Translate this page into:
An outbreak of acute diarrhoeal disease caused by Shigella sonnei in a village in Dibrugarh district, Assam
For correspondence: Dr Pallab Sarmah, Division of Microbiology and Immunology, ICMR-Regional Medical Research Centre, NE Region, Dibrugarh 786 010, Assam, India e-mail: pallabsarmah007@gmail.com
-
Received: ,
Abstract
Background & objectives
Food and waterborne illnesses remain a neglected public health issue in India. Events with large gatherings frequently witness outbreaks of acute diarrheal diseases due to consumption of contaminated food or water or poor food handling practices. In the present study, an outbreak of acute diarrhoeal disease (ADD) occurring among the attendees of a birthday party in rural Dibrugarh district in the northeastern Indian State of Assam was investigated.
Methods
Sociodemographic information along with details of ADD outbreak that included information about source of foods, food handlers, illness details, etc., were collected using an outbreak investigation form for descriptive and analytical epidemiology. Rectal swabs from affected individuals and food handlers were collected along with bore-well water samples and tested in the laboratory by performing bacterial culture, biochemical analysis and polymerase chain reaction. Due to the delayed report on the outbreak, collecting leftover food for laboratory testing and analysis was impossible.
Results
A total of 25 cases of ADD had similar signs and symptoms. The mean incubation period for developing acute diarrhoea was 26.36±8.76 (± standard deviation) hours from food consumption. The overall attack rate was 60.04 per cent (25/41); 20 per cent (5/25) required hospitalization. Thirteen rectal swab samples were tested for pathogens and found positive for Shigella sonnei. Antibiotic susceptibility test of isolated S. sonnei showed resistance to nalidixic acid, ciprofloxacin and cefotaxime. Consumption of one of the food items - chicken curry was significantly associated with illness (Odds Ratio=14.8; 95% Confidence Interval: 2.75-85.11); P value<0.05 and Population Attributable Fraction (PAF) was 70.18 per cent. The water samples were found satisfactory for human consumption.
Interpretation & conclusions
The findings suggested that S. sonnei infection could be implicated in the investigated food-borne diarrhoeal disease outbreak and that there was a potential for human-poultry cross-infection. Additionally, the study revealed concerning levels of S. sonnei resistance to recommended antibiotics and drew attention to their public health relevance.
Keywords
Acute diarrhoeal disease
epidemiology
outbreak June 2022
food-borne illness
Shigella sonnei
Food and water-borne illnesses caused by bacteria, viruses and parasites are a global public health problem1,2. Poor hygiene, inadequate sanitation, and contaminated water sources contribute to the high prevalence of diarrhoea in many countries. Among various diarrhoeagenic pathogens, Salmonella and Shigella are the main outbreak-causing organisms worldwide and more than 200000 deaths have been associated globally with Shigella infection3. Collectively, food-borne pathogens are a leading cause of infection in children below five yr of age causing more than 500000 deaths every year globally1,2,4. Food and waterborne disease outbreaks are quite common in India, particularly in north-east India, where outbreaks occur in spite of improvements of sanitation conditions due to Swatch Bharat Abhiyan implemented by government of India5. Moreover, the occurrence of food-borne diseases in the community is frequently reported during festivals, rallies, religious congregations, etc.6,7. In India, Shigella sonnei is more commonly associated with acute diarrhoeal diseases as per the Integrated Disease Surveillance Programme (IDSP)8. However, detailed epidemiological investigation, research and reporting of such outbreaks are limited in northeastern region of the country. This results in delayed medical aid and lack of timely control measures to halt the spread of disease in the community. Here, we report an acute diarrhoeal disease (ADD) outbreak following a birthday party in Ougurigaon, Moran area in the Dibrugarh district of the northeastern Indian State of Assam.
Material & Methods
The investigation was jointly conducted by the Integrated Disease Surveillance Programme (IDSP), Dibrugarh District and ICMR-Regional Medical Research Centre (RMRC), NE Region, Dibrugarh under the ongoing ICMR-Food borne Pathogen Surveillance Project (ICMR-FoodNet) (https://www.icmrfoodnet.in/) after the approval from the Institutional Ethics Committee. Data was collected from all the birthday party attendees aged ≥18 yr after obtaining written informed consent. In the case of the minors, written informed consent was obtained from parents/legal guardians. In the case of the children 7-17 yr, verbal assent and informed written consent were obtained from the children and parents/legal guardians, respectively.
