Translate this page into:
Anti-angiogenic therapy in ovarian cancer: current situation & prospects
For correspondence: Dr Yaping Zhu, Department of Obstetrics & Gynecology, Shanghai General Hospital, Shanghai Jiaotong University School of Medicine, No. 650 Xin Songjiang Road, Shanghai, 200 080, PR China e-mail: zhuyp63@126.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Ovarian cancer (OC) is one of five leading causes of cancer related death among women worldwide. Although treatment has been improving, the survival rate has barely improved over the past 30 years. The fatality rate is due to asymptomatic early signs and the lack of long-term effective treatment strategies for advanced disease. Angiogenesis is an important process in tumour growth and metastasis and is the creation of new blood vessels from existing blood vessels. It is a dynamic and complex process involving various molecular regulatory pathways and multiple mechanisms. The inhibition of angiogenesis has become a recognized therapeutic strategy for many solid tumours. While benefits in progression-free survival have been observed, the OS is far from satisfactory for OC patients who receive antiangiogenic therapy. In this article, the present research status of angiogenesis in OC was reviewed and the reasons for poor antiangiogenic therapeutic effects was explored with the aim to identify potential therapeutic targets that may improve the effect of antiangiogenic therapies.
Keywords
Angiogenesis
antiangiogenic therapy
bevacizumab
ovarian cancer
therapeutic targets
Ovarian cancer (OC) is the most lethal of gynaecological malignancies, and high-grade serous ovarian carcinoma (HGSOC) has the highest degree of malignancies1. Although the methods of surgery and chemotherapy have improved in the recent decades, the progression-free survival (PFS) of OC is only 16 to 22 months, and the five-year survival rate is less than 30 per cent2. Furthermore, 60 to 80 per cent of patients with OC can achieve complete remission after surgery and first-line chemotherapy, but 80 per cent of these patients will eventually die due to drug resistance or recurrence34. Because of the poor prognosis and limited treatment options, researchers are constantly looking for new ways to treat advanced OC. Angiogenesis, the process of generating new capillaries from existing blood vessels, is the key to the growth and metastasis of many solid tumours, including OC5. During the process of rapid growth and metastasis, tumour cells continuously secrete many related factors that promote angiogenesis, such that new vascular networks are continuously generated in the tumour tissues for the rapid proliferation of tumour cells6. If there is no blood supply, tumours cannot grow to more than 1~2 mm7. Therefore, tumour blood vessels are an important target for tumour treatment89.
Pathways of vessel formation in OC
Four main means of a tumour constructing new vessels are shown in Figure 1.
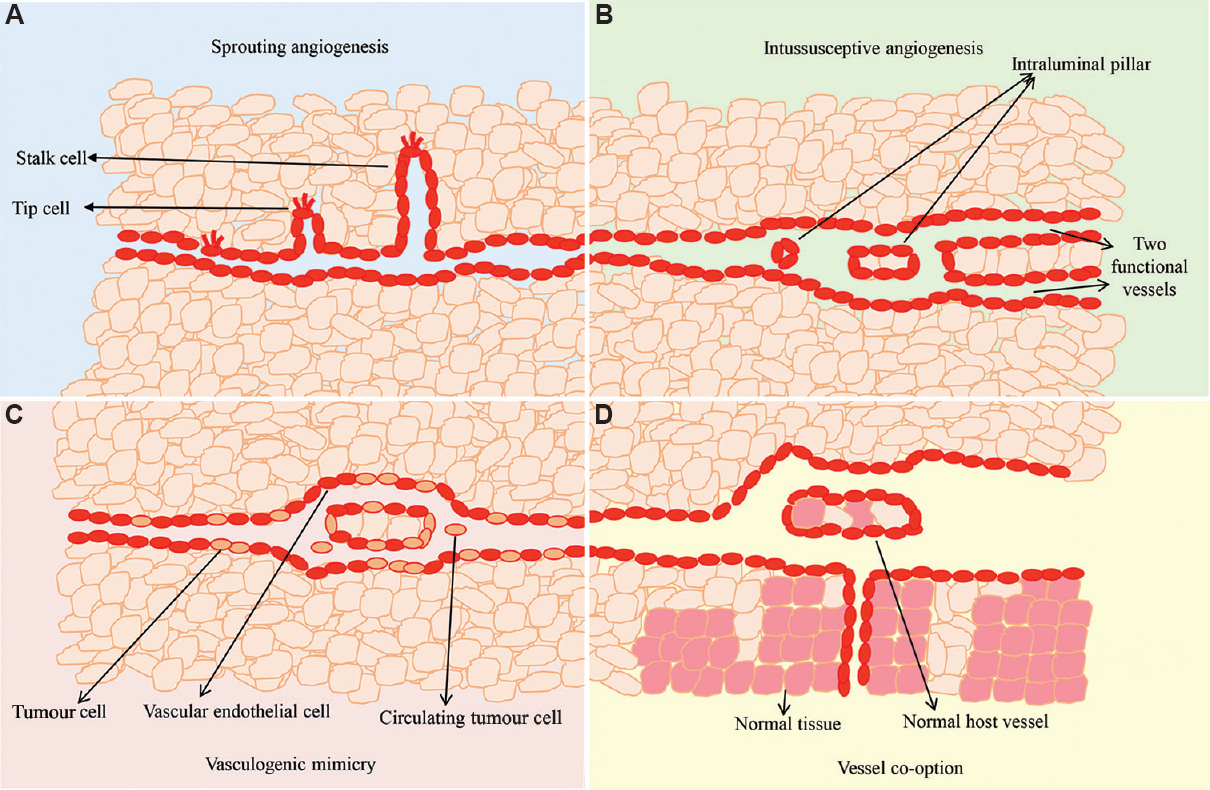
- Tumour neovascularization. (A) Sprouting angiogenesis: new blood vessels are produced from an existing blood vessel and are elongated. (B) Intussusceptive angiogenesis: insertion of interstitial tissue pillars into the lumen of pre-existing vessels to split it into two new functional vessels. (C) Vessel cooption: tumour cells obtain their blood supply by hijacking and moving along the pre-existing vasculature of the host organ. (D) Vasculogenic mimicry: tumour cells simultaneously express endothelial and tumour cell markers to form a channel structure for blood perfusion. Source: Refs 6,11,12,15,16,17,20.
