Translate this page into:
An analytical observational study for diagnostic accuracy of volume, conductivity & scatter (VCS) indices of neutrophils for diagnosis of sepsis in an emergency hospital setting
For correspondence: Dr Anshu Palta, Department of Pathology, Government Medical College and Hospital, Chandigarh 160 030, India e-mail: anshupalta@yahoo.com
-
Received: ,
Accepted: ,
Abstract
Background & objectives
The newer technique using an innovative volume conductivity scatter (VCS) technology is emerging as a surrogate for sepsis diagnosis. The VCS technology offers a more objective method to measure cell volume (V), characterize conductivity (C) and light scatter (S) directly from more than 8,000 white blood cells (WBCs). However, diagnostic performance of VCS parameters in sepsis has not been extensively tested in routine hospital emergency settings. Therefore, the present study aimed to investigate the diagnostic and prognostic performance of VCS markers of neutrophils in our local hospital emergency setting.
Methods
It was an observational analytical study with 41 cases of sepsis and 43 healthy controls aged above 18 yr. Individuals with acute coronary syndrome and individuals with already diagnosed Human Immunodeficiency Virus (HIV) infection were excluded from the study.
Results
The mean neutrophil volume (MNV) values were not significantly different between cases and controls (P 0.138) whereas mean neutrophil conductance (MNC) and mean neutrophil scatter (MNS) measurements were significantly higher among cases as compared to controls (both P-values <0.001). According to Receiver Operating Characteristics (ROCs) curve analysis, MNV in our study failed to show statistically significant discriminatory ability in sepsis (AUC 0.54) whereas MNC (AUC 0.98) and MNS (AUC 0.95) showed marked discriminatory ability in diagnosing sepsis in this study cohort.
Interpretation & conclusions
Among VCS parameters, MNV failed as a standalone biomarker of sepsis in routine emergency setting whereas MNC and MNS had statistically significant diagnostic and discriminatory accuracies among hospitalized affected individuals with sepsis.
Keywords
Accuracy
conductivity
neutrophil
scatter
sepsis
volume
Bacterial infections are notably common among hospitalized patients in low-and-middle-income country (LMICs) settings1-6. Early diagnosis of bacterial sepsis is of paramount importance for its appropriate management3-5. The gold standard for confirming sepsis in patient with fever is blood culture7. The usual delay of a minimum of 36-48 h in obtaining a blood culture report is overwhelming for the treating unit and the patients2-3. Other bottlenecks in using blood culture are low yield due to prior antibiotic treatment, risk of bacterial contamination and technical difficulties in collecting blood samples7-9. An increasing battery of haematological and biochemical markers complement the clinical signs and symptoms in diagnosing sepsis10-11. These include total lymphocyte count (TLC), total neutrophil count (TNC), immature neutrophil count, immature neutrophil over total neutrophil (I/T) ratio, erythrocyte sedimentation rate (ESR) along with acute phase reactants like C-reactive protein (CRP), elastase, procalcitonin (PCT), IL-6, etc12-13. Recently with the advent of automated haematology analyzers, there is a notable increase in the ability to assess functional activity of neutrophils14-17. Besides calculating total and differential leucocyte counts, new machines can easily capture the degree of maturity and activity of circulating neutrophils based on flow cytometric principles of intensity and degree of light scattered.
The newer technique using an innovative volume conductivity scatter (VCS) technology is emerging as a surrogate for sepsis diagnosis16-17. The VCS technology offers a more objective method to measure cell volume (V) and characterize conductivity (C) and light scatter (S) directly from more than 8,000 WBCs. This is considered as a more objective alternative to microscopic evaluation of peripheral smear16-17. However, diagnostic performance of VCS parameters in sepsis has not been extensively tested in routine hospital emergency settings. These constraints limit the widespread implementation of this newer diagnostic marker. Therefore, the present study was aimed to investigate the diagnostic and prognostic performance of novel VCS markers of neutrophil in our local hospital emergency setting.
