groups. Relative risk and odds ratio were determined to compare the risk factors in the different groups of interest (UTI due to ciprofloxacin resistant E. coli and UTI due to E. coli). Statistical softwares GraphPadInStat3 software (GraphPadInc, San Diego, USA) and SPSS 16.0 (SPSS Inc, Chicago, Illinois, USA) were used to analyse the data.
Results
Data from a total of 19,050 consecutive urine samples were included in the study. Of these, 11,811 (62%) were sterile, 4956 (26.01%) showed significant growth, 438 (2.3%) showed insignificant growth and 1828 (9.6%) were found contaminated. Wet mount microscopy for presence of bacteria or pus cells in significant amount per field had sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of 83, 58, 44 and 89 per cent, respectively in detecting infections.
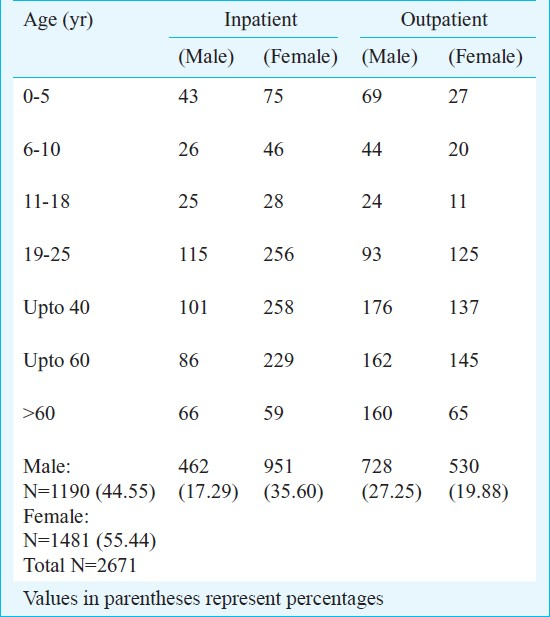
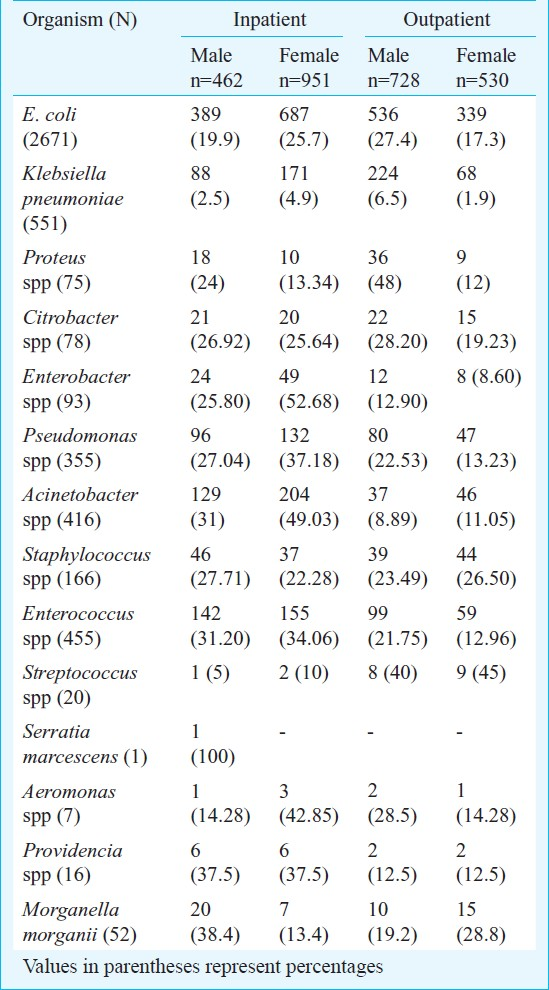
Of the 4956 culture positives, E. coli was the most common (59%) isolate. (Table I). The percentage of Klebsiella pneumoniae, Acinetobacter spp, Pseudomonas spp, Staphylococcus spp, Enterococcus faecalis was higher in patient females and patients with history of prior treatment with antibiotics, compared to the rest. In addition to these isolates, the percentage of Proteus spp was more in males, especially in cases with prior history of antibiotics administration (Table I).