Sprouting angiogenesis: The most common form of angiogenesis in the development of most cancers, including OC, is when new blood vessels are produced from an existing blood vessel and are elongated. In the process of neovascularization, there are two basic cell types involved, the tip and the stalk cells. Tip cells are located at the front of blood vessels and stimulate angiogenesis in the microenvironment through their motile filopodia, while the stalk cells align right behind the tip cells and proliferate speedily, by which the sprouting branch is elongated and the process of lumenization begins6. The differentiation of both these cell types is mainly controlled by the vascular endothelial growth factor A (VEGFA) and Notch signalling pathways10.
Intussusceptive angiogenesis: A typical feature of this type of angiogenesis is the formation of intraluminal pillars. The intussusceptive process is characterized by the insertion of interstitial tissue pillars into the lumen of existing vessels to divide the pre-existing vessel into two new functional vessels11. Intussusceptive angiogenesis is significantly better than angiogenic sprouting. Neovascularization is faster and has less metabolic requirements than those generated by angiogenic sprouting because these are independent of EC proliferation, membrane degradation and tissue invasion. Tumours use this strategy to rapidly adapt to changing environments12. Intussusceptive angiogenesis has been found in many tumours, such as intestinal cancer and melanoma1314. However, to date, the underlying molecular mechanisms of this process remains unclear.
Vessel cooption: Vessel co-option is a mechanism through which tumour cells obtain blood supply by hijacking and moving along the pre-existing vascular system of host organs. This mechanism often occurs in organs with abundant blood supply, such as the brain and liver151617.
Vasculogenic mimicry: Vasculogenic mimicry (VM) refers to the behaviour of tumour cells, which is similar to that of endothelial cells; tumour cells simultaneously express endothelial and tumour cell markers to form a channel structure for blood perfusion. In VM, tumour cells express markers of vascular endothelial cells, such as VE-cadherin1819, which play an important role in maintaining pipeline integrity by establishing connections between cells20. In addition, tumour cells can be directly integrated into the tumour-associated blood vessels, which allows the tumour cells to be directly exposed to the bloodstream, leading to tumour metastasis; this process has been reported in a variety of malignant tumour types, including breast, ovarian, prostate, bladder and lung cancers, as well as sarcomas and gliomas202122. In addition, tumour VM is correlated with metastatic tumours and closely associated with poor prognosis in cancer patients232425.
Tumour vascular characteristics
Aggressive growth of the tumour cell population and overexpression of proangiogenic factors lead to vascular network disorder. The typical characteristics of tumour vascular system are abnormal structural dynamics, immature, tortuous and hyperpermeable vasculature92627. The abnormal characteristics lead to aberrant microenvironmental conditions that obstruct traditional anticancer treatment strategies28. Microregional hypoxia can lead to resistance to radiotherapy and chemotherapy. Despite these phenomena, the unique characteristics of the tumour vascular system also present an opportunity for selective therapeutic interventions compared to the normal tissue vasculature29.
Current status of antiangiogenic agents in OC
Bevacizumab is a humanized anti-VEGF monoclonal antibody that was the first to be widely studied and is currently the most widely applied antiangiogenic drug in a variety of tumours, including epithelial OC30. In 2014, bevacizumab was approved by the US Food and Drug Administration for the treatment of platinum-resistant epithelial OC. There have been several randomized phase III clinical trials that evaluated bevacizumab combined with first-line chemotherapy for recurrent OC. The results of these trials showed that the PFS of OC patients improved, but the benefit in patient OS was modest (Table I)31. ICON-7 and GOG-218 were the first two front-line phase III trials that tested bevacizumab in combination with chemotherapy (carboplatin and paclitaxel)3233. ICON-7 enrolled 1528 OC patients, 70 per cent of whom were stage IIIc or IV, the median PFS of bevacizumab arm was significantly improved (19.8 months vs. 17.4 months). GOG-218 was a placebo-controlled three-arm study (paclitaxel + carboplatin + placebo; placebo maintenance versus paclitaxel + carboplatin + bevacizumab; placebo maintenance versus paclitaxel + carboplatin + bevacizumab; bevacizumab maintenance), the median PFS of bevacizumab throughout the arm was significantly improved (3.8 months). Three randomized phase III trials (GOG-213, OCEANS and AURELIA) evaluated bevacizumab in recurrent cases of OC. GOG-213 enrolled 674 platinum sensitive relapse patients. The addition of bevacizumab significantly extended PFS (13.8 vs 10.4 months), but there was no significant improvement in OS (P=0.56, HR: 0.83)36. OCEANS trial evaluated the benefits of adding bevacizumab to gemcitabine and carboplatin in platinum sensitive patients. The results showed that PFS improved for four months, but no improvement was observed in OS35. The AURELIA trial showed that combined bevacizumab with cytotoxic regimens extended the PFS of platinum resistant patients (6.7 vs 3.4 months)34. Other antiangiogenic agents that target the VEGF/VEGF receptor (VEGFR) signalling pathway are multitarget tyrosine kinase inhibitors (TKIs) and corresponding phase III clinical trials have been carried out, including nintedanib (OVAR-12)37, cediranib (ICON6)38 and pazopanib (OVAR-16)39. These TKIs have multiple targets that are different from those of bevacizumab (Table II); although their mechanism of action is more attractive than that of bevacizumab, TKIs do not show significant advantages in terms of patient prognosis compared with drugs that target VEGF alone41, such as bevacizumab (Table III). In addition, because of the wide range of targets, there is the possibility of severe toxic side effects. OC has been treated with a variety of antiangiogenic drugs so far, but the results so far have not been promising. Although the PFS has been observed to be prolonged in some clinical trials, the OS has not improved significantly.
