Translate this page into:
Alleviation of metabolic abnormalities induced by excessive fructose administration in Wistar rats by Spirulina maxima
Reprint requests: Prof. G.B.K.S. Prasad, SOS in Biochemistry & Biotechnology, Jiwaji University, Gwalior 474 011, India e-mail: gbksprasad@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Diabetes mellitus is a metabolic disorder characterized by hyperglycaemia. Several natural products have been isolated and identified to restore the complications of diabetes. Spirulina maxima is naturally occurring fresh water cyanobacterium, enriched with proteins and essential nutrients. The aim of the study was to determine whether S. maxima could serve as a therapeutic agent to correct metabolic abnormalities induced by excessive fructose administration in Wistar rats.
Methods:
Oral administration of 10 per cent fructose solution to Wistar rats (n=5 in each group) for 30 days resulted in hyperglycaemia and hyperlipidaemia. Aqueous suspension of S. maxima (5 or 10%) was also administered orally once daily for 30 days. The therapeutic potential of the preparation with reference to metformin (500 mg/kg) was assessed by monitoring various biochemical parameters at 10 day intervals during the course of therapy and at the end of 30 days S. maxima administration.
Results:
Significant (P<0.001) reductions in blood glucose, lipid profile (triglycerides, cholesterol and LDL, VLDL) and liver function markers (SGPT and SGOT) were recorded along with elevated level of HDL-C at the end of 30 days therapy of 5 or 10 per cent S. maxima aquous extract. Co-administration of S. maxima extract (5 or 10% aqueous) with 10 per cent fructose solution offered a significant protection against fructose induced metabolic abnormalities in Wistar rats.
Interpretation & Conclusions:
The present findings showed that S. maxima exhibited anti-hyperglycaemic, anti-hyperlipidaemic and hepatoprotective activity in rats fed with fructose. Further studies are needed to understand the mechanisms.
Keywords
Diabetes mellitus
fructose
hyperglycaemia
hyperlipidaemia
Spirulina maxima
Diabetes mellitus is a chronic degenerative disorder with increasing morbidity and mortality, characterized by hyperglycaemia and often associated with hyperlipidaemia and oxidative stress. Diabetes is the metabolic disorder, which can affect nearly every organ system in the body and is generally viewed as a clinical syndrome with variable phenotypic expression rather than a single disorder with a specific aetiology. It is often associated with hyperlipidaemia and hypertension1. Patients with type 2 diabetes suffer from chronic oxidative stress leading to complications2.
Emerging evidence from recent epidemiological and biochemical studies suggest that exposure to high fructose leads to rapid stimulation of lipogenesis and triglyceride accumulation, which in turn contributes to reduced insulin sensitivity and hepatic insulin resistance/glucose intolerance3. Fructose feeding has been shown to promote oxidative damage and exert detrimental effects by reducing antioxidant defenses, and increasing generation of free radicals4.
Spirulina maxima, a filamentous edible alga, is a cyanobacterium belongs to the family Oscillatoriaceae, which blooms well in alkaline waters5. It is characterized by high protein content (60-70%) and also contains considerable levels of chlorophyll, carotenoids, phycocyanin and vitamins6. Besides the organism's role in the treatment of protein malnutrition disorders, it also reduced body weight in obese humans7 and cholesterol level8 in experimental rats. S. maxima prevented fatty liver in rats after the administration of fructose rich purified diet9, or by carbon tetrachloride treatment10. S. maxima has also been shown to decrease vascular tone of aortic rings11. The present study was undertaken to investigate the protective and therapeutic potential, if any, of S. maxima against fructose induced diabetes and other metabolic abnormalities in Wistar rats.
Material & Methods
Animals: Randomly bred healthy male rats (Rattus norvegicus; Wistar strain; age: 10-14 wk; weight: 180-220 g) were obtained from the animal facility of Defence Research & Development Establishment, Gwalior (MP). The rats were housed in polypropylene cages, maintained on standard pellet diet (M/s Ashirwad Industry, Mohali, India) and water ad libitum. After randomization into various groups and before initiation of experiment, the rats were acclimatized for a period of seven days to laboratory conditions [temperature (25 ± 2°C) and 12 h light/dark cycle]. The study protocol was approved by Institutional Animal Ethics Committee (IHEC-09/2008), Jiwaji University, Gwalior during 2008.