Study setting and location
Five acute diarrhoeal cases were admitted to Assam Medical College from Ougurigaon village in Moran area of Dibrugarh district, Assam from June 8-9, 2022, with a history of food consumption at a birthday party on June 7, 2022, before the episode. The admitted cases were suspected to be part of an acute diarrhoeal disease outbreak. All admitted ADD cases who attended the birthday party were interviewed on June 9, 2022, to confirm the occurrence of food-borne outbreak. In this episode, we defined the case of ADD including acute gastroenteritis (AGE) as any affected individual who presented with complaints of passage of three or more episodes of loose or liquid stools in last 24 h with or without dehydration within three days of eating at the birthday party as per the case definition of IDSP p-form9,10.
Epidemiological study
On June 14, a retrospective cohort study was carried out on individuals attending the above-mentioned birthday party held on June 7, 2022. An interdisciplinary team comprising an epidemiologist, a medical microbiologist, a biological scientist, field workers, a laboratory technician, a data entry operator and ASHA (Accredited Social Health Activist) workers from the village visited the households. The kitchen, where the birthday party food was prepared, was examined, and the residences of those who attended the event were also surveyed. Sociodemographic information such as age and gender, along with details about the birthday party, including the source of foods, a list of prepared items, information about food handlers, food exposure, timing of food consumption, illness details (onset date and time), signs and symptoms, interventions and outcomes were collected using a pre-designed outbreak investigation form (Supplementary Material). For statistical analysis, Statistical Package for Social Sciences (SPSS) Statistical Software Version 14.0 (IBM corp., Armonk, NY, USA) was used for data management and analytics. The spot map and the line list were prepared, and the epidemic curve was plotted by the date and time of the onset of ADD along with the calculation of age- and gender-specific attack rates. Univariate analysis was carried out using the variables such as age groups, gender and food items [cake, rice and daal (lentils), egg curry, chicken curry, chicken intestine fry, pork fry and fish fry]. The P value <0.05 was considered as statistically significant. The association measures were calculated by odds ratios (OR; unadjusted and adjusted) and their 95% confidence interval (CI) using the logistic regression method. For calculating the attributable fraction, the sum of the category-specific differences between the total number of observed cases and the total number of expected cases was divided by the sum of the observed cases.
Laboratory investigations
Thirteen rectal swabs were saved in Cary Blair Medium, and stool samples were collected using sterile cotton swabs from the affected individuals and were examined at the Microbiology and Immunology Laboratory of ICMR-RMRC, Dibrugarh within 3 h of collection11,12. Briefly, stools were cultured on MacConkey, Xylose-lysine deoxycholate (XLD), and Hektoen enteric (HE) agars (HiMedia Laboratories, Mumbai) to isolate Escherichia coli, Shigella spp., and Salmonella spp., respectively. Sucrose-Xylose-lactose non-fermenting red colonies on XLD agar with green colour colonies on Hektoen Enteric agar were suspected as Shigella spp., were further tested biochemically for differentiating between different strains11,13. Antimicrobial susceptibility was tested using commercial antibiotic discs (HiMedia Laboratories, Mumbai) and the inhibition zone diameters were interpreted as resistant to a particular antimicrobial agent as per standard CLSI guidelines12.