| Study | Agent | n | Setting | Treatment arm | Clinical outcomes (media PFS, media OS, months) |
|---|---|---|---|---|---|
| GOG-21832 | Bevacizumab | 1873 | Front-line and maintenance | Paclitaxel+carboplatin+placebo; placebo maintenance versus paclitaxel+carboplatin+bevacizumab; placebo maintenance versus paclitaxel+carboplatin+bevacizumab; bevacizumab maintenance | PFS: 10.3 versus 11.2 versus 14.1 (HR, 0.908; P=0.16)1 (HR, 0.717; P<0.001)2 OS: 39.3 versus 38.7 versus 39.7 (HR, 1.036; P=0.76)1 (HR, 0.915; P=0.45)2 |
| ICON-733 | Bevacizumab | 1528 | Front-line and maintenance | Paclitaxel+carboplatin versus paclitaxel+carboplatin+bevacizumab; bevacizumab maintenance | PFS: 17.4 versus 19.8 (HR: 0.87; P: 0.04) OS: 58.6 versus 58.0 (HR: -; P: -) |
| AURELIA34 | Bevacizumab | 361 | Recurrent, platinum-resistant | Chemotherapy (paclitaxel–-weekly, topotecan–-daily ×5 or weekly, PLD) versus chemotherapy+bevacizumab | PFS: 3.4 versus 6.7 (HR: 0.48; P<0.001) OS: 13.3 versus 16.6 (HR, 0.85; P=0.174) |
| OCEANS35 | Bevacizumab | 484 | Recurrent, platinum-sensitive | Gemcitabine+carboplatin+placebo (combination and maintenance) versus gemcitabine+carboplatin+bevacizumab (combination and maintenance) | PFS: 8.4 versus 12.4 (HR=0.484; P<0.0001) OS: 33.6 versus 32.9 (HR=0.96, P=0.736) |
| GOG-21336 | Bevacizumab | 674 | Recurrent, platinum-sensitive | Paclitaxel+carboplatin versus paclitaxel+carboplatin+bevacizumab; bevacizumab maintenance | PFS: 10.4 versus 13.4 (HR=0.61; P<0.0001) OS: 37.3 versus 42.2 (HR=0.829; P=0.056) |
Source: Adapted with permission from ref 31. 1Paclitaxel+carboplatin+placebo; placebo maintenance versus paclitaxel+carboplatin+bevacizumab; placebo maintenance, 2Paclitaxel+carboplatin+placebo; placebo maintenance versus paclitaxel+carboplatin+bevacizumab; bevacizumab maintenance. PFS, progression-free survival; OS, overall survival; HR, hazard ratios
| Agent | Route of administration | Targets |
|---|---|---|
| Nintedanib37 | Oral | VEGFR, FGFR, and PDGFR |
| Pazopanib39 | Oral | VEGFR, PDGFR, FGFR, c-Kit, and c-Fms |
| Cediranib40 | Oral | VEGFR |
VEGFR, vascular endothelial growth factor receptor; FGFR, fibroblast growth factor receptor; PDGFR, platelet-derived growth factor receptor
| Study | Agent | n | Setting | Treatment arm | Clinical outcomes [media PFS, months, HR (95%CI)] |
|---|---|---|---|---|---|
| OVAR-1237 | Nintedanib | 1366 | Front-line and maintenance | Paclitaxel+carboplatin+placebo; placebo maintenance versus paclitaxel+carboplatin+nintedanib; nintedanib maintenance | PFS: 16.6 vs. 17.3 (HR=0.84, P=0.024) |
| OVAR-1639 | Pazopanib | 940 | Maintenance | Placebo versus pazopanib | PFS: 12.3 vs. 17.9 (HR=0.77, P=0.0021) |
| ICON638 | Cediranib | 456 | Recurrent, platinum-sensitive | Chemotherapy (paclitaxel or gemcitabine combination) or single agent carboplatin+placebo; placebo maintenance versus chemotherapy+cediranib; placebo maintenance versus chemotherapy+cediranib; cediranib maintenance | PFS: 8.7 vs. 10.1 vs. 11.1 HR=0.67 (0.53-0.87)# HR=0.57 (0.44-0.74)† |
Source: Adapted with permission from Ref 31. #Chemotherapy (paclitaxel or gemcitabine combination) or single agent carboplatin+placebo; placebo maintenance versus chemotherapy+cediranib; placebo maintenance; †Chemotherapy (paclitaxel or gemcitabine combination) or single-agent carboplatin+placebo; placebo maintenance versus chemotherapy+cediranib; cediranib maintenance; PFS, progression-free survival; OS, overall survival; HR, hazard ratios; CI, confidence interval
Mechanisms of resistance and biomarkers
Because of the high cost and potential side effects of these drugs, only a small percentage of patients can benefit; therefore, a better understanding of bevacizumab resistance mechanisms and the identification of predictive biomarkers are essential.