All the chemicals used in the experiments were of analytical grade, the kits and chemicals used in this experiment were purchased from E. Merck India Limited (Mumbai, India).
Culturing of Spirulina maxima: The pure culture of S. maxima was obtained from National Facility for Blue Green Algae, Indian Agriculture Research Institute, New Delhi. The culture was grown in Zarrouk's medium (pH 8.5-9.5) under controlled conditions of light and temperature (2500 lux, 16/8 h light/dark, at 30˚C). The cultures were maintained for 20 days and harvested after exponential algal growth phase. The biomass was filtered through a screen filter of pore size 305 nm. The Spirulina mat was collected in the drying trays and dried at 40˚C for 12 h.
Induction of hyperglycaemia and hyperlipidaemia in rats: Hyperglycaemia and hyperlipidaemia were induced in Wistar rats by feeding 10 per cent fructose solution in drinking water for 30 days. All biochemical parameters in blood/plasma were estimated at an interval of 10 days. Animals were divided into seven groups of five rats each and treated as below: Group I: Healthy rats (Normal control); Group II: Hyperglycaemic rats without any treatments (Diabetic control, DC); Group III: Hyperglycaemic rats administered with 5 per cent S. maxima extract (SM) once daily for the following 30 days (Diabetic rats treated with 5% SM); Group IV: Hyperglycaemic rats co-administered with 10 per cent fructose solution continuously (CF) and 5 per cent S. maxima extract once daily for the following 30 days (Diabetic rats treated with 5% SM and 10% CF concurrently); Group V: Hyperglycaemic rats administered with 10 per cent S. maxima extract once daily for the following 30 days (Diabetic rats treated with 10% SM); Group VI: Hyperglycaemic rats co-administered with 10 per cent fructose solution and 10 per cent S. maxima extract once daily for the following 30 days (Diabetic rats treated with 10% SM and 10% CF concurrently); Group VII: Hyperglycaemic rats administered with metformin (500 mg/kg) once daily for the following 30 days (Metformin).
The dried S. maxima powder was suspended in distilled water to achieve a concentration of 5 and 10 per cent (i.e. 5 and 10 g of Spirulina powder was suspended in 100 ml of distilled water). The homogenous suspension of S. maxima was prepared and administered orally with a dose of 1.0 ml/day. The doses were administered once a day to experimental rats with the help of intragastric tube (cannula).
Blood collection: The animals were kept on 6-8 h fasting and then the blood samples (0.5-1.0 ml) were collected from retro orbital plexus of rat eyes under mild ether anaesthesia once before and at 10 day intervals during the course of study for biochemical analyses. Plasma was separated, aliquoted and used for analyses of glucose, triglyceride and cholesterol, remaining plasma sample was assayed for HDL cholesterol, LDL, VLDL and for serum glutamate pyruvate transaminase (SGPT), serum glutamate oxaloacetate transaminase (SGOT). All estimations were carried out on freshly collected blood/plasma samples.
Biochemical analyses: Blood glucose was estimated by glucose oxidase-peroxidase method12. Plasma triglyceride was estimated by glycerol-3-phosphate oxidation-peroxidase method13. Total cholesterol was estimated by cholesterol-oxidase and p-amino-phenazone method14. HDL-cholesterol level was estimated by phosphotungstate method15. The LDL and VLDL levels were calculated by Friedewald's equation. Serum glutamate pyruvate transaminase (SGPT) and serum glutamate oxaloacetate transaminase (SGOT) were estimated by 2, 4-dinitrophenyl hydrazine (2, 4-DNPH) method16.
Statistical analysis: Data were analyzed using one-way ANOVA (Bonferroni t-test) employing Sigma Stat, statistical software, version 1.0 (Jandal Corporation, USA).
Results
Fructose administration to experimental animals (Groups II-VII) resulted in hyperglycaemia (190-235 mg/dl), hypertriglyceridaemia (107-156 mg/dl), hypercholesterolaemia (80-112 mg/dl) and significant increases in liver function markers (SGPT and SGOT).
Effect of S. maxima administration on blood glucose level: At a dose of 5 per cent S. maxima, the reduction in blood glucose levels observed was 60 per cent (215-87 mg/dl) (P<0.001) and with 10 per cent S. maxima, the reduction was 54 per cent (202-92 mg/dl) (P<0.001) at the end of the 30 days therapy when compared to the pre-treatment levels. The diabetic rats administered with S. maxima and 10 per cent fructose in drinking water concurrently showed decrease in blood glucose levels by 45-48 per cent. When the diabetic rats were treated with metformin (500 mg/kg), the blood glucose level was reduced to 46 per cent of pre-treatment levels after 30 days of the therapy (Fig. 1).