Material & Methods
The study was carried out in the Haematology section of the department of Pathology in collaboration with the departments of Medicine and Blood Transfusion, Government Medical College and Hospital (GMCH 32, Chandigarh) from September 2021 to November 2021. The study was initiated after receiving approval from the Institute’s Research and Ethical Committee. It was an observational analytical study. Affected participants with sepsis of more than 18 yr of age and of either sex who were willing to give consent were included in the study. All consecutive acutely ill individuals presenting to the adult emergency/ICU who met the eligibility criteria were enrolled throughout the study. The diagnosis relied on bedside patient assessments and file records personally reviewed by the investigators. Experienced clinicians treating the study participants in the unit further validated any discrepancies or uncertainties. Individuals with acute coronary syndrome and patients with already diagnosed human immunodeficiency virus (HIV) infection were excluded from the study. The comparison group (control) was constituted by the blood samples of healthy donors available in the blood bank.
Sample size
A sample size was computed using MedCalc software based on the assumptions that the discriminatory ability of our proposed tests in sepsis would have an area under the Receiver Operating Characteristic (ROC) curve of 0.85 against the null hypothesis of 0.70 with an alpha error of five per cent and a beta error of 20 per cent . This gave us a sample size of 41 cases of clinical sepsis and 41 cases of controls. Even when we computed the sample sizes using Stata software with package “diagsampsi” based on the sensitivity of mean neutrophil volume (MNV) as 95 per cent and specificity as 80 per cent keeping the prevalence of sepsis as 28 per cent, maximum sample size was 77.
The study participants’ basic demographic details, including the name, age, sex and registration no., were recorded. A detailed history was taken, and clinical examination was performed. Study participants with suspected clinical sepsis were then enrolled in this study. Controls were healthy donors who presented to the blood bank at the same time. Confirmatory or gold standard for sepsis diagnosis was considered to be blood culture6,7. In the absence of blood culture positivity, clinical diagnosis of sepsis was made based on the Sepsis 3 definition and raised inflammatory markers (Fig. 1) defined by the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM)4.
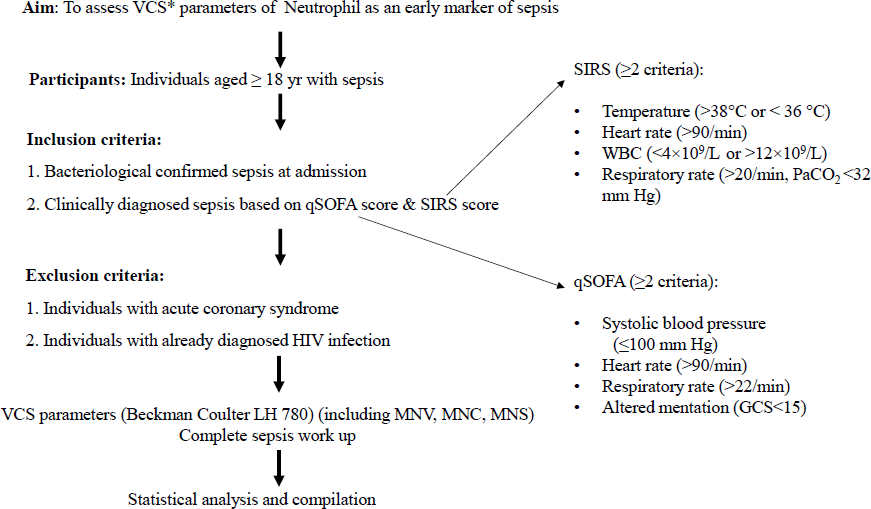
- Diagnostic accuracy of volume conductivity & scatter (VCS) neutrophil indices in sepsis: a schematic of study. MNV, mean neutrophil volume; MNC, mean neutrophil conductivity; MNS, mean neutrophil scatter; SIRS, systemic inflammatory response syndrome; qSOFA, quick sequential organ failure assessment.