Endogenous or acquired resistance is considered to be the main cause of failure in antiangiogenic therapy42. Based on some preclinical studies, several drug resistance mechanisms against vascular therapy have been proposed. First, antiangiogenic therapy aggravates tumour hypoxia, which leads to elevated hypoxia inducible factor 1A (HIF1A) levels, the stimulation of angiogenesis-related factors, such as fibroblast growth factors (FGFs), angiotensins (ANGs) and interleukin-8, and a high risk of tumour invasion and metastasis434445. Second, when VEGF activity is neutralized, blood perfusion in the tumour tissue is significantly reduced, thereby impairing the killing effect of chemotherapy and radiotherapy on the tumour. Third, anti-VEGF therapy blocks the VEGF/VEGFR-dependent angiogenic pathways but results in the upregulation of VEGF-independent angiogenesis mechanisms, such as those associated with angiopoietin 1 (Ang1), Dll4/Notch and microRNAs (miRNA)46. Fourth, immune responses can lead to the recruitment of pro-angiogenic monocytes from the bone marrow, induction of hypoxia, or high pericyte coverage of the tumour vascular system47. According to data from 2011 from the Cancer Genome Atlas, HGSOC is divided into four subtypes, and among them proliferative and mesenchymal subtypes are associated with poorer survival, but derive a comparably greater benefit from treatment of bevacizumab than the other two subtypes. In contrast, bevacizumab conferred modest improvements in the PFS of patients with the immunoreactive subtype or differentiated subtype48. The above data indicate that patients should be classified when receiving vascular-targeted drugs, such as bevacizumab, to ensure the efficacy of the targeted drugs, reduce side effects, as well as medical costs. Fifth, the heterogeneity of tumour cells allows some tumour cell subsets to survive under hypoxic conditions, thereby increasing the risk of invasion and metastasis. The heterogeneity of the tumour vasculature itself in tumour tissue represents a difference in demand for VEGF4950. This may be the most critical mechanism to explain endogenous antiangiogenic resistance. Four angiogenic patterns are present in OC tissues, as mentioned above, three of which are nonvascular endothelium dependent. Differences between individuals, different proportions of vascular subtypes in different tumour tissues, and changes in the ratio between VEGF-dependent and VEGF-independent vascular subtypes during antiangiogenic therapy lead to resistance to antiangiogenic therapy. Several other candidate markers, such as plasma protein levels, circulating endothelial cells, and free DNA, have also been proposed, but have not been verified51.
Prospects for antivascular therapeutic targets
The application of vascular-targeted therapy in OC is not successful. In addition, in breast cancer and liver cancer, studies have shown that antivascular therapy increases the risk of tumour invasion and metastasis525354, but the reason is so far unclear. One possible mechanism is the lack of oxygen. Hypoxia is a typical characteristic of most solid tumours and is related to the overexpression of hypoxia response pathway molecules. Overexpression of HIF1 protein leads to enhanced tumour angiogenesis, proliferation, invasion, metastasis and apoptosis resistance. Therefore, the HIF1 protein is a promising target for improving the sensitivity to chemoradiotherapy in cancer patients and improving the survival rate. However, no HIF1 protein inhibitor has been applied in clinical research55.
The phase III clinical trials of OC that combined inhibitors targeting VEGF/VEGFR angiogenic pathways with various chemotherapy drugs found that PFS was significantly improved in patients, but the difference in OS was not significant34. In response to this phenomenon, some scholars have proposed the concept of tumour vascular normalization which indicates that chemotherapy and radiotherapy combined with VEGF/VEGFA angiogenesis signalling pathway inhibitors can promote tumour vascular maturation and improve clinical symptoms in a short span of time56. The secretion of large amounts of VEGF by tumour cells results in the formation of immature blood vessels that lack pericyte coverage. A certain dose of VEGF/VEGFR inhibitor can restore the tumour angiogenesis signal to a certain extent. By strengthening the tight link between cells, actively recruiting pericytes, reducing tumour vascular permeability, and increasing blood flow perfusion of tumour tissue, the sensitivity to radiotherapy or chemotherapy can be increased. However, not all patients can benefit from this finding. Studies have shown that patients with high microvessel density (MVD) expression have high sensitivity to VEGF inhibitors, so the basal expression of MVD can be used as a marker to determine whether bevacizumab will be effective5758. In addition, antagonizing VEGFR2 activates the Ang- 1/Tie2 signalling pathway, thereby recruiting pericytes59. At the same time, inhibition of VEGF can upregulate PDGFRB signalling and promote the recruitment and maturation of pericytes60. Another challenge in vascular normalization with VEGF inhibitors is achieving what is refered to as a ‘window of opportunity’, which is the time frame and dose of VEGF inhibitor that are required to observe normalization of tumour blood vessels. The dose time frame and the amount of VEGF inhibitors required to attain normalization were relatively narrow, depending on the type of tumour used, dose planning and VEGF signalling inhibitors. The effect is usually transient (7-10 days), but could last 1-4 months, depending on the drug used and the type of tumour616263. To improve the sensitivity of chemotherapy, radiotherapy and immunotherapy, it is necessary to maintain normalization of the tumour blood vessels for a long time; therefore, it is essential to develop an effective and long-term strategy for stabilizing the tumour blood vessels63. Three major functional imaging techniques can be used to evaluate patients undergoing antiangiogenic therapy: dynamic contrast-enhanced (DCE)-US, DCE computed tomography (CT), and DCE magnetic resonance imaging (MRI) which can determine the function of the tumour blood vessels after antiangiogenic therapy and help determine the ideal treatment dosage for normalization64656667. In addition, tracers can also be used to monitor vascular normalization after VEGF inhibitor treatment. A specific radiotracer 99mTcRGD binds to integrin avb3, which is expressed during active angiogenesis and has been shown to help in monitoring vascular normalization after bevacizumab treatment and helps to identify the solution required for an ideal efficacy rate68.