Polymerase chain reaction (PCR) assay
The DNA was isolated from the S. sonnei isolates using a Bacterial DNA isolation kit (HiPure Genomic DNA Purification Kit, HiMedia Laboratories, Mumbai). PCR was performed using virulence genes of EIEC pathotype for virF and ipaH genes using a simplex PCR14. For amplification of the virF gene Forward 5’ AGC TCA GGC AAT GAA ACT TTG AC 3’ primer and Reverse 5’ TGG GCT TGA TAT TCC GAT AAG TC 3’ primer was used that amplifies a product of 618 bp. For the ipaH gene Forward 5’CTC GGC ACG TTT TAA TAG TCT GG 3’ and Reverse 5’ GTG GAG AGC TGA AGT TTC TCT GC 3’ primers were used to amplify a PCR product of 933 bp. The thermal profile used for PCR was initial denaturation at 94°C for 5 min, samples were amplified for 35 cycles, with each cycle consisting of 1.5 min at 94°C for denaturation, 1.5 min at 60°C for primer annealing and 1.5 min at 72°C for strand elongation and a final elongation at 72°C for 7 min. The PCR products were visualized in 1.5% agarose gel stained with ethidium bromide. However, genomic sequencing was not done. Confirmation of the identified pathogen was done by the ICMR-National Institute of Cholera and Enteric Diseases, Kolkata using biochemical tests and serotyping for identification of Shigella spp.
Environment investigation
The sole food handler and the cook was one – the father of the birthday child. He was interviewed about the recent illness, and a rectal swab was collected for analysis. Borewell groundwater samples were collected from the household that hosted the event for laboratory testing as per the standard operating procedures (SOP)11.
Results
Descriptive and analytical epidemiology
The index case was a 14 yr old boy, who was admitted to the department of Medicine, Assam Medical College, Dibrugarh on June 8, 2022, with chief complaints of severe vomiting, bloody dysentery, high fever, nausea, and loss of consciousness resulting in a fall in the bathroom. The affected boy was severely dehydrated during admission due to the passage of loose, watery stools and required administration of intravenous IV fluids and antibiotics. Interviews with both the affected boy and the guardian revealed that the boy had attended the Ougurigaon birthday party before experiencing illness. We recorded through further investigation that 41 people had attended the birthday party on June 7, 2022, at Ougurigaon village. The birthday celebration event including food consumption lasted from 12:30 PM to 4:30 PM, following which 25 people developed ADD [overall attack rate 60.04% (25/41)]. The family members of the host were also affected, and one required hospitalization. Among the affected individuals all had weakness, 19 developed fever, 18 abdominal cramps and 17 cases had vomiting. The mean incubation period for the onset of symptoms from the time of food consumption was 26.36±8.76 h (±SD) and indicated a common source of contamination with no secondary cases. The last case was reported on June 10, 2022, at 9:00 AM as shown in the Epidemic curve (Figure). Two outlier cases were also traced five days post-cessation of outbreak, though they did not attend the event, but they had consumed only chicken curry on June 8, 2022, that was sent to them by the host on June 7, 2022 and fell ill on June 10, 2022. However, no chicken intestine fry was sent to them by the host. Table I shows the age and gender distribution among ill and non-ill persons. The highest attack rate (AR; 100%) was in the age group 0-4 yr followed by AR of 66.7 per cent in the age group 40-64 yr. The lowest AR was 50 per cent in the 25-39 yr age group (Table I). Females had higher rate of infection (64%) compared to males; no case fatality was reported. Table I also presents the distribution of the explanatory variables among the 16 people who did not fall ill after attending the event.