Neutrophil VCS parameters were evaluated on a 2 ml EDTA sample including MNV, mean neutrophil conductance (MNC) and mean neutrophil scatter (MNS). For all the above-mentioned laboratory investigations, the concerned instruments were periodically calibrated to ensure reliable test results. Internal quality control (IQC) and external quality assessment (EQA) were being done in the laboratory. Intra and inter assay coefficients of variation (CV) were also computed.
Statistical analysis
Descriptive statistics was used to describe quantitative variables (using mean, SD, median, interquartile range) and categorical variables (using frequencies and proportions). Given unequal sample sizes for comparison, non-parametric univariate analysis included comparison of means (using Mann-Whitney U test) as well as proportions (using chi square test/Fisher exact test). Diagnostic accuracy measures of sensitivity and specificity were also computed. The discriminatory abilities of MNV, MNC and MNS were evaluated using ROC curve analysis. All statistical tests were two-tailed and P value <0.05 was considered as statistically significant. MedCalc software version 19.5.1. was used to analyze the collected data.
Results
A total of 41 cases of clinical sepsis were enrolled from the Emergency Medicine department of a Government Medical College and Hospital, Chandigarh. All study participants qualified the criteria of Sepsis -3 definition based on systemic inflammatory response syndrome (SIRS) and quick sequential organ failure assessment (qSOFA) parameters (Fig. 1). Of all the cases, 31 (75.6%) cases had sepsis alone whereas 10 (24.4%) cases presented with septic shock at admission to emergency. A total of 43 healthy controls were taken from the voluntary blood donation camp. The underlying illnesses which predisposed study participants to sepsis/septic shock are enumerated in Table I.
| Disease | Number | Percentage (%) |
|---|---|---|
| Respiratory illness | 15 | 36.6 |
| Infections | 12 | 29.3 |
| Diabetes mellitus | 7 | 17.1 |
| Gastrointestinal illness | 5 | 12.2 |
| Renal disease | 2 | 4.9 |
| Total | 41 |
In 36.6 per cent of study participants admitted with sepsis, underlying respiratory illnesses were present, followed by infections (29.3%). Diabetes and gastrointestinal illnesses contributed 17.1 per cent and 12.2 per cent of sepsis cases, respectively. Overall, 12 cases out of 41 had underlying chronic systemic comorbidities (Diabetes, renal, hypertension and COPD). Mean age of study participants was 53.07 yr (SD±18.41 yr). Youngest case enrolled was 18 yr old and the oldest was 92 yr. There were 26 men (63.4%) and 15 women (36.6%) among our study cases (n=41).
VCS measurements were estimated using an automated haematology cell counter: Beckman Coulter LH 780 B. The intra-assay coefficient of variation (based on 10 samples of the same subject in the same assay) was one per cent, whereas the inter-assay coefficient of variation (based on repeated daily samples of the same study participan for the next 7 days) was 6.17 per cent. Both intra-assay and inter-assay coefficients of variation were within acceptable limits.
Comparison of VCS measurements between cases and controls
The descriptive summary estimates MNV, MNC & MNS values between cases and controls are shown in Table II The mean MNV values were statistically not significantly different between cases and controls (P value 0.138). In contrast, the mean MNC and MNS measurements were significantly higher among cases as compared to controls (P values <0.001).