The Dll4/Notch signalling pathway is crucial to the development of embryonic blood vessels, and studies have shown that it is also closely related to tumour angiogenesis69. Changes in the Notch signalling pathway are common in HGSOC and are associated with low OS70. Dll4 is an endothelial-specific ligand that is highly expressed in tumour blood vessels, and 72 per cent of OC patients exhibit Dll4 overexpression, which is an independent risk factor for poor prognosis. The expression of Dll4 is low in patients who are sensitive to VEGF inhibitors, and knockdown of the Dll4 gene in ovarian tumour cells and tumour-associated endothelial cells leads to low tumour growth and angiogenic capacity71. Therefore, Dll4/Notch may be a potential target for vascular-targeted therapy in OC.
Numerous studies have shown that inflammation promotes tumour progression. Tumour-associated macrophages (TAMs) are the main inflammatory components of tumour stroma, which are associated with tumour development and anti-vascular resistance727374757677. Cytokines in the tumour microenvironment polarize TAMs toward an M2 phenotype, which is characterized by high expression of IL-10, TGFβ, VEGF, MMPs and other cytokines that inhibit adaptive immunity, stimulate metastasis and angiogenesis787980. In addition, TAMs accumulated in tumour hypoxia areas, and hypoxia induced by antiangiogenic therapy was associated with high TAM infiltration63. In vivo experiments have shown that compared to control groups, groups with macrophages depleted from the abdominal cavity have reduced ascites production and inhibited tumour growth and angiogenesis81. Compared with sorafenib alone, ZA combined with sorafenib can obviously inhibit tumour progression, metastasis and tumour angiogenesis in a mouse model of liver cancer by targeting macrophages82. Neferine inhibits tumour growth by inhibiting the differentiation of M2 macrophages and inducing autophagy to inhibit angiogenesis in high-grade serous OC83. Hence, TAMs are a promising target for the treatment of OC.
MiRNA is a noncoding small RNA, and an increasing number of studies have shown that miRNA plays an important role in tumour angiogenesis848586. A single miRNA can target hundreds of mRNA transcripts for translational inhibition, mRNA degradation, or induction of mRNA instability to regulate target gene expression87. This feature allows miRNAs to simultaneously target multiple angiogenesis-related pathways. Wu et al88 showed that miR-192 can target multiple angiogenesis-related genes and mediate potent antiangiogenic and antitumour effects in multiple orthotopic mouse models of ovarian and renal cancer and that the antiangiogenic and antitumour effects of miR-192 were stronger than those of VEGF antibodies. Through large-scale patient data, lower levels of miR-192 in tumours were shown to be associated with high angiogenesis and low overall survival in patients with HGSOC or renal clear cell carcinoma. Chen et al5 proved that miR-204-5p could promote angiogenesis in ovarian tumours through THBS1. Therefore, miRNA is a potential target for the treatment of OC.
Status of targeted therapy for OC
The combination of paclitaxel and carboplatin every three weeks with or without bevacizumab remains the first-line standard treatment for advanced OC patients89. At present, it is believed that the survival rate of OC patients cannot be improved by either dose-dense chemotherapy or adding a third chemotherapy drug or intraperitoneal (IP) therapy administration90. GOG-172 was a phase III trial with OS is reported to improve by 15.9 months (65.6 vs 49.7 months) in the IP arm compared with the intravenous (IV) arm. Due to increased catheter-related complications and toxicity, only 42 per cent patients completed the six cycles of the assigned therapy. GOG-252 is a phase III trial to further evaluate the role of IP compare with IV chemotherapy, the results failed to show a survival benefit from IP chemotherapy91 due to which it is not universally accepted9293. In addition, hyperthermic intraperitoneal chemotherapy (HIPEC) or neoadjuvant chemotherapy is not better than standard chemotherapy. Although HIPEC is widely used, up to now, there was no randomized and convincing evidence for HIPEC versus surgery without HIPEC for OC94. Based on studies on the pathogenesis of OC, several target agents have been used in the treatment of OC. Targeted agents are less toxic than chemotherapy drugs and can be combined9596. Currently, several combination trials are ongoing, including trials of antiangiogeneic drugs with poly-(ADP)-ribose polymerase inhibitors (PARPi) [PAOLA-1 or ENGOT-ov25 trial (NCT0247764)], immune checkpoint inhibitors plus antiangiogenic agents [IMaGYN050 (NCT03038100)] and the combination of antiangiogenic agents, PARPi and checkpoint inhibitors (ENGOT-ov46 trial)9798. These ongoing OC trials certainly show great promise, and we eagerly await the results of these studies.