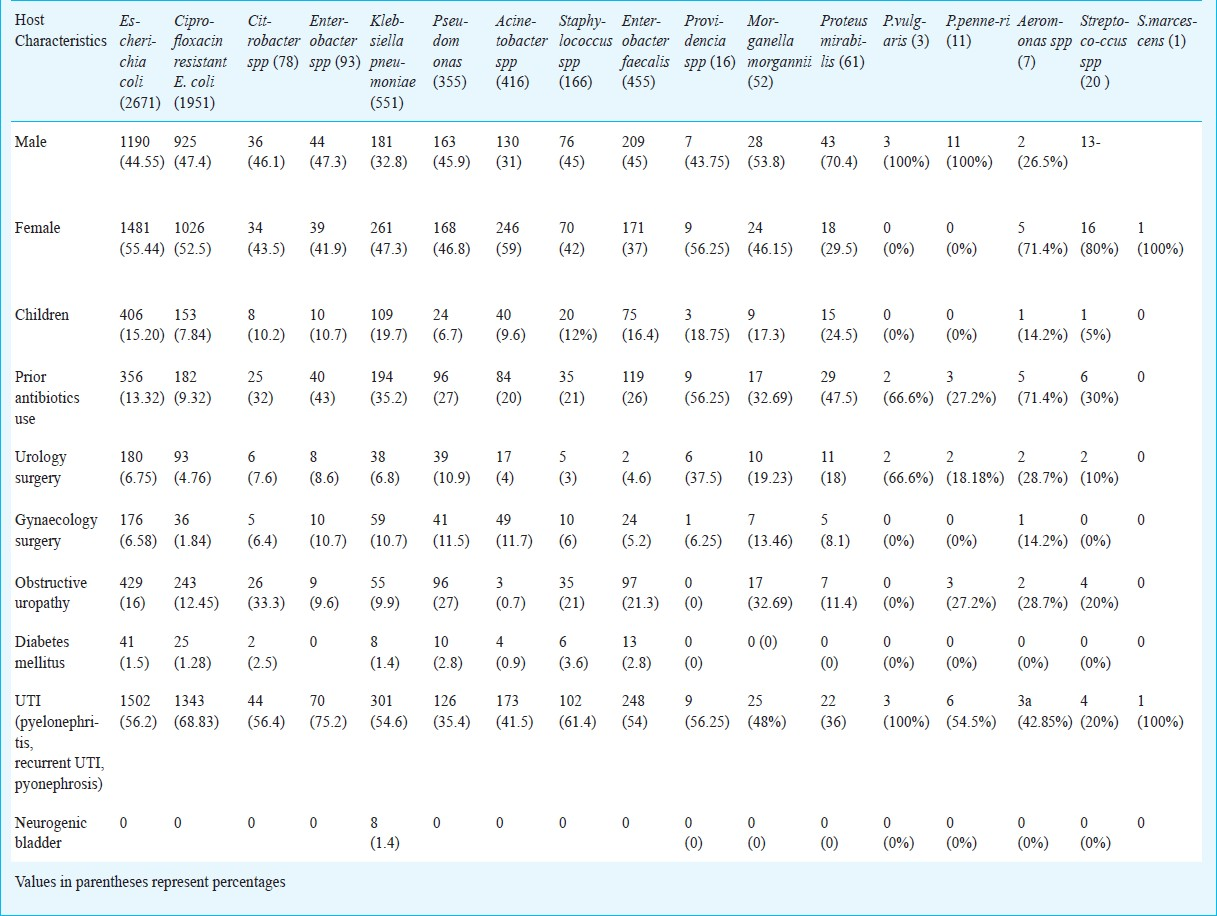
Seventy three per cent of all E.coli isolates were found to be resistant to ciprofloxacin.Ciprofloxacin resistance was comparatively less among the other Gram-negative uropathogens like Pseudomonas spp, Proteus spp and K. pneumoniae as mentioned (Table II). Resistance to the aminoglycosides amikacin and gentamicin was also considerable especially among isolates of Acinetobacter spp with as many as 68.9 per cent of all Acinetobacter isolates showing resistance to gentamicin and 58.6 per cent to amikacin. The percentage of isolates of E. coli resistant to ampicillin was found to be as much as 80.6 per cent.