| Groups of study participants with sepsis |
MNV Mean (±SD) |
MNC Mean (±SD) |
MNS Mean (±SD) |
|---|---|---|---|
| Study group | |||
| Cases (n=41) | 163.65 (19.55) | 143.52 (9.19)* | 138.50 (9.28)* |
| Controls (n=43) | 158.79 (8.19) | 130.54 (4.09) | 120.76 (2.88) |
P*<0.001 in comparison to control group; MNV, mean neutrophil volume; MNC, mean neutrophil conduction; MNS, mean neutrophil scatter; SD, standard deviation; CI, confidence interval
The diagnostic accuracy measures as well as discriminatory abilities of VCS measurements in sepsis were also analyzed using the ROC curve (Table III). According to ROC curve analysis, MNV in our study failed to show statistically significant discriminatory ability in sepsis whereas MNC and MNS showed marked discriminatory ability in diagnosing sepsis in our study cohort. The ROC plots are presented in Figure 2.
| VCS parameter | Cutoff | Sensitivity | Specificity | PPV | NPV | AUC (95% CI) |
|---|---|---|---|---|---|---|
| MNV | 168 | 39 | 88.4 | 76.2 | 60.3 | 0.54 (0.41-0.67) |
| MNC | 136.2 | 92.7 | 90.7 | 90.5 | 92.8 | 0.98 (0.95-1) |
| MNS | 125.8 | 92.7 | 100 | 100 | 93.5 | 0.95 (0.89-1) |
VCS, volume conductivity scatter; PPV, positive predictive value; NPV, negative predictive value; AUC, area under curve
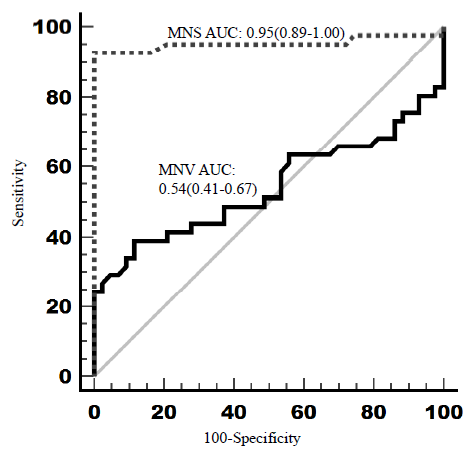
- Receiver operating characteristic (ROC) curve analysis. Figure generated using MedCalc® statistical software version 20.305.
We also compared MNV values among cases stratified by various clinical characteristics (Table IV). The sepsis study participants with comorbidities had significantly higher mean MNC values as compared to those without comorbidities (P value 0.01). There was also trend for significance for higher mean MNC values among septic patients who died as compared to the survivors (P value 0.06).
| Groups of study participants with sepsis |
MNV Mean (±SD) |
MNC Mean (±SD) |
MNS Mean (±SD) |
|---|---|---|---|
| Age (yr) | |||
| ≤65 (n=31) | 163.88 (17.04) | 143.52 (10.51) | 138.67 (10.67) |
| >65 (n=10) | 158.31 (23.09) | 142.45 (5.35) | 137.02 (4.98) |
| TLC | |||
| ≤11,000 (n=16) | 163.32 (16.88) | 142.83 (5.42) | 137.58 (11.6) |
| >11,000 (n=25) | 163.86 (21.43) | 143.96 (11.04) | 139.09 (7.64) |
| Polymorph count | |||
| ≤85% (n=20) | 161.70 (20.03) | 142.46 (4.41) | 139.05 (11.13) |
| >85% (n=21) | 165.51 (19.39) | 144.53 (12.17) | 137.98 (7.33) |
| Comorbidities | |||
| Absent (n=26) | 162.35 (20.33) | 140.86 (3.58) | 139.346 (8.01) |
| Present (n=15) | 165.91 (18.6) | 148.13 (13.51)* | 137.03 (11.29) |
| Sepsis at admission | |||
| Without shock (n=31) | 160.80 (18.53) | 143.023 (5.01) | 138.57 (9.14) |
| With shock (n=10) | 172.50 (20.98) | 145.07 (16.97) | 138.28 (10.18) |
| Final outcome | |||
| Survived (n=34) | 162.36 (18.21) | 142.02 (5.83) | 138.03 (9.96) |
| Died (n=7) | 163.31 (21.57) | 149.29 (18.94) | 139.40 (7.93) |
| Culture | |||
| Sterile (n=34) | 163.16 (19.11) | 143.52 (9.73) | 138.19 (9.49) |
| Positive (n=7) | 166.06 (23.07) | 143.54 (6.5) | 140.00 (8.65) |
P*<0.05 in comparison to subjects with comorbidities; TLC, total leukocyte count
Discussion