Conclusion
The main contents of this review are summarized in Fig. 2. All in all, antiangiogenic strategies are key for OC therapeutic management, with less toxicity than conventional chemotherapy methods, and can be used as a potential maintenance therapy to reduce or delay recurrence. However, to date, there is a lack of effective measures to classify OC patients and effectively determine which patients may benefit from VEGF/VEGFR inhibitors, which makes these interventions costly, minimizes efficacy and increases side effects. To improve this shortcoming, the following aspects should be considered in future studies of OC angiogenesis. First, there are multiple mechanisms involved in OC angiogenesis. To effectively inhibit angiogenesis, multiple angiogenic pathways need to be blocked simultaneously, and miRNAs may be an ideal therapeutic approach in this context. Second, there is high heterogeneity of tumour blood vessels in tumour tissues, and future research directions should include identifying predictable biomarkers to identify which patients are responders. Third, vascular normalization is a new therapeutic strategy, which has great clinical potential in improving the local immunosuppressive microenvironment, enhancing drug delivery, and improving the hypoxia status of tumours. Therefore, the challenges in future studies will involve determining the optimal duration and the scheduling of vascular normalization agents and how to combine different agents effectively without significant toxicity. Ultimately, to achieve this goal, a close cooperation between basic researchers and clinicians is essential.
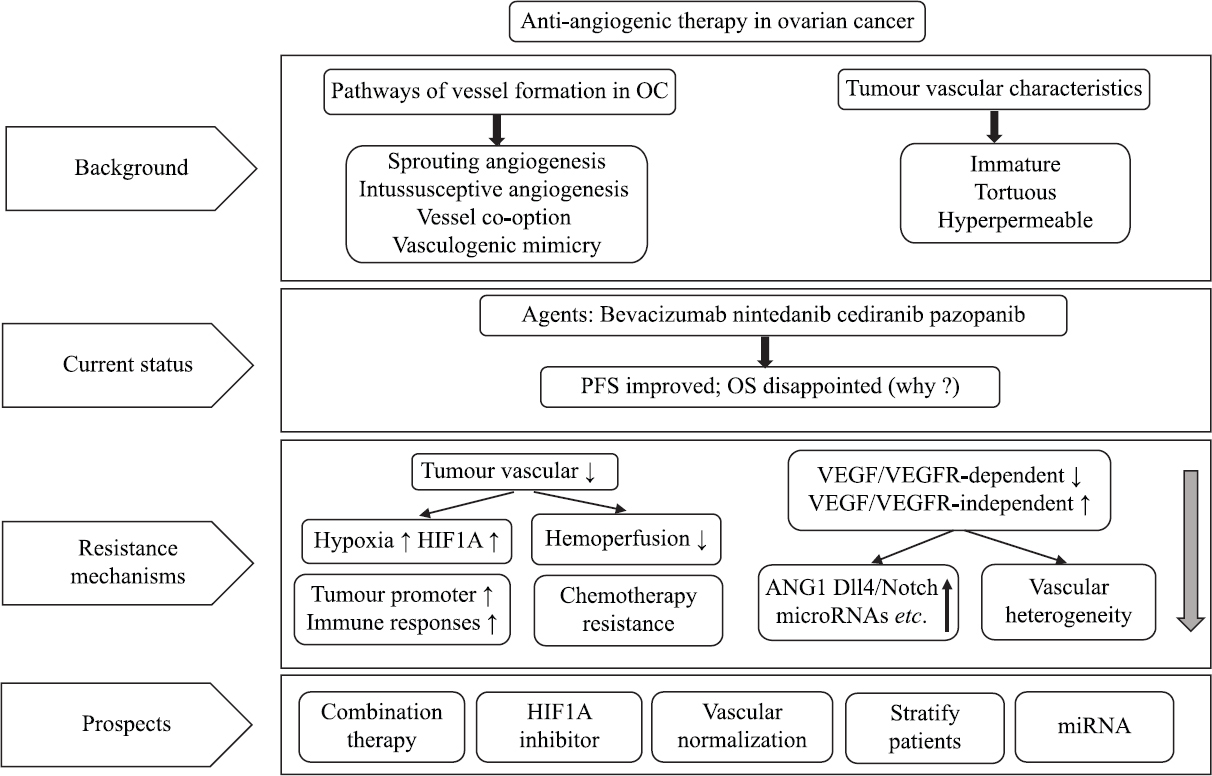
- An outline diagram summarizes the entire review narrative on angiogenesis.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3-14.
- [Google Scholar]
- Ovarian cancer:Strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502-16.
- [Google Scholar]
- Identifying and targeting angiogenesis-related microRNAs in ovarian cancer. Oncogene. 2019;38:6095-108.
- [Google Scholar]
- Tumor angiogenesis and vascular normalization:Alternative therapeutic targets. Angiogenesis. 2017;20:409-26.
- [Google Scholar]
- Randomized trial of oral cyclophosphamide versus oral cyclophosphamide with celecoxib for recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancer. Cancer Treat Res Commun. 2019;21:100155.
- [Google Scholar]
- Long-term progression-free survival of apatinib monotherapy for relapsed ovarian cancer:A case report and literature review. Onco Targets Ther. 2019;12:3635-44.
- [Google Scholar]
- Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943-53.
- [Google Scholar]
- Angiogenesis and vascular remodeling by intussusception:From form to function. News Physiol Sci. 2003;18:65-70.
