Adult vaccination in India: A rapid review of current status & implementation challenges
For correspondence: Prof. Arunaloke Bhattacharyya, Department of Pediatrics, Institute of Child Health, Kolkata 700 017, West Bengal, India e-mail: arunalokeb@yahoo.com
-
Received: ,
Accepted: ,
Abstract
Background & objectives
The expanded programme on immunization launched in India in 1978, with its focus on preventing six diseases in children (tetanus, diphtheria, pertussis, poliomyelitis, typhoid, and childhood tuberculosis), was widened in its scope in 1985-86. This new avtaar, the Universal Immunization Programme (UIP), incorporated measles vaccine for children and rubella and adult diphtheria vaccines for pregnant women. We conducted this rapid review on adult immunization relevant for India, as recent COVID-19 experience revealed how newly emergent or re-emergent pathogens could have their onslaughts on the elderly and adults with comorbidities.
Methods
Three different bibliographic databases, namely PubMed, Scopus and Ovid were searched electronically to access the articles published in peer-reviewed journals. Relevant consensus guidelines by in-country professional groups were also collated. We conducted deduplication and screening of the outputs of these searches (1242 bibliographical records). Finally, 250 articles were found eligible for inclusion. As trials on the reduction of morbidities, mortalities and hospitalizations in adults due to proposed vaccines under Indian consensus guidelines were not available, no meta-analysis was conducted.
Results
Evidence from articles finally included in this synthesis were grouped under (i) preventing viral and bacterial infections in adults; (ii) adult vaccination and awareness tools; (iii) vaccine hesitancy/acceptance; and (iv) adult vaccination guidelines. In-country research revealed the need for introducing the Human Papilloma Virus (HPV) vaccine in adolescence or early-adulthood to prevent ano-genital cancers in elderly and later life. Importantly HPV prevalence among cervical cancer patients varied between 88 to 98 per cent in Andhra Pradesh, Odisha and Delhi. The importance of conducting regular surveillance of pneumococcal diseases and influenza, as well as tweaking the vaccines accordingly, was revealed in other articles. A poor uptake of influenza vaccine (≤2%) in adults (≥45 yr) was documented.
The uptake of hepatitis B vaccine in Health Care Workers (HCWs) in Delhi and Mumbai was of concern and ranged from 55 to 64 per cent. The vulnerability of HCWs to rubella was investigated in a paediatric ophthalmic hospital in Madurai: a tenth of the selected HCWs were rubella seronegative and mounted good protective immunity following RA 27/3 vaccine administration. An outbreak of measles in college students in Pune emphasized the phenomenon of waning immunity. Similarly, a study in the infectious disease hospital in Kolkata and in-patients in Delhi revealed a lack of protective immunity against diphtheria and tetanus in adults. The researchers estimated the economic benefits of providing a typhoid vaccine to a household to be US$ 23 in a middle-income neighbourhood and US$ 14 in slum settings. The authors highlighted the importance of preventive strategies, finding that the cost of severe typhoid fever was US$ 119.1 in 18 centres across India. Both qualitative and quantitative investigations explored vaccine hesitancy, which was studied more during the COVID-19 pandemic than earlier.
Interpretation & conclusions
Vaccination programmes in India would require (i) increasing awareness around vaccine-preventable diseases among adults and HCWs; (ii) actively engaging health care systems and community-based organizations; and (iii) developing and producing affordable, safe, and country-appropriate vaccines. Effective communication strategies and tools will be the key to the success of such interventions.
Keywords
Adult
gaps in immunization
Indian guidelines
pandemic preparedness
vaccine preventable infections
The Expanded Programme on Immunization (EPI) was launched by the World Health Organization (WHO) in 19741. The idea was to ensure access to life-saving vaccines for every child, irrespective of their geographical origin and socioeconomic status. Since then, its impact has grown over the last five decades. There are now thirteen vaccines recommended for different age groups1. EPI was launched in India in 1978, with the objective to reduce the mortality and morbidity from six diseases i.e., tetanus, diphtheria, pertussis, poliomyelitis, typhoid and childhood tuberculosis by providing immunization services to all eligible children. Its new incarnation, the Universal Immunization Programme (UIP) was launched in 1985-86, in India, with the inclusion of measles vaccine2 and exclusion of the injectable typhoid vaccines, which had several side effects and poor protection.