This was an observational analytical study where diagnostic accuracy of VCS parameters of neutrophils were tested in routine hospital emergency setting. Forty-one cases with clinically diagnosed sepsis (admitted to emergency), and 43 healthy controls were included in the study. Our study failed to show significantly higher MNV values of neutrophils among subjects with sepsis necessitating admission to emergency. The mean MNV value among cases was 163.65 fL [95% confidence interval (CI): 157.48-169.83] as compared to 158.79 fL (95% CI: 156.27-161.31) among healthy controls, but this difference failed to reach statistical significance (P value 0.14). The area under the curve (AUC) for ROC curve analyses was 0.54 (95% CI: 0.43 to 0.65). Even the upper bound of 95% CI of AUC failed to reach the minimal cut off of 0.7 for statistically significant AUC. There was no significant difference correlation of MNV values with TLC, neutrophil count and length of stay in hospital. On the other hand, MNC and MNS values as well as their AUC were significantly higher among study participants with sepsis as compared to healthy controls. The latter findings contradicted the findings of a retrospective study by Arora et al16. where mean MNC and MNS were significantly lower in adult sepsis subjects compared to controls. However, another study by Ganesan et al.18 mirrored our study findings with significantly higher means as well as AUCs for MNC and MNS among neonates with sepsis as compared to controls.
The poor diagnostic accuracy of MNV in our study is contrary to the previously generated evidence. In a recent meta-analysis17 diagnostic performance of MNV was high. Their meta-analysis included seven studies with 994 participants where AUC of ROC analysis was 0.87 (95% CI: 0.83-0.89), quite well above the required minimal cut off 0.7. At the MNV cut off value of 153.15 fL, the pooled sensitivity and specificity were 0.82 (0.71, 0.89), and 0.78 (0.68, 0.86) respectively in their meta-analysis17. The AUC in the meta-analysis was 0.87 (95% CI: 0.83-0.89), which was substantially higher than our study result. One possible reason for enhanced diagnostic performance of MMV might be the inclusion of only blood culture positive septic patients in all their metanalytical studies, whereas our study had bacterial cultural positivity in only 17 per cent (7/41) of cases. In literature also, only 12 per cent of adults (range 1–51%) had been recorded to have bacteremia when they reported to hospital with acute febrile illness6-7. Also, in routine care, patients are referred to referral centres after administering oral/parenteral antibiotics further compromising the bacterial culture positivity2,3,5. Moreover, the results of meta-analysis were marred with moderate quality studies (on GRADE evaluation) and the overall large effect size was undermined by substantial bias and marked heterogeneity among included studies.
Another possible reason for the suboptimal diagnostic performance of MNV could be a smaller number of bacterial culture positive cases in our study. Most of the subjects had localized infections with complications. But study by Lee et al19 even showed the superiority of MNV among subjects with localized infections compared to controls19. This finding was not replicated in our study, where most subjects with sepsis had localized infections like pneumonia or urosepsis, where we failed to show the predictive ability of MNV compared to controls.
Our study also failed to show any significant correlation between neutrophil count and MNV value. This finding contrasted with Bagdasaryan et al20 findings, which showed a statistically significant positive correlation between absolute neutrophil count and MNV values (P<0.05)20. We also tested the association of MNV values with length of hospital stay to evaluate its role in prognosticating the illness of admitted study participants and arranging for necessary resources in advance, which also did not attain statistical significance.