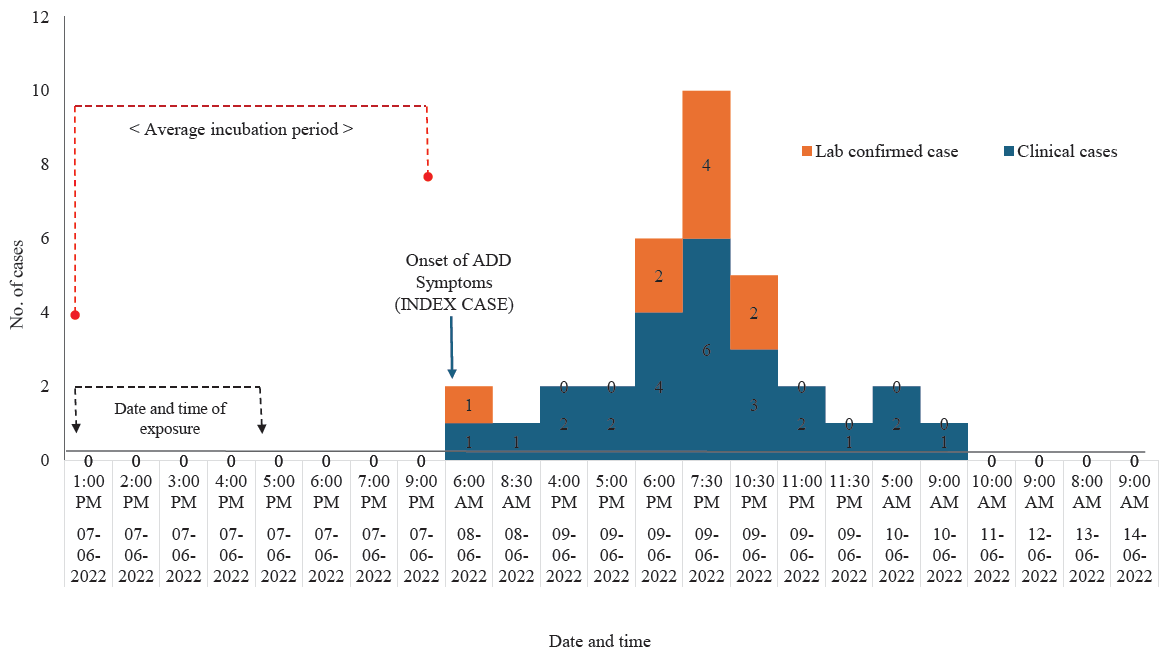
- Epidemic curve of the outbreak from exposure in the event and the appearance of symptoms of Acute Gastrointestinal Disease (AGE) among those who were affected during the outbreak. ADD, acute diarrhoeal disease.
| Variables | No. of cases, n (%) | Total attendees (n) | Attack rate (%) | AOR | 95 % CI | P value |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 9 (36) | 16 | 56.3 | 1 | -- | |
| Female | 16 (64) | 25 | 64 | 0.44 | (0.12-1.58) | 0.213 |
| Age (yr) | ||||||
| 0-4 | 4 (16) | 4 | 100 | 1 | -- | 0.964 |
| 5-14 | 5 (20) | 8 | 62.5 | 807737421 | (0-0) | 0.999 |
| 15-24 | 8 (32) | 15 | 53.3 | 0.83 | (0.09-7.67) | 0.871 |
| 25-39 | 4 (16) | 8 | 50 | 0.57 | (0.08-4.13) | 0.577 |
| 40-64 | 4 (16) | 6 | 66.7 | 0.5 | (0.06-4.47) | 0.532 |
AOR, adjusted odds ratio; CI, confidence interval
The food served at the birthday party was prepared by the father of the birthday child and consisted of cake, rice, daal (lentils), egg curry, chicken (broiler) curry, fried chicken intestine, fried pork and fried fish. The fish was cut and dressed by a vendor in the nearby market. Moreover, he bought 10-12 live broiler chickens from a local retailer, which was relatively smaller in size. Family members mentioned that the chickens appeared unwell, exhibiting signs of apparent illness- such as being underweight, having ruffled feathers, and showing exposed skin patches. He had culled all the chickens, eviscerated the intestines and prepared the chicken curry and fried intestines with red gram, onion and green chilli flakes. As per the host, the food was kept on the serving tables covered but not kept under refrigeration as all the food items were to be consumed within the same day. There was no leftover food in the party. Further, both parents did not suffer from a similar illness before the event.
Out of the eight food items served at the party, only one food item was significantly associated with ADD: chicken (broiler) curry, which was consumed by the 30 individuals of which 76.6 per cent (23) developed ADD. Thirteen people consumed chicken intestine, of which nine (69.2%) developed ADD. However, it was not significantly associated with the illness (Table I). The OR was statistically significant for exposure to chicken curry consumption and occurrence of ADD (OR=14.8; 95% CI: 2.75-85.11); P<0.05. The analysis of the food-specific population attributable fraction (PAF) was highest for chicken curry 70.18 per cent as shown in the Table I. All the other food items were not significantly associated with the occurrence of ADD. Further, the logistic regression was performed to explore the effects of gender, age groups, food items consumed and occurrence of ADD in the cohort. The Model summary of the logistic regression showed Nagelkerke R2 value of 0.714 showing good fit predicting 71.4 per cent of the variance in the ADD cases and correctly classified 71 per cent of the cases. The consumption of chicken curry (OR=43.89; CI: 1.83-1054.35); P<0.05 was significantly associated with occurrence of ADD. The association of other independent variables such as gender, age groups, food items consumed at the party (cake, rice and daal, egg curry, chicken intestine fry, pork fry and fish fry) and occurrence of ADD was not statistically significant (Table II).