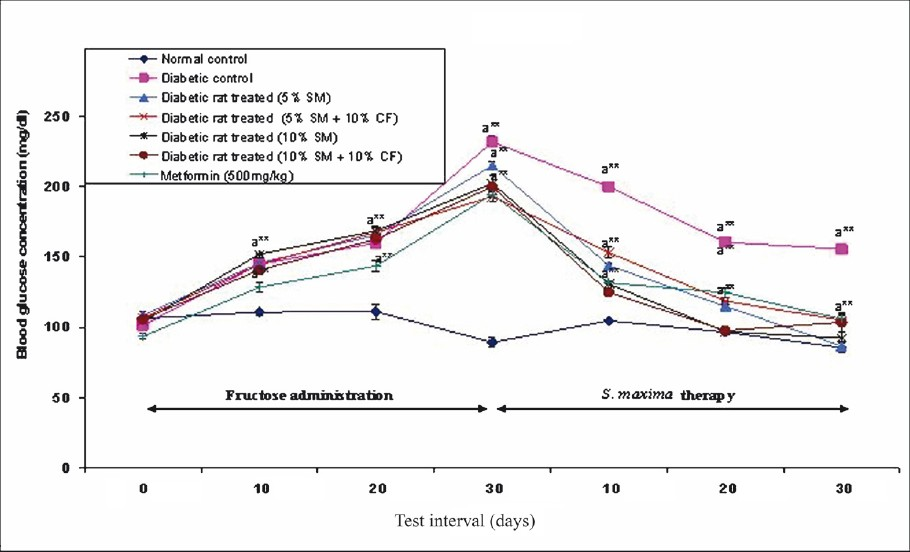
- Effect of S. maximaadministration on blood glucose level. SM, Spirulina maxima; CF, continuous fructose. The values expressed as mean ± SE, n=5. P*<0.05, **<0.001 compared to NC group.
Effect of S. maxima administration on triglyceride level: Administration of 5 or 10 per cent S. maxima suspension resulted in a significant (P<0.001) reduction in plasma triglyceride level by 51 and 39 per cent of pre-treatment levels of 162 and 136.18 mg/dl, respectively. Concurrent administration of S. maxima and 10 per cent fructose to hypertriglycedaemic rats also exhibited significant decrease in plasma triglyceride level by 28-34 per cent of pre-treatment levels. In metformin (500 mg/kg) treated group, the triglyceride level was reduced by 43 per cent of pre-treatment levels within 30 days of the therapy (Fig. 2).
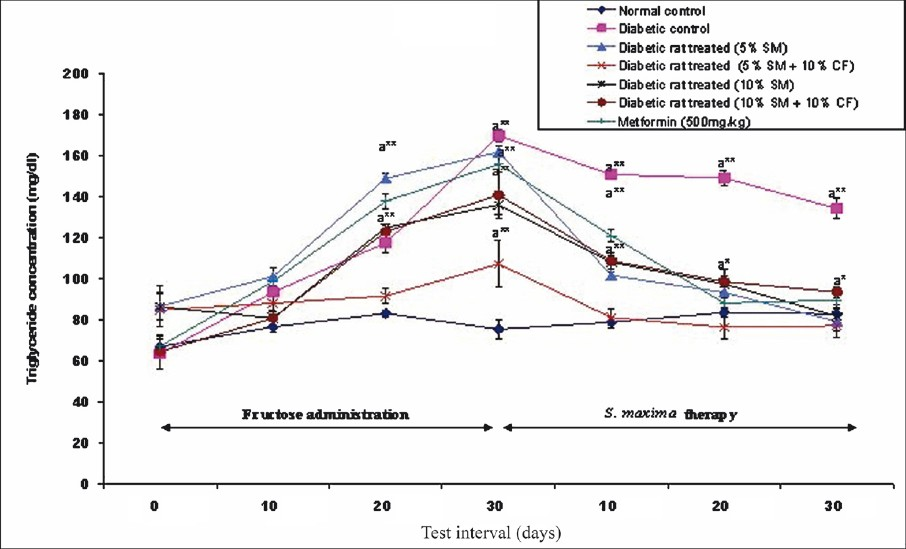
- Effect of S. maximaadministration on triglyceride level. SM, Spirulina maxima; CF, continuous fructose. The values expressed as mean ± SE, n=5. P*<0.05, **<0.001 compared to NC group.