The enrolment of acutely sick study participants only from emergency with myriad of infective illnesses enhanced the generalizability of our study to other similar settings, which was the strength of our investigation. Evaluating the results of VCS neutrophil markers among cases with ‘clinical’ sepsis (without positive blood culture) hospitalized in medical emergency made our study results more robust and generalizable. Robust statistical methods for assessment of diagnostic accuracy (sensitivity, specificity, positive and negative predictive values, as well as likelihood ratios for positive and negative tests) and discriminatory ability (ROC curve analysis) used in the present study further enhanced its internal validity. Use of standard state of the art equipment for VCS parameter estimation, acceptable intra-assay and inter-assay coefficient of variations were other key strengths of our investigation. Limitations of our study include failure to study VCS parameters of monocytes and lack of randomization design. Also, correlations of these markers were not tested with the biochemical parameters of sepsis. Data on CRP and D-Dimer were only available for a limited number of study participants due to the unavailability of these investigations outside routine working hours. Lack of bacterial culture positivity could be explained due to the fact that most patients were referred from other hospitals where they had already undergone antibiotic treatment. Due to the logistic difficulties, sampling of VCS parameters was not possible at admission for all cases; however testing for VCS markers was done within 72 h of hospitalization in most cases.
Overall, mean neutrophil volume has not shown standalone diagnostic accuracy as an early marker of sepsis in our clinical setting, which included study participants hospitalized due to medical emergencies. On the contrary, MNC and MNS had higher diagnostic as well as discriminatory accuracy. To conclude, the utility of diagnostic biomarkers cannot undermine the vital role of careful clinical evaluation and assessment in individuals with sepsis.
Financial support & sponsorship
None.
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the global burden of disease study. The Lancet. 2020;395:200-11.
- [Google Scholar]
- Epidemiology of adult-population sepsis in India: A single center 5 year experience. Indian J Crit Care Med. 2017;21:573-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801-10.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Recognizing sepsis as a global health priority - A WHO resolution. N Engl J Med. 2017;377:414-7.
- [CrossRef] [PubMed] [Google Scholar]
- Community-acquired bacterial bloodstream infections in developing countries in south and southeast Asia: A systematic review. Lancet Infect Dis. 2012;12:480-7.
- [CrossRef] [PubMed] [Google Scholar]
- How to Optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol. 2016;7:697.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Blood culture utilization in the hospital setting: A call for diagnostic stewardship. J Clin Microbiol. 2022;60:e0100521.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Modern blood culture: Management decisions and method options. Clin Lab Med. 2020;40:379-92.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin Infect Dis. 2004;39:206-17.
- [CrossRef] [PubMed] [Google Scholar]
- Biomarkers of sepsis. Crit Rev Clin Lab Sci. 2013;50:23-36.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Use of immature-to-total-neutrophil ratio in early neonatal sepsis. Pediatr Infect Dis J. 2012;31:1101-2.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnostic roles of neutrophil in bloodstream infections. Immunobiology. 2020;225:151858.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil dysregulation during sepsis: an overview and update. J Cell Mol Med. 2017;21:1687-97.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Volume, conductivity, and scatter parameters of leukocytes as early markers of sepsis and treatment response. J Lab Physicians. 2019;11:29-33.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The accuracy of mean neutrophil volume relative to blood culture for the diagnosis of sepsis: A meta-analysis. PJP. 2017;2:18-22.
- [CrossRef] [Google Scholar]
- Volume, conductivity and scatter parameters of neutrophils in neonatal sepsis – Is it a cost-effective tool? Iran J Ped Hematol Oncol. 2022;12:174-81.
- [Google Scholar]
- Mean cell volumes of neutrophils and monocytes are promising markers of sepsis in elderly patients. Blood Res. 2013;48:193-7.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil VCS parameters are superior indicators for acute infection. Lab Hematol. 2007;13:12-6.
- [CrossRef] [PubMed] [Google Scholar]