| Food Item | Ate | Did not eat | PAF | Crude OR | 95 % CI | P value | AOR | 95 % CI | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADD | No ADD | Total | Attack rate (%) | ADD | No ADD | Total | Attack rate (%) | ||||||||
| Cake | 22 | 13 | 35 | 62.9 | 3 | 3 | 6 | 50 | 18 | 1.7 | 0.30-9.65 | 0.277 | 0.05 | (0-4.30) | 0.194 |
| Rice and daal | 22 | 15 | 37 | 59.5 | 3 | 1 | 4 | 75 | -23 | 0.5 | 0.05-5.16 | 0.276 | 0.62 | (0-83.54) | 0.839 |
| Egg curry | 23 | 13 | 36 | 63.9 | 2 | 3 | 5 | 40 | 34.4 | 2.7 | 0.39-18 | 0.159 | 4.39 | (0.11-173.64) | 0.431 |
| Chicken curry | 23 | 7 | 30 | 76.7 | 2 | 9 | 11 | 18.2 | 70.18 | 14.8 | 2.57-85.11 | 0.001 | 43.89 | (1.83-1054.35) | 0.02 |
| Chicken intestine fry | 9 | 3 | 12 | 75 | 16 | 13 | 29 | 55.2 | 9.52 | 2.4 | 0.55-10.9 | 0.122 | 6.77 | (0.11-416.11) | 0.363 |
| Pork fry | 7 | 2 | 9 | 77.8 | 18 | 14 | 32 | 56.3 | 7.75 | 2.7 | 0.49-15.2 | 0.127 | 0.01 | (0-0.73) | 0.049 |
| Fish fry | 20 | 13 | 33 | 60.6 | 5 | 3 | 8 | 62.5 | -2.5 | 0.9 | 0.19-4.54 | 0.461 | 0.25 | (0.01-9.53) | 0.453 |
ADD, acute diarrhoeal disease; PAF, population attributable fraction
Laboratory confirmation
The physical examination of the stool showed presence of mucous (100%) and blood (12). Thirteen samples tested positive for S. sonnei as the causative pathogen and no other enteric pathogen was detected. The water sample collected from the borewell was found satisfactory. S. sonnei isolated from the cases showed highest resistant to nalidixic acid (71.42%), ceftazidime, ceftriaxone and cefotaxime (64.28%). The high sensitivity was seen for gentamicin (64.28%), chloramphenicol (57.14 %) and least resistance to chloramphenicol (14.28%), gentamicin (28.57%), cefoxitin (42.86%) followed by azithromycin (50%) and tetracycline (50%). The PCR results for all S. sonnei isolates were positive for virulence encoding regions of the invasion plasmid antigen H (ipaH) gene.
Environmental findings
The hygienic condition of the room where the food was prepared and stored seemed satisfactory to the investigators with no apparent sight of flies or unclean areas nearby. The hygienic practices of the food handlers were found satisfactory as nails were trimmed adequately. Also, all the family members followed safe hand-washing practices. The investigators also inspected the overall sanitary conditions in the household, which were satisfactory- with separate toilet, no mixed dwelling and availability of bore-well in the house. The borewell groundwater samples on laboratory testing and analysis were found satisfactory for drinking, and no family members fell ill by drinking that water before or after the event.
Discussion
The prevailing global epidemiological impact of shigellosis is primarily linked to two species, S. flexneri and S. sonnei, which were associated with developing and developed regions, respectively15. Nevertheless, recent evidence indicates S. flexneri and S. sonnei are responsible for endemic disease in India and S. dysenteriae causes epidemics or outbreaks at different intervals16-18. We present an outbreak investigation of shigellosis in rural Assam, affecting all age groups, after consumption of food served at a birthday party. Our findings implicated S. sonnei as an important emerging food-borne pathogen as was found in investigations by other researchers from the northeastern (NE) region of India.