By incorporating Rubella and adult Diphtheria vaccines for pregnant women, the UIP expanded its horizon to the adult population3. However, immunization coverage, thus achieved to address the vulnerability of adults to various infections, was inadequate. Notably, the shape of the population pyramid of India has changed considerably between 1978 and 2024. The percentage of the population ≥65 yr of age has increased during this period from 0.9 (both male and female), to 1.4 in males and 1.5 per cent in females4.
With the world population ageing rapidly, partly due to life saving impact of childhood immunization, it is estimated that by 2030, there will be 34 nations with over 20 per cent population ≥65 yr5. Due to the immune-senescence and declining functional reserve and resilience, older people, especially those with non-communicable diseases (NCDs), such as cardiovascular diseases, chronic obstructive pulmonary disease, diabetes, and cancers, are more prone to infections6. Therefore, the incidences of various vaccine preventable infections (VPIs) are increasing in the elderly (≥60 yr)7. Inclusion of adult vaccines within the ambit of UIP, in this context appears crucial.
Against this background, we conducted a rapid review of the adult vaccination issues in India. The purpose was to understand their nuances and inform policymakers about evidence-based programming. Moreover, worldwide, there is ongoing discussion about pandemic preparedness. In this context, adult vaccination comprises an important intervention element because the recent COVID-19 experience revealed how the elderly in general and adults with comorbidities could suffer most with the emergence of new viral pathogens. We, therefore, conducted this rapid review of adult vaccination to inform the current country-specific programme planning in India and beyond.
Materials & Methods
To conduct a rapid review on various domains around adult immunization in India and to synthesize evidence available in the published articles, an electronic search was conducted. Three different bibliographic databases, namely PubMed, Scopus and Ovid, were used for this purpose. The search was guided by a framed research topic using the PICOS framework. In PICOS, the Population (P) under scrutiny was adults aged ≥18 yr (PubMed allows grouping of population aged 19+ yr). The Intervention (I) was ‘immunization’, further divided into two sub-domains; one was ‘Intervention at individual level’, which included vaccination, vaccine-preventable diseases (VPDs), and different vaccines available for adults. The other sub-domain was ‘Intervention at the policy and programme level’, including issues like vaccination programmes, EPI, UIP, National Technical Advisory Group on Immunization (NTAGI), etc. The Outcome (O) was captured under qualitative concepts such as hesitancy, denial, anxiety, etc. In quantitative terms, the outcome was represented through vaccine uptake, vaccination coverage, out-of-pocket expenses, complications avoided, etc. India at the country level and ‘its States and union territories’ at the regional level served to characterize the Settings (S). No time restriction was imposed on the search strategy. All possible keywords under the domains, as mentioned earlier, were used to develop a comprehensive search strategy (Supplementary Table). Results of the searches (1242 bibliographic records) conducted on June 27, 2024 from the above-mentioned databases were exported to Rayyan software that helped conduct the literature search, deduplication (259 duplicate records), and screening based on pre-decided eligibility criteria for inclusion in our investigation. Initially, the title and abstract screening of 983 articles were undertaken independently by the two authors (AB and SMS) to select only the eligible ones. Any conflict regarding this, between the two authors were resolved through mutual consensus. The access of full texts of 448 articles, thus selected, was needed for final inclusion in the study. However, 46 articles could not be accessed for full text, and 152 articles were excluded following full-text reading due to various reasons (Figure). Finally, 250 articles were found eligible to be included in this study, which were evaluated for their design and implementation details during the qualitative synthesis of evidence. The entire scheme of workflow is presented in the flow diagram (Figure) based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). As trials on the reduction of morbidities, mortalities and hospitalizations in adults due to proposed vaccines under Indian consensus guidelines were not available, no meta-analysis was conducted.