The rates of resistance among Gram-negative uropathogens to third generation cephalosporins like ceftriaxone and ceftazidime were high. Ceftriaxone resistance were seen in 60.5 and 59.3 per cent of all isolates of E. coli and K. pneumoniae. Resistance to ceftazidime among the Gram- negative non-fermenters was also considerable. 60 per cent of all Acinetobacter isolates and 44.5 per cent of all Pseudomonas spp isolates were found to be resistant to ceftazidime (Table II). Compared to the other Gram-negative uropathogens, resistance to the urinary antiseptic nitrofurantoin was comparatively less among isolates of E. coli.
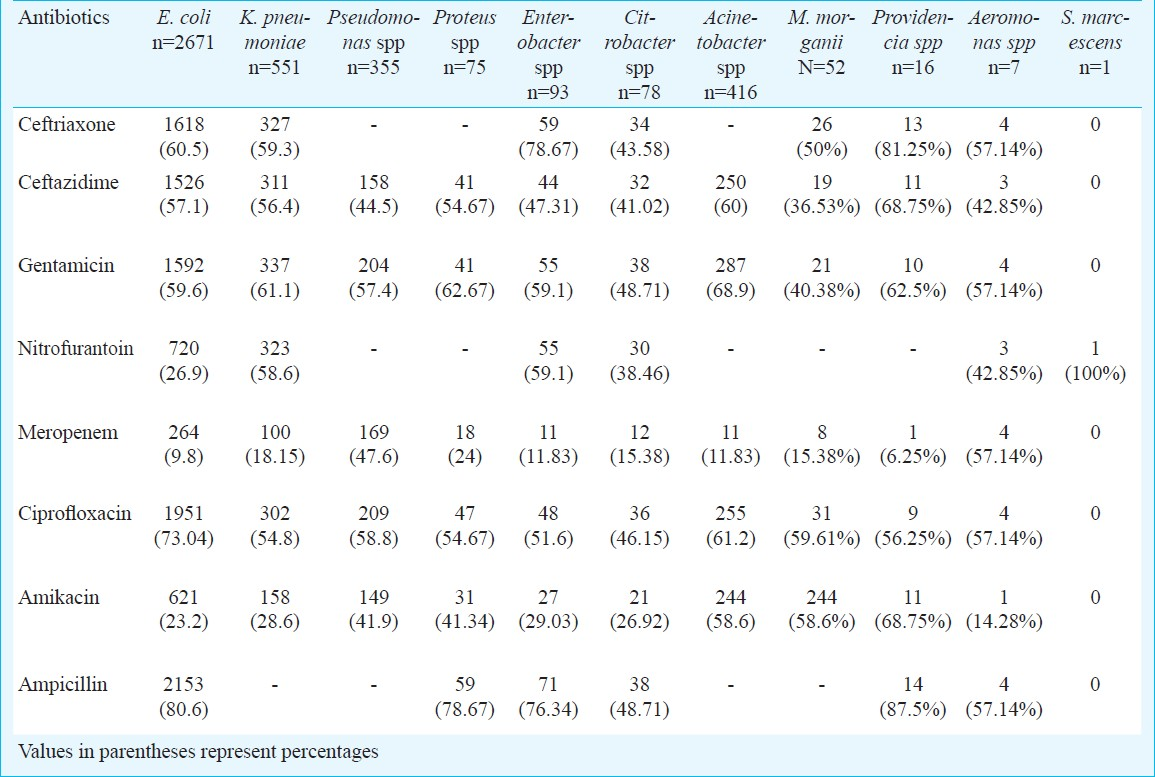
Amongst the Gram-positive isolates, Enterococcus faecalis was the most commonly isolated organism with 3.2 per cent resistance to vancomycin. Amongst the Streptococcus spp, 3 (15%) were identified as S. agalactiae, which were isolated from antenatal women and were sensitive to all the antimicrobial agents. Substantial number of Staphylococcus [109 (65.6%)] and Streptococcus [11 (55%)] isolates were resistant to ciprofloxacin. Resistance to nitrofurantoin was comparatively more amongst the Enterococcus spp (Table III).