- [Google Scholar]
- A new mechanism for pillar formation during tumor-induced intussusceptive angiogenesis:Inverse sprouting. Am J Pathol. 2011;179:1573-85.
- [Google Scholar]
- Microvascular density, vascular endothelial growth factor immunoreactivity in tumor cells, vessel diameter and intussusceptive microvascular growth in primary melanoma. Oncol Rep. 2005;14:81-4.
- [Google Scholar]
- The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin Exp Metastas. 2012;29:541-9.
- [Google Scholar]
- Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016;22:1294-302.
- [Google Scholar]
- Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J Pathol. 2017;241:362-74.
- [Google Scholar]
- Tumour vasculogenic mimicry is associated with poor prognosis of human cancer patients:A systemic review and meta-analysis. Eur J Cancer. 2013;49:3914-23.
- [Google Scholar]
- Tumor cell vasculogenic mimicry:From controversy to therapeutic promise. Am J Pathol. 2012;181:1115-25.
- [Google Scholar]
- Signalling pathways in vasculogenic mimicry. Biochim Biophys Acta. 2010;1806:18-28.
- [Google Scholar]
- Mosaic blood vessels in tumors:Frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. 2000;97:14608-13.
- [Google Scholar]
- Hypoxia promotes vasculogenic mimicry formation by inducing epithelial-mesenchymal transition in ovarian carcinoma. Gynecol Oncol. 2014;133:575-83.
- [Google Scholar]
- Tumor vasculogenic mimicry predicts poor prognosis in cancer patients:A meta-analysis. Angiogenesis. 2016;19:191-200.
- [Google Scholar]
- Tumor cell-mediated neovascularization and lymphangiogenesis contrive tumor progression and cancer metastasis. Biochim Biophys Acta. 2013;1836:273-86.
- [Google Scholar]
- A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature. 2015;520:358.
- [Google Scholar]
- Transport of drugs from blood vessels to tumour tissue. Nat Rev Cancer. 2017;17:738-50.
- [Google Scholar]
- Implications of Increase in vascular permeability in tumors by VEGF:A commentary on the pioneering work of harold dvorak. Cancer Res. 2016;76:3118-20.
- [Google Scholar]
- Quantification of longitudinal tissue pO2 gradients in window chamber tumours:Impact on tumour hypoxia. Br J Cancer. 1999;79:1717-22.
- [Google Scholar]
- The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat Rev. 2011;37:63-74.
- [Google Scholar]
- State of the science:Emerging therapeutic strategies for targeting angiogenesis in ovarian cancer. Gynecol Oncol. 2015;138:223-6.
- [Google Scholar]
- Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473-83.
- [Google Scholar]
- Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer:The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302-8.
- [Google Scholar]
- OCEANS:A randomized, double-blind, placebo-controlled Phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039-45.
- [Google Scholar]
- A phase III randomized controlled clinical trial of carboplatin and paclitaxel alone or in combination with bevacizumab followed by bevacizumab and secondary cytoreductive surgery in platinum sensitive, recurrent ovarian, peritoneal primary and fallopian tube cancer (Gynecologic Oncology Group 0213) Gynecol Oncol. 2015;137:3-4.
- [Google Scholar]
- Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12):A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016;17:78-89.
- [Google Scholar]
- Randomised double-blind phase III trial of cediranib (AZD 2171) in relapsed platinum sensitive ovarian cancer:Results of the ICON6 trial. EJC. 2013;49:S5-6.
- [Google Scholar]
- Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol. 2014;32:3374-82.
- [Google Scholar]
- Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6):A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;387:1066-74.
- [Google Scholar]
- Phase II trial of nintedanib in patients with bevacizumab-resistant recurrent epithelial ovarian, tubal, and peritoneal cancer. Gynecol Oncol. 2019;153:555-61.
- [Google Scholar]
- Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371-5.
- [Google Scholar]
- Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70:1063-71.
- [Google Scholar]
- Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299-309.
- [Google Scholar]
- Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell Rep. 2014;8:696-706.
- [Google Scholar]
- Therapeutic approaches of resveratrol on endometriosis via anti-inflammatory and anti-angiogenic pathways. Molecules. 2019;24:667.
- [Google Scholar]
- Combinatorial therapy of immune checkpoint and cancer pathways provides a novel perspective on ovarian cancer treatment. Oncol Lett. 2019;17:2583-91.
- [Google Scholar]
- Bevacizumab may differentially improve ovarian cancer outcome in patients with proliferative and mesenchymal molecular subtypes. Clin Cancer Res. 2017;23:3794-801.
- [Google Scholar]
- Heterogeneity of the tumor vasculature:the need for new tumor blood vessel type-specific targets. Clin Exp Metastas. 2012;29:657-62.
- [Google Scholar]
- cRGD-functionalized nanoparticles for combination therapy of anti-endothelium dependent vessels and anti-vasculogenic mimicry to inhibit the proliferation of ovarian cancer. Acta Biomater. 2019;94:495-504.
- [Google Scholar]
- Predictive and prognostic angiogenic markers in a gynecologic oncology group phase II trial of bevacizumab in recurrent and persistent ovarian or peritoneal cancer. Gynecol Oncol. 2010;119:484-90.
- [Google Scholar]
- Increasing incidence of brain metastasis in patients with advanced hepatocellular carcinoma in the era of antiangiogenic targeted therapy. Oncologist. 2011;16:82-6.
- [Google Scholar]
- Antiangiogenic therapy promoted metastasis of hepatocellular carcinoma by suppressing host-derived interleukin-12b in mouse models. Angiogenesis. 2013;16:809-20.