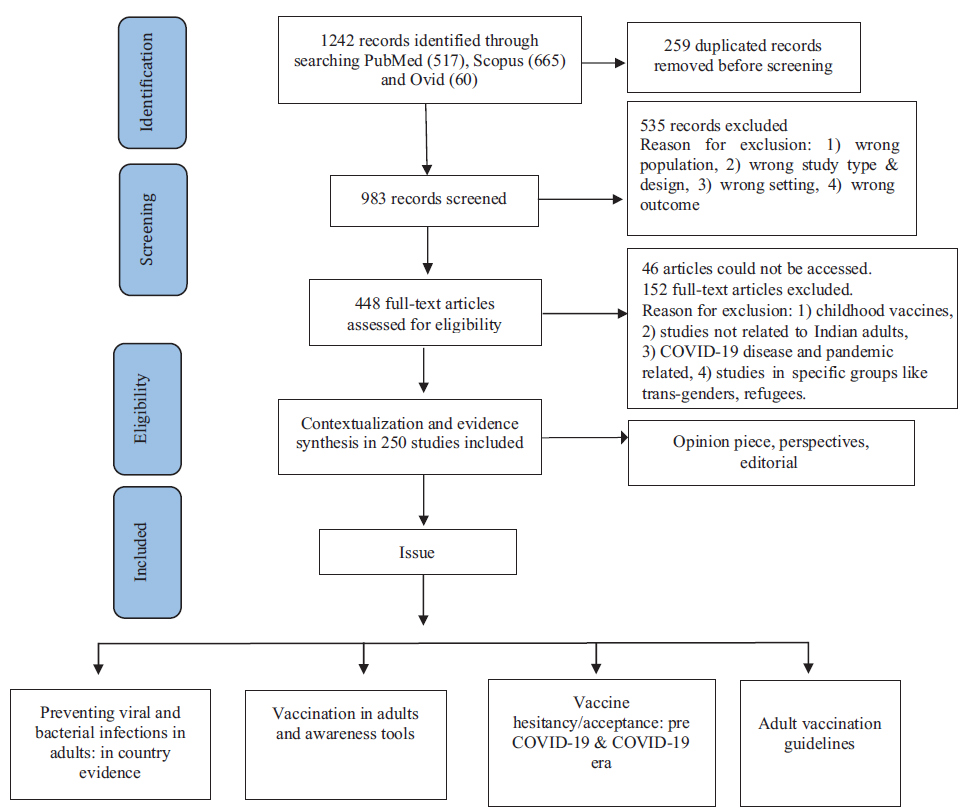
- PRISMA scheme of workflow.
Results
Data categorization
Two hundred and fifty publications were used to synthesize evidence under the following four subtitles: (i) Preventing viral and bacterial infections in adults: in-country evidence, (ii) Vaccination in adults and awareness tools, (iii) Vaccine hesitancy/acceptance: pre-COVID-19 & COVID-19 era, and (iv) Adult vaccination guidelines.
Preventing viral and bacterial infections in adults; in-country evidence
In the following section, viral infection in adults caused by human papillomavirus (HPV), influenza, Japanese encephalitis (JE), measles and rubella (MR) and hepatitis B (HBV), have been dealt with. Subsequently relevant bacterial diseases such as pneumococcal infection, typhoid, diphtheria, and tetanus have been discussed for their relevance to adult vaccination.
HPV-early vaccination in early adulthood & opportunities to avert adult cancer
HPV is a sexually transmitted infection which is acquired through unprotected sex during adolescence or later. The infection predisposes ano-genital cancers in males and females after a long latent phase of incubation. In India, the HPV prevalence has been recorded as 93.8, 87.8 and 98.1 per cent among patients with cervical cancers in Odisha, Andhra Pradesh and Delhi, respectively8-10. In contrast, according to the NFHS 5 (2019-2021) report, only two per cent of the women of urban population and 1.7 per cent of the rural population underwent screening test for cervical cancer11. Research has emphasized that immunization with the HPV vaccine in adolescents may help address these issues12.
In a multi-centric cluster randomized trial of quadrivalent HPV vaccination in girls (10-18 yr), 25 per cent of the participants received one dose of HPV vaccine. The trial demonstrated a robust and sustained immune response against HPV 16 and 18, and the antibody levels were stable over a four-yr period. The frequencies of cumulative incidence and persistent HPV 16 and 18 infections up to seven yrs of follow-up were also low in all the vaccinated groups12. Noticeably, modelling studies have shown that a single-dose HPV vaccine has the potential to provide short and long term protection13, and is likely to be cost effective than two dose schedule14. Dandapat and colleagues15 highlighted the need to add HPV vaccine to the UIP. Importantly, the Federation of Obstetric and Gynaecological Societies of India (FOGSI), the Indian Academy of Paediatrics (IAP), and the NTAGI during 2018-2022, endorsed the introduction of two-dose (for girls aged 10-12 yr) and three-dose (for those aged ≥15 yr) schedules of the HPV vaccine16-18.