Effect of S. maxima administration on cholesterol level: Administration of S. maxima suspension (5 or 10%) to hypercholesterolaemic rats reduced the cholesterol level by 33 per cent of pre-treatment levels. Co-administration of S. maxima and 10 per cent fructose in drinking water resulted in reduction in cholesterol level by 33-36 per cent of pre-treatment levels. Treatment of diabetic rats with metformin (500 mg/kg) reduced the cholesterol level by 55 per cent of pre-treatment levels (Fig. 3).
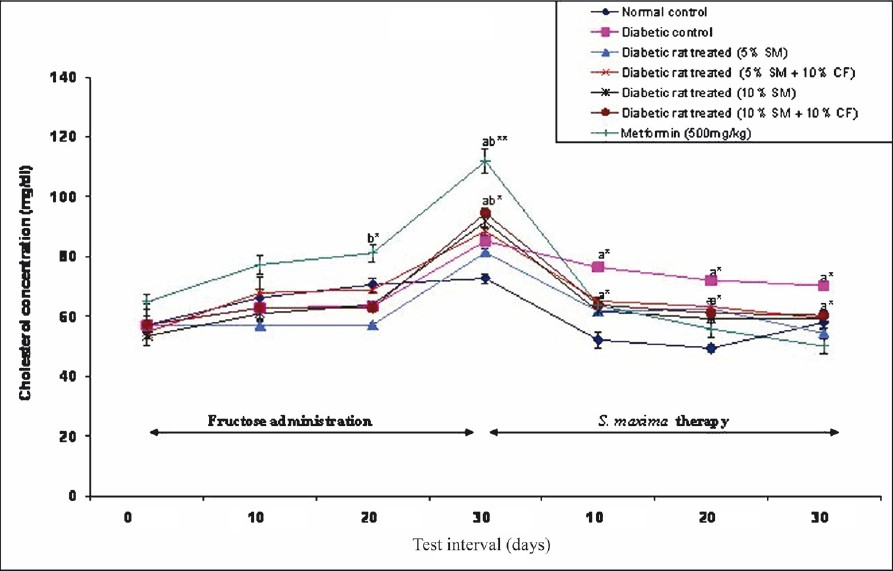
- Effect of S. maximaadministration on cholesterol level. SM, Spirulina maxima; CF, continuous fructose. The values expressed as mean ± SE, n=5. P*<0.01, **<0.001 compared to NC group.
Effect of S. maxima administration on HDL-C, LDL and VLDL levels: Administration of S. maxima to diabetic rats significantly (P<0.001) reduced the LDL and VLDL levels by 79 and 23 per cent of pre-treatment levels, respectively, and elevated the HDL-C level by 55 per cent. Co-administration of S. maxima and 10 per cent fructose in drinking water also reduced the LDL and VLDL levels by 39 and 28 per cent of pre-treatment levels and elevated the HDL-C level by 43 per cent of pre-treatment levels. On the other hand, administration of metformin (500 mg/kg) to diabetic rats reduced the LDL and VLDL levels by 80.10 and 49.18 per cent, respectively and elevated the HDL-C level by 53.76 per cent compared to diabetic control group (Fig. 4).

- Effect of S. maxima administration on HDL-C, LDL and VLDL levels. SM, Spirulina maxima; CF, continuous fructose. The values expressed as mean SE, n=5. P*<0.05, **<0.001 compared to NC group.
Effect of S. maxima administration on body weight: There was no significant intra-group variation in the body weight of animals. The diabetic rats, when treated with either 5 or 10 per cent S. maxima suspensions gained the body weight within 30 days of therapy. There was a significant increase in the body weight of the fructose fed animals (diabetic control) as well as metformin (500 mg/kg) treated group also. In general, the weight gain in animals show a mixed trend and is found to increase on fructose administration or S. maxima or metformin therapies (Fig. 5).