All age groups in this study were equally affected; however, females were mostly affected during the outbreak (64%). Debnath et al18 (2018) reported a S. sonnei outbreak in West Bengal, where out of 31 invitees 25 developed acute gastroenteritis (AGE; attack rate of 73%) similar to our findings and females had a higher attack rate at 81 per cent in their study18. Further, the signs and symptoms and mean incubation time of 12-48 h reported was also similar to our findings18. The aetiology of the mode of transmission of Shigella is predominantly by the faecal–oral route or through person to person, however in our study cooked broiler chicken was probably the source of infection, as two cases were exclusively found to be affected who did not attend the party but had the same chicken curry dish that was sent to them by the host. This led us to infer that the chicken curry could be the incriminated food item that caused the outbreak. Moreover, as shown in Table II, the OR for the chicken curry consumption was strongly associated with disease status. Also, the high PAF of 70.18 per cent among the affected individuals further suggested that consumption of chicken curry at the event highly attributed to the occurrence of ADD in the affected individuals.
It may be highlighted that the chickens were not healthy in appearance, as the family members informed us during our interview. It might be possible that the host got them at a cheaper price due to the sick condition of the broilers. Previous reports showed infection of fowl with Shigella19. Experimental studies of inoculation of Shigella in chickens via intraperitoneal injection resulted in severe clinical signs such as weakness, somnolence, droopy wings, retracted heads, ruffled feathers, loss of appetite, and standing still with closed eyes20. The possibility of human-poultry cross-infection was previously indicated as the pathogenicity was found to be similar in the strains isolated from humans or chicken15,20. Shi et al21, studied the pathogenicity of Shigella in chickens and the possibility of cross-infection between humans and chickens. Their results demonstrated that Shigella could invade primary chicken intestinal epithelial cells in vitro and chicken intestinal mucosa in vivo, resulting in pathogenicity and even death. The findings also suggested that Shigella isolated from humans or chicken shared similar pathogenicity20.
In a study from Chennai, India, chicken meat was reported to contain Shigella spp. with a frequency as high as 28 per cent19. In another outbreak survey conducted in Orissa, India presence of more than one diarrhoea causing pathogens (Vibrio spp. and Shigella) was indicated, causing ADD in a population22,23. In the neighbouring country Nepal, it was documented that six per cent of the meat samples were contaminated with Shigella spp14,18,19,21.
As profiling of antimicrobial resistance (AMR) of the clinically relevant food-borne pathogens is of utmost importance, especially during outbreaks, this component was included in the ICMR-Food-borne pathogen surveillance initiative in the NE region in 2020. S. sonnei isolated from this outbreak samples showed resistance to nalidixic acid, ceftazidime, ceftriaxone and cefotaxime. In another study by Jain et al17, resistance of S. sonnei isolates in south India to ciprofloxacin and cotrimoxazole was reported. Further, recent reports of Shigella isolates showing resistance to multiple antibiotics such as ampicillin, cefotaxime, ceftriaxone, ceftazidime, nalidixic acid as well as third-generation cephalosporins as well as reduced susceptibility to extended-spectrum cephalosporins and fluoroquinolones in Shigella isolates carried significant clinical and therapeutic implications15,24-26.
Limitation of this study was the lack of leftover food samples for laboratory testing. There were no leftovers of the epidemiologically implicated foods, due to the time lag between the event and the outbreak investigation. Nonetheless, we tried to compensate for this limitation by conducting in-depth interviews with all the attendees of the event and by analyzing 13 clinical samples from the 25 affected individuals to gather comprehensive outbreak data. In the absence of laboratory confirmation of the causative agent in food samples, a comprehensive assessment of the outbreak’s epidemiological and clinical features served as a valuable substitute.
Overall, food-borne diarrhoeal disease outbreak investigations are important in order to halt the spread of disease and reduce morbidity and mortality. A few evidence-based recommendations that included regular monitoring of the health of the animals entering into the human food chain and animal-origin food products and creating awareness by routine Information, Education and Communication (IEC) activities for practicing safe food handling practices, particularly during handling of animal meat during cooking to avoid risk of contamination were communicated to the local health administration to prevent any possible future outbreaks.