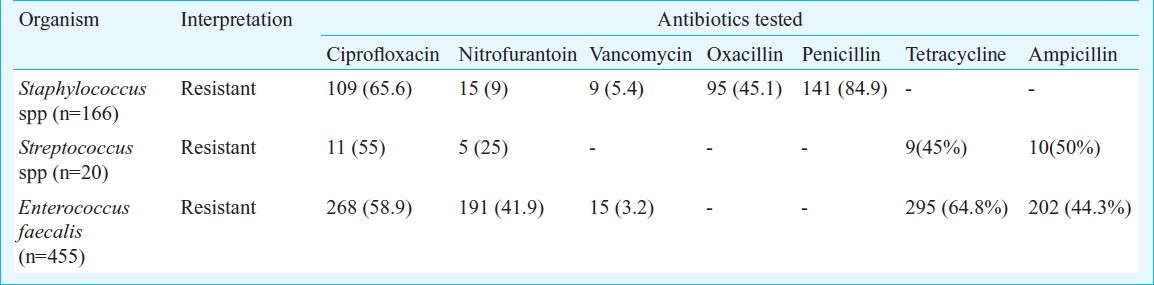
Our findings indicate that prior antibiotic therapy especially flouroquinolone therapy and post-operative patients of gynecological surgeries, were significant risk factors for the emergence of fluoroquinolone resistant E. coli (Table IV). The difference between the resistance patterns amongst the inpatients (IP) and the outpatients (OP) was very minimal and no significant difference (P=0.0981) was noted between the two groups (Tables V and VI). But, there was a significant difference (P=0.0058) noted between the IP and the OP groups particularly in the age group of 19 to 25 yr with UTI due to ciprofloxacin resistant E. coli. Though E. coli was the commonest organism associated with UTI in both the IP and OP patients, there were many other unusual isolates like Psuedomonas spp which were isolated both from the OP patients (Table VII).

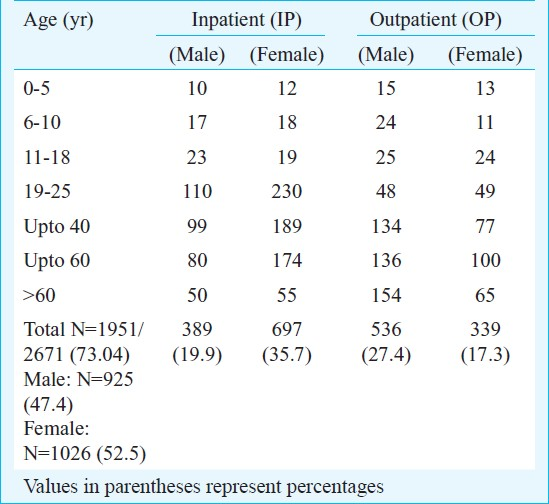
A total of 150 consecutive isolates of E. coli were subjected to MIC determination. Of these, 137 were ciprofloxacin resistant while 23 were sensitive. MIC50 was found to be between 32 and 64 μg/ml, while MIC90 was determined to be at 128 μg/ml for ciprofloxacin. It was noted that 5 of 150 (3.33%) isolates had MIC at 256 μg/ml, 106 (70.67%) had MIC at 64 μg/ml, 58 (38.66%) at 32 μg/ml, 37 (24.66%) at 16 μg/ml, 26 (17.33%) had MIC at 8 μg/ml and 23 (15.33%) had MIC at 4 μg/ml. The resistance pattern varied in different age groups. In the 0-5 years group, it was noted that (10 out of 150) 6.6 per cent isolates of E. coli were ciprofloxacin resistant whereas the number of isolates resistant to ciprofloxacin in the 6-10 and 10-18 yr groups was equal i.e. (7 of 150) 4.6 per cent. In the adults, in the age group of 19-25 yr, the resistance was noted in (32 out of 150) 21.3 per cent. In the age group of >25 yr up to 40 yr, the resistance was found to be (39 out of 150) 26 per cent and in the age group of >40 up to 60 yr, the resistance was found to be 27 (18%). In the elderly (>60 yr), the resistance was found to be 18.6 per cent (28 out of 150).
Discussion
Ciprofloxacin and ofloxacin are the most extensively used fluoroquinolones for the treatment of UTIs.