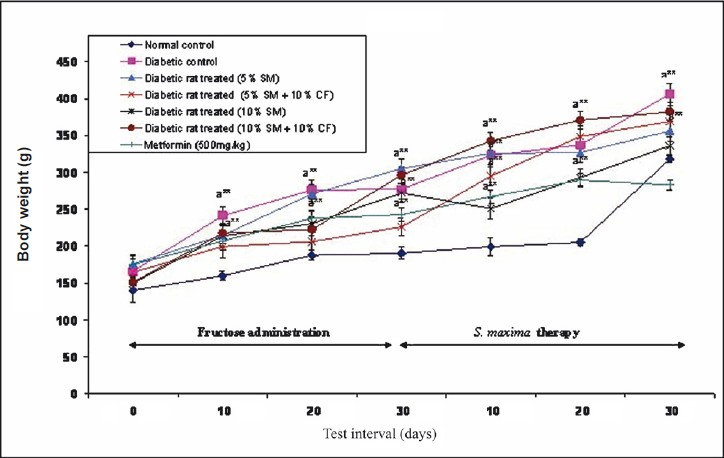
- Effect of S. maxima administration on body levels. SM, Spirulina maxima; CF, continuous fructose. The values expressed as mean ± SE, n=5. P*<0.05, **<0.001 compared to NC group.
Effect of S. maxima administration on liver function markers: Analysis of liver function markers viz., SGPT and SGOT activities were significantly (P<0.05) increased in fructose fed rats. Administration of 5 per cent S. maxima to experimental rats reduced the SGPT activity by 24.78 per cent (Fig. 6) and SGOT activity by 33.42 per cent of pre-treatment levels (Fig. 7). While concurrent administration of 5 per cent S. maxima and 10 per cent fructose reduced the SGPT and SGOT activities by 16.01 and 29.19 per cent, respectively compared to diabetic control group. Administration of 10 per cent S. maxima suspension to experimental rats reduced SGPT activity by 29 per cent (P<0.05) (Fig. 6) and SGOT activity by 33.57 per cent of pre-treatment levels respectively (Fig. 7). Treatment of experimental rats with metformin (500 mg/kg) reduced the SGPT and SGOT activities by 14.44 and 19.60 per cent of pre-treatment levels, respectively.
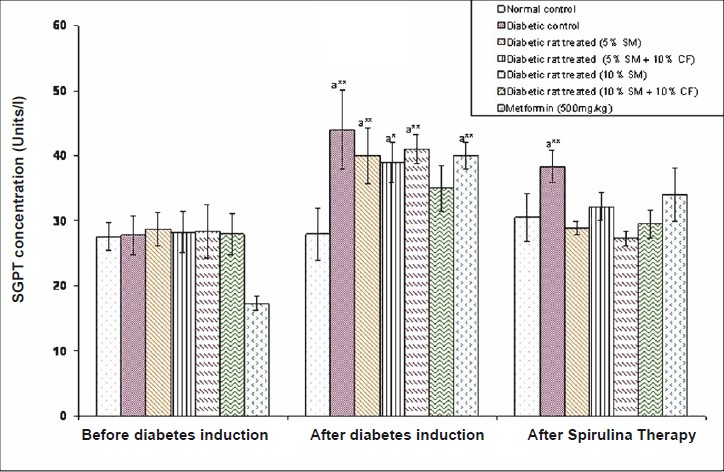
- Effects of S. maxima administration on serum glutamate pyruvate transaminase (SGPT) activity. SM, Spirulina maxima; CF, continuous fructose. The values expressed as mean ± SE, n=5. P*<0.05, **<0.001 compared to NC group.
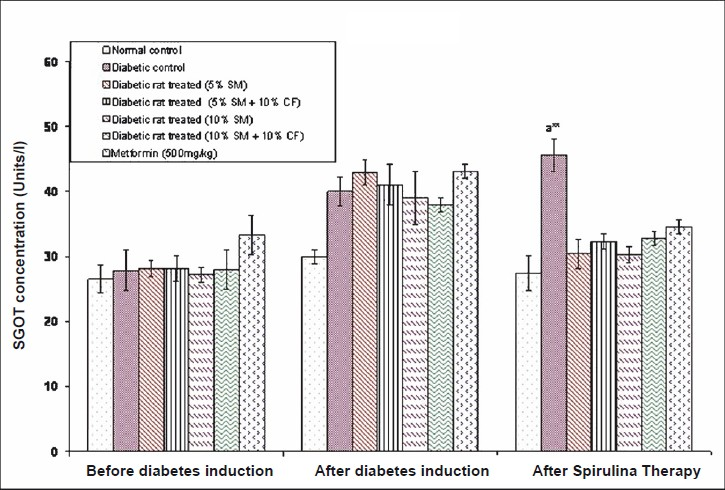
- Effect of S. maxima administration on serum glutamate oxaloacetate transaminase (SGOT) levels. SM, Spirulina maxima; CF, continuous fructose. The values expressed as mean ± SE, n=5. P*<0.05, **<0.001 compared to NC group.