- [Google Scholar]
- Antiangiogenic therapy using sunitinib combined with rapamycin retards tumor growth but promotes Metastasis. Transl Oncol. 2014;7:221-9.
- [Google Scholar]
- Hypoxia-inducible factor-1 (HIF-1) inhibitors from the last decade (2007 to 2016):A “structure-activity relationship”perspective. Med Res Rev. 2018;38:1404-42.
- [Google Scholar]
- Normalizing tumor vasculature with anti-angiogenic therapy:A new paradigm for combination therapy. Nat Med. 2001;7:987-9.
- [Google Scholar]
- Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc Natl Acad Sci USA. 2015;112:14325-30.
- [Google Scholar]
- Emerging biomarkers in ovarian granulosa cell tumors. Int J Gynecol Cancer. 2019;29:560-5.
- [Google Scholar]
- Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation:Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553-63.
- [Google Scholar]
- A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809-13.
- [Google Scholar]
- Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88-91.
- [Google Scholar]
- Antiangiogenic therapy:Impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:210-21.
- [Google Scholar]
- Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561-6.
- [Google Scholar]
- A pilot study to determine the timing and effect of bevacizumab on vascular normalization of metastatic brain tumors in breast cancer. BMC Cancer. 2016;16:466.
- [Google Scholar]
- Hypoxia imaging markers and applications for radiation treatment planning. Semin Nucl Med. 2012;42:343-52.
- [Google Scholar]
- Do imaging biomarkers relate to outcome in patients treated with VEGF inhibitors? Clin Cancer Res. 2012;18:6588-98.
- [Google Scholar]
- (68) Ga tagged dendrimers for molecular tumor imaging in animals. Hell J Nucl Med. 2019;22:78-9.
- [Google Scholar]
- Bevacizumab enhances efficiency of radiotherapy in a lung adenocarcinoma rodent model:Role of alphavbeta3 imaging in determining optimal window. Nucl Med Biol. 2015;42:923-30.
- [Google Scholar]
- The expression of VEGF and Dll4/Notch pathway molecules in ovarian cancer. Clin Chim Acta. 2014;436:243-8.
- [Google Scholar]
- Biological roles of the Delta family Notch ligand Dll4 in tumor and endothelial cells in ovarian cancer. Cancer Res. 2011;71:6030-9.
- [Google Scholar]
- The regulation of angiogenesis by tissue cell-macrophage interactions. Front Physiol. 2014;5:262.
- [Google Scholar]
- Therapeutic strategies for targeting the ovarian tumor stroma. World J Clin Cases. 2014;2:194-200.
- [Google Scholar]
- Macrophages facilitate resistance to anti-VEGF therapy by altered VEGFR expression. Clin Cancer Res. 2017;23:7034-46.
- [Google Scholar]
- The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618-31.
- [Google Scholar]
- Matrix metalloproteinases:Regulators of the tumor microenvironment. Cell. 2010;141:52-67.
- [Google Scholar]
- Cysteine cathepsin proteases:Regulators of cancer progression and therapeutic response. Nat Rev Cancer. 2015;15:712-29.
- [Google Scholar]
- Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71-8.
- [Google Scholar]
- Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression:Potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717-27.
- [Google Scholar]
- Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559.
- [Google Scholar]
- Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Res. 2007;67:5708-16.
- [Google Scholar]
- Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420-30.
- [Google Scholar]
- Anti-angiogenesis effect of Neferine via regulating autophagy and polarization of tumor-associated macrophages in high-grade serous ovarian carcinoma. Cancer Lett. 2018;432:144-55.
- [Google Scholar]
- A microRNA signature defines chemoresistance in ovarian cancer through modulation of angiogenesis. P Natl Acad Sci USA. 2013;110:9845-50.
- [Google Scholar]
- Lung endothelial microRNA-1 regulates tumor growth and angiogenesis. Am J Respir Crit Care Med. 2017;196:1443-55.
- [Google Scholar]
- A miR-192-EGR1-HOXB9 regulatory network controls the angiogenic switch in cancer. Nat Commun. 2016;7:11169.
- [Google Scholar]
- Major clinical research advances in gynecologic cancer in 2018. J Gynecol Oncol. 2019;30:18.
- [Google Scholar]
- Hopes and failures in front-line ovarian cancer therapy. Crit Rev Oncol Hematol. 2019;143:14-9.
- [Google Scholar]
- Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma:An NRG oncology/gynecologic oncology group study. J Clin Oncol. 2019;37:1380-90.
- [Google Scholar]
- Phase III trials in ovarian cancer:The evolving landscape of front line therapy. Gynecol Oncol. 2019;153:436-44.
- [Google Scholar]
- Hyperthermic intraperitoneal chemotherapy does not improve survival in advanced ovarian cancer. Cancer. 2019;125((Suppl 24)):4594-7.
- [Google Scholar]
- Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer. Ann Oncol. 2019;30:551-7.
- [Google Scholar]
- Cediranib suppresses homology-directed DNA repair through down-regulation of BRCA1/2 and RAD51. Sci Transl Med. 2019;11:4508. eaav
- [Google Scholar]
- PARP inhibitors for BRCA wild type ovarian cancer;gene alterations homologous recombination deficiency and combination therapy. Jpn J Clin Oncol. 2019;49:703-7.
- [Google Scholar]
- Treatment of patients with recurrent epithelial ovarian cancer for whom platinum is still an option. Ann Oncol. 2019;30:721-32.
- [Google Scholar]