This study showed that E. coli was the commonest pathogen causing complicated and uncomplicated UTI as described previously1–3. There are several organisms known to cause UTIs, including P. aeruginosa, S. saprophyticus, S.epidermidis, Enterococcus spp, P. mirabilis, Klebsiella spp., Citrobacter spp, etc. as reported by earlier workers1718. This study also demonstrates (Table VII) the emergence of E. faecalis and the non-fermenters Acinetobacter spp and Pseudomonas spp as major uropathogens especially in the patients admitted in the hospitals, more so in the intensive care units. Such findings have been documented elsewhere5–1619–22. The percentage of isolates of E.coli resistant to ampicillin was found to be as much as 80 per cent in our set up. Such high levels of resistance to ampicillin have been quoted by many other studies from different parts of India. Gupta et al23 in a study from the northern part of the country reported 76 per cent resistance to E.coli isolates for ampicillin. A more recent study from Karnataka reported a resistance rate of 80.6 per cent for ampicillin24.
Our MIC results showed that fluoroquinolone resistance increased significantly with patient's age. An MIC of 256 μg/ml was noted in the age group of >60 yr of age. There could be two explanations for this. Firstly, as a consequence of frequent exposure to fluoroquinolones resulting from the treatment of repeated infections in elderly leads to increase in MIC of fluoroquinolone19. Secondly, unlike urinary tract infections (UTIs) in females, UTIs in males are frequently complicated and are more likely to require prolonged antimicrobial therapy, especially in the elderly, potentially explaining the fluoroquinolone the higher MIC2526. Moreover, fluoroquinolones are used to treat chronic prostatitis, even though these do not all readily penetrate the prostate192728. Such doses of fluoroquinolones produces “sub-inhibitory concentration effects” which leads to the selection of mutants exhibiting resistance. All the elderly males, from whom the resistant isolates with high MIC were obtained, had prostatitis. In isolates from children below 18 yr, the MIC was 4 μg/ml. Amongst the cases with MIC >4 μg/ml, 14.6 per cent had obstructive uropathy, 6.6 per cent had undergone uro-surgery, 13.3 per cent had undergone gynaecological procedures, 22 per cent had indwelling catheter, and 28 per cent had history of prior antibiotic therapy.
The emergence of resistance for fluoroquinolones is multifactorial7171920. Resistance to ciprofloxacin has emerged in a variety of genera of the family Enterobacteriaceae2728. Apart from the notable resistance of E. coli to ciprofloxacin, other organisms were also found to be resistant to ciprofloxacin especially K. pneumoniae, Citrobacter spp, Pseudomonas spp, Acinetobacter spp, Proteus spp and Enterobacter spp, Staphylococcus spp, and E. faecalis. Also, fluoroquinolone resistance in E. coli has emerged particularly in patients with urinary tract infections who have received fluoroquinolone prophylaxis5–12. An association between the increase in quinolone prescriptions and an increase in bacterial resistance has been reported from several countries5–812. Usually, the prevalence of fluoroquinolone resistance is related to the intensity of antibiotic use5. Resistance rates for ciprofloxacin against uncomplicated UTI pathogens were reported as 0-14.7 per cent in the ECO-SENS Project, 2.5 per cent in the USA and 1.2 per cent in outpatients in Canada278.
In conclusion, the present results in increasing antibiotic resistance trends in UTI patients indicate that it is imperative to rationalize the use of antimicrobials and to use these conservatively.30
References
- Bacterial infections of the urinary tract. In: Borriello P, Murray PR, Funke G, eds. Topley ' Wilson's microbiology & microbial infections (10th ed). London: Hodder Arnold Publishers; 2007. p. :671-83.
- [Google Scholar]
- Prevalence of anti microbial resistance among urinary tract pathogens isolated from female outpatients across the US in 1999. Int J Antimicrob Agents. 2001;18:121-7.
- [Google Scholar]
- Urinary tract. In: Gorbach SL, Bartlett JG, Balcklow NR, eds. Infectious diseases. Philadelphia: Lippincott Williams & Wilkins Publisher; 2004. p. :861-81.
- [Google Scholar]
- Guidelines for the treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Clin Infect Dis. 1999;29:745-58.
- [Google Scholar]
- Relationship between fluoroquinolone use and changes in susceptibility to fluoroquinolones of selected pathogens in 10 United States teaching hospitals, 1991-2000. Clin Infect Dis. 2003;37:1643-8.
- [Google Scholar]
- Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J Antimicrob Chemother. 2005;56:914-8.
- [Google Scholar]
- Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrob Agents Chemother. 2002;46:2540-5.