Discussion
Wistar rats fed with excessive fructose diet develop hyperglycaemia and hypertriglyceridaemia as well as oxidative damage17. The currently available anti-diabetic drug regimens viz. sulphonylureas, biguanides and α-glucosidase inhibitors fail to address all the ailments associated with diabetes mellitus and are associated with side reactions18. Hence, the search is on for new anti-diabetic compounds with multiple targets and without any side effects. Studies carried out by earlier researchers revealed modulatory effects of Spirulina on lipid and carbohydrate metabolisms19. Feeding of 10 per cent fructose solution (in drinking water) to Wistar rats for 30 days resulted in hyperglycaemia and dyslipidaemia in the present study. After 30 days, the animals in groups II, III IV and VII were shifted to normal drinking water and were then administered S. maxima extract, while, the rats in groups IV and VI, were continued with 10 per cent fructose solution along with S. maxima administration. This was done to examine differential effect, if any, on continuous fructose feeding. The present study revealed that administration of S. maxima extract was effective in alleviating the abnormalities of carbohydrate and lipid metabolisms induced by excess fructose administration in Wistar rats.
Several therapeutically important compounds/molecules like ß-carotene, phycocyanin, γ-linolenic acid, etc. identified in S. maxima are shown to possess immunomodulatory and biomodulatory functions20. Our study showed that the administration of S. maxima suspension for 30 days to hyperglycaemic rats produced a marked decrease in the blood glucose level and the effect was found superior to metformin as also shown earlier20. Mani et al21 showed a significant decrease in the fasting blood sugar level of patients after 21 days of 2 g/day Spirulina supplementation. In another study the oral administration of 15 mg/kg Spirulina for 45 days to diabetic rats significantly reduced the blood glucose level22. This could perhaps be due to high fibre content that interfered with the absorption of glucose23. Another theory was based on the possible action of peptides and polypeptides generated by the digestion of Spirulina proteins24.
S. maxima was found effective in normalizing the triglyceride levels also. This could be due to decreased VLDL triglyceride production or increased VLDL clearance in the peripheral tissues. The reduction in triglyceride level could also be through lipoprotein lipase, a key enzyme in the metabolism of triglyceride rich lipoprotein25.
In our study, cholesterol, LDL and VLDL showed a significant reduction with an increase in HDL-C following S. maxima administration for 30 days. Similar observations were made by Rodriguez-Hernandez26, who found that the dietary administration of 5 per cent S. maxima for four weeks to diabetic mice reverted the LDL and VLDL levels to normal level. In another study, rats fed with a 20 per cent water-soluble fraction of Spirulina exhibited a high HDL to LDL ratio and a low fasting serum glucose level compared with rats fed a water insoluble fraction27. A significant increase in HDL cholesterol has been reported earlier28. This effect might be due to the presence of Gamma-linoleic acid in Spirulina, which prevents accumulation of fats and cholesterol in human body23. Ramamoorthy & Premkumari29 found that Spirulina inhibited jejunal cholesterol absorption and ileal bile acid reabsorption in hypercholesterolaemic patients, proposing that C-phycocyanin present in Spirulina might be responsible for this effect.
Elevated serum activities of SGOT and SGPT reflect hepatocellular necrosis as these are released into the blood after cell membrane damage. Administration of S. maxima for 30 days resulted in significant decrease in plasma SGPT and SGOT activities in fructose fed Wistar rats. The restoration of SGOT and SGPT activities to their respective normal levels after administration of Spirulina showed hepatoprotective effect of this alga. The presence of β-carotene in Spirulina , a fat soluble pigment, the precursor of vitamin A, known for its antioxidant activity helps in the hepatoprotective activity7.
In conclusion, the present results showed the anti-hyperglycaemic, anti-hyperlipidaemic and hepatoprotective effects of Spirulina and further studies are needed to elucidate the underlying mechanism(s).