Financial support & sponsorship
The study received funding support from the Indian Council of Medical Research, New Delhi, India [ICMR grant No. 5/8-I(3)/2019-20 ECD-II (Part –’A’)].
Conflicts of Interest
None.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Diarrhoeal disease. Available from: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease, accessed on March 3, 2024.
- Characterization of Shiga-toxigenic Escherichia coli isolated from cases of diarrhoea & haemolytic uremic syndrome in north India. Indian J Med Res. 2014;140:778-84.
- [PubMed] [PubMed Central] [Google Scholar]
- Morbidity and mortality due to Shigella and enterotoxigenic Escherichia coli diarrhoea: the global burden of disease study 1990–2016. Lancet Infect Dis. 2018;18:1229-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Syndromic surveillance during religious mass gatherings, southern India 2015-2018. Travel Med Infect Dis. 2022;47:102290.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehending the risk of foodborne and waterborne disease outbreaks: Current situation and control measures with special reference to the Indian scenario. Heliyon. 2024;10:e36344.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Weekly Outbreaks. Available from: https://idsp.mohfw.gov.in/index4.php?lang=1&level=0&linkid=406&lid=3689, accessed on March 3, 2024.
- Modified case definitions of the P form under IDSP (Jan 2017). Available from: https://idsp.mohfw.gov.in/showfile.php?lid=3744, accessed on March 3, 2024.
- Acute gastroenteritis. Prim Care. 2013;40:727-41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Standard operating procedures ICMR Foodborne pathogen survey and research Network (North-East India). Switzerland: Zenodo; 2022.
- CLSI M100TM. Performance standards for antimicrobial susceptibility testing 34th edition. Available from: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf, accessed on March 3, 2024.
- Paniker CKJ, ed. Ananthanarayan and Paniker’s textbook of microbiology (7th ed). Hyderabad, India: Orient Longman Pvt. Ltd; 2006.
- Single multiplex PCR assay to identify simultaneously the six categories of diarrheagenic Escherichia coli associated with enteric infections. J Clin Microbiol. 2005;43:5362-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Shigellosis: Epidemiology in India. Indian J Med Res. 2016;143:565-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Shigella dysenteriae type 1 with reduced susceptibility to fluoroquinolones. Lancet. 2003;361:785.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and antimicrobial profile of Shigella isolates in a tertiary care hospital of North Karnataka: a 12-year study. Indian J Med Microbiol. 2020;38:101-8.
- [CrossRef] [PubMed] [Google Scholar]
- An outbreak of foodborne infection caused by Shigella sonnei in West Bengal, India. Jpn J Infect Dis. 2018;71:162-6.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological study on Shigella from meat and its public health significance. Int J Curr Microbiol Appl Sci. 2021;10:433-43.
- [CrossRef] [Google Scholar]
- Pathogenicity of Shigella in chickens. PLoS One. 2014;9:e100264.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antibiotic resistance patterns of Staphylococcus aureus, Escherichia coli, Salmonella, Shigella and Vibrio isolated from chicken, pork, buffalo and goat meat in eastern Nepal. BMC Res Notes. 2019;12:1-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Drug resistant Shigella flexneri in & around Dibrugarh, north-east India. Indian J Med Res. 2013;137:183-6.
- [PubMed] [PubMed Central] [Google Scholar]
- Rapid situation & response assessment of diarrhoea outbreak in a coastal district following tropical cyclone AILA in India. Indian J Med Res. 2011;133:395-400.
- [PubMed] [PubMed Central] [Google Scholar]
- Prevalence and antibiotic susceptibility pattern of fluoroquinolone resistant Shigella species isolated from infants stool in Gulbarga district, Karnataka, India. Asian Pac J Trop Dis. 2015;5:116-20.
- [CrossRef] [Google Scholar]
- Molecular characterization of extended-spectrum cephalosporin and fluoroquinolone resistance genes in Salmonella and Shigella isolated from clinical specimens in Thailand. Heliyon. 2022;8:e12383.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Foodborne disease outbreak in a resource-limited setting: a tale of missed opportunities and implications for response. Pan Afr Med J. 2016;23:69.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