- [Google Scholar]
- An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO·SENS Project. J Antimicrob Chemother. 2003;51:69-76.
- [Google Scholar]
- A study of recurrent urinary tract infection in women attending the outpatient department of SMHS hospital, Srinagar, Kashmir, India. JK - Practitioner. 2004;11:272-3.
- [Google Scholar]
- Virulence factors, serotypes and antimicrobial susceptibility patterns of Escherichia coli in urinary tract infections. AJMS. 2009;2:47-51.
- [Google Scholar]
- Urinary tract infections: A retrospective survey of causative organisms and antibiotics prescribed in a tertiary setting. Indian J Pharmacol. 2002;34:278-80.
- [Google Scholar]
- Risk factors for acquisition of urinary tract infections caused by ciprofloxacin-resistant Escherichia coli. J Urol. 1995;153:117-20.
- [Google Scholar]
- Laboratory strategy in the diagnosis of infective syndromes. In: Collee JG, Duguid JP, Fraser AG, Marmion BP, Simmons A, eds. Mackie & McCartney practical medical microbiology (14th ed). New York: Churchill Livingstone; 1999. p. :84-90.
- [Google Scholar]
- Susceptibility Test Methods: Dilution and Disk Diffusion methods. In: Murray PR, Baron EJ, Jorensen JH, Landry ML, Michael AP, eds. Manual of clinical microbiology (10th ed). Washington, D.C: American Society for Microbiology Press; 2007. p. :1152-72.
- [Google Scholar]
- Clinical Laboratories Standards Institute (CLSI) In: Performance of standards for antimicrobial disk susceptibility tests;approved standards Vol 29. (10th ed). Wayne, PA: CLSI; 2009. M02-A10
- [Google Scholar]
- Emerging mechanisms of fluoroquinolone resistance. Emerg Infect Dis. 2001;2:338-41.
- [Google Scholar]
- Antibiotic prescribing and urinary tract infection. Int J Antimicrob Agents. 2002;20:407-11.
- [Google Scholar]
- Increased fluoroquinolone resistance with time in Escherichia coli from >17,000 patients at a large county hospital as a function of culture site, age, sex, and location. BMC Infect Dis. 2008;8:4-10.
- [Google Scholar]
- Fluoroquinolones and resistance in the treatment of uncomplicated urinary tract infection. Int J Antimicrob Agents. 2003;22:S65-S72.
- [Google Scholar]
- Prevalence and antibiotics susceptibility of uropathogens in patients from a rural environment, Tamil Nadu. Am J Infect Dis. 2010;6:29-33.
- [Google Scholar]
- Increasing emergence of antibacterial resistance mainly in uropathogens:southeast part of India. Intl J Microbiol Res. 2010;2:1-6.
- [Google Scholar]
- Antimicrobial susceptibility of uropathogens in India. J Infect Dis Antimicrob Agents. 2007;24:13-8.
- [Google Scholar]
- Changing trends in the spectrum of antimicrobial drug resistance pattern of uropathogens isolated from hospitals and community patients with urinary tract infections in Tumkur and Bangalore. Int J Biol Med Res. 2011;2:504-7.
- [Google Scholar]
- Antibiotic resistance of urinary pathogens in female general practice patients. Scand J Infect Dis. 2005;37:256-61.
- [Google Scholar]
- Antibiotic resistance of Escherichia coli from community-acquired urinary tract infections in relation to demographic and clinical data. Clin Microbial Infect. 2005;11:199-203.
- [Google Scholar]
- Concentrations of gatifloxacin in plasma and urine and penetration into prostatic and seminal fluid, ejaculate, and sperm cells after single oral administrations of 400 milligrams to volunteers. Antimicrob Agents Chemother. 2001;45:293-7.
- [Google Scholar]
- Fluoroquinolone antimicrobial agents in the treatment of prostatitis and recurrent urinary tract infections in men. Curr Infect Dis Rep. 2005;7:9-16.
- [Google Scholar]
- Effect of subinhibitory concentrations of ciprofloxacin on Mycobacterium fortuitum mutation rates. J Antimicrob Chemother. 2005;56:344-8.
- [Google Scholar]
- Comparison of the postantibiotic and postantibiotic sub-MIC effects of levofloxacin and ciprofloxacin on Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:950-5.
- [Google Scholar]