Acknowledgment
The study was supported in part by research grants from University Grants Commission, DRDO, New Delhi and Biotech Council of Madhya Pradesh, Bhopal.
References
- Epidemiology of diabetes in India-current perspective and future projections. Indian Med Assoc. 2002;100:144-8.
- [Google Scholar]
- The role of oxidative stress in development of complications in diabetes. Curr Opinion Endo. 1996;7:277-84.
- [Google Scholar]
- Vitamin E improves the free radical defense system potential and insulin sensitivity of rat fed high fructose diets. J Nutr. 1997;127:103-7.
- [Google Scholar]
- Clinical and biochemical evaluations of the algae Spirulina with regards to its application in treatment of obesity: A double blind cross over study. Nutr Rep Int. 1986;4:565-74.
- [Google Scholar]
- Effect of Spirulina platensis on plasma lipoprotein lipase activity in fructose induced hyperlipidemic rats. J Nutr Sci Vitaminol. 1990;36:165-71.
- [Google Scholar]
- Preventive effects of Spirulina maxima on the fatty liver induced by a fructose rich diet in the rat, a preliminary report. Life Sci. 1993;53:57-61.
- [Google Scholar]
- Studies on the preventive effect of Spirulina maxima on fatty liver development induced by carbon tetrachloride, in the rat. J Ethanopharmacol. 1999;64:141-7.
- [Google Scholar]
- Effects of dietary Spirulina maxima on endothelium dependent vasomotor responses of rat aortic rings. Life Sci. 1997;61:211-9.
- [Google Scholar]
- Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Anal Clin Biochem. 1969;24:24-30.
- [Google Scholar]
- Prevention of coronary heart disease in clinical practice. J Euro Heart. 1998;19:1434-503.
- [Google Scholar]
- Tietz textbook of clinical chemistry (3rd ed). Philadelphia: W.B. Saunders Company; 1999. p. :809-61.
- Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977;23:882-4.
- [Google Scholar]
- A colorimetric method for the determination of serum glutamate oxaloacetic and glutamic pyruvic transaminases. Am J Clinic Pathol. 1957;28:56-63.
- [Google Scholar]
- Insulin, oral hypoglycemic agents, and the pharmacology of endocrine pancreas. In: Hardman JG, Limberd LE, Malinoff PB, Ruddon RW, Gilman AG, eds. Goodman and Gilman's the pharmacological basis of therapeutics (9th ed). New York: McGraw Hill; 1996. p. :1487-518.
- [Google Scholar]
- Glycolipid isolated from Spirulina maxima: structure and fatty acid composition. Agricul Biol Chem. 1983;47:2349-55.
- [Google Scholar]
- Effect of water soluble and insoluble fractions of Spirulina over serum lipids and glucose resistance of rats. J Jpn Soc Nutr Food Sci. 1991;44:273-7.
- [Google Scholar]
- Studies on the effect of Spirulina supplementation in control of diabetes mellitus. In: Subramanian G, ed. Cyanobacterial biotechnology. USA: Science Publishers Inc; 1998. p. :301-4.
- [Google Scholar]
- Hypocholesterolemic effect of Spirulina in patients with hyperlipidemic nephrotic syndrome. J Med Food. 2002;5:91-6.
- [Google Scholar]
- Studies on long term effect of Spirulina supplementation on serum lipid profile and glycated proteins in NIDDM patients. J Nutraceut. 2000;2:25-32.
- [Google Scholar]
- Age related response to dietary fructose in the rat: discrepancies in triglyceride and apolipoprotein B synthesis as a possible mechanism for fatty liver induction in adult rat. Proc Soc Exp Biol Med. 1993;204:180-3.
- [Google Scholar]
- Spirulina maxima prevent fatty liver formation in CD-1 male and female mice with experimental diabetes. Life Sci. 2001;69:1029-37.
- [Google Scholar]
- Effects of water-soluble and insoluble fractions of Spirulina on serum lipid components and glucose tolerance in rats. J Jpn Soc Nutr Food Sci. 1991;44:273-7.
- [Google Scholar]
- Effect of Spirulina platensis on glucose, uric acid and cholesterol levels in the blood of rodents. Int J Exp Bot. 1995;57:93-6.
- [Google Scholar]
- Effect of supplementation of Spirulina on hypercholesterolemic patients. J Food Sci Technol. 1996;33:124-8.
- [Google Scholar]