Influenza; importance of including circulating strains in the vaccine
In Kashmir, north India, 1219 patients admitted with severe acute respiratory illness (SARI) were tested for influenza viruses. About one-third of them were influenza-positive. Sequencing of the HA genes revealed about fifty per cent of them were infected with influenza B/Victoria, followed by about forty seven per cent with influenza A/H1N1 and the rest were infected with influenza A/H3N2 strain. Among influenza positive patients, 6.8 per cent were vaccinated, whereas vaccination proportion among influenza negative, was 8.6 per cent. Vaccine effectiveness (VE) in this study, for any influenza strain was found to be 13 per cent (95% CI - 42 to 47), and for influenza B, it was zero per cent. Poor VE was due to genetic mismatch between the circulating strain and the strain included in the vaccine19. Studies on acceptance, awareness, knowledge, attitude, etc., related to influenza vaccine among adults have also been conducted from different parts of India20-27.
JE, MR & HBV vaccine; unaddressed issues
A case-control study was conducted among participants aged 15-65 yr to estimate VE in the Sivasagar and Dibrugarh districts, of the northeastern State of Assam. The overall VE was 77 per cent (95% CI: 67–83). The VE decreased from 91 per cent in the first year of vaccination to 71 per cent at six yr post-vaccination28. It was found that, adults were more affected in a JE outbreak29 in Assam. We could retrieve a few more research articles highlighting the evidence of the occurrence of JE in adults and preventive strategies in India30-32.
In 2024, a retrospective cohort study was conducted to investigate the outbreak of measles among young college students (18-24 yr) in Pune, India. Among 31 suspected cases, 12 were tested, of which seven were positive for measles-specific IgM antibodies. All these cases received the measles vaccine in childhood33, indicating the waning of immunity over a period of time following childhood immunization. The investigators highlighted the importance of adult measles vaccination. Studies on the measles vaccine were few. They dealt mainly with seroprevalence and susceptibility to measles among adults in India34-36.
A cross-sectional study37, from a paediatric ophthalmology hospital in Madurai, south India, highlighted the proneness of healthcare workers (HCWs) to rubella infection. About a tenth of the selected HCWs in this hospital were found to be rubella seronegative and thus vulnerable. Vaccinating these seronegative HCWs with RA 27/3 rubella vaccine mounted good protective immune responses. Such result prompted the researchers to raise an advocacy point highlighting the need for rubella vaccination among hospital staff. A few other studies from our search reiterated the need for rubella vaccine in adult females before pregnancy in India38,39.
In two studies from New Delhi, only about 55 per cent of the HCWs were vaccinated against HBV40,41. This highlighted the existing gap in vaccine coverage. Another investigation from an institute in Mumbai, recorded the incidence of needle stick injury among HCWs to be 10.4/100 occupied bed per year, and only 64.2 per cent of the HCWs in this investigation had prior vaccination against Hepatitis B42.
Pneumococcal infection; serotypes included in vaccines vs. circulating types
A systematic review and meta-analysis revealed that approximately one fifth of adult Indian patients with community-acquired pneumonia (CAP) had S pneumonae infection, contributing a significant burden of CAP in India43. Three cross-sectional studies during 2007-201744-46, investigated if circulating pneumococcal strains (serotypes) in the communities were covered by the existing pneumococcal vaccines. A study from Vellore44 revealed that serotype coverage offered by the pneumococcal conjugate vaccines (PCV) PCV7, PCV10, PCV13, and pneumococcal polysaccharide vaccine (PPV) PPV23 were 29, 53, 64, and 73 per cent, respectively. The coverage for the same serotypes was 16, 24, 48 and 66 per cent, respectively in New Delhi45. This study also found 30 per cent of the isolates were of non-vaccine serotypes among patients ≥50 yr of age with CAP. In a prospective laboratory survey of Invasive Pneumococcal Diseases (IPDs) among individuals aged ≥18 yr in a South Indian tertiary care referral centre46, the protective coverage was 58.7 and 67.4 per cent respectively for PCV13 and PPV 23.
Typhoid; benefits of adult vaccination
In 2004, a contingent valuation (CV) survey was conducted in Kolkata, India to generate information on private demand for cholera and typhoid vaccines. The median private economic benefits of providing a typhoid vaccine to a household with five members were estimated to be about US$ 23 in a middle-income neighbourhood, and for a cholera vaccine, it was US$ 27. In this investigation, the estimated benefits were different in slum settings; US$ 14 for typhoid and US$ 15 for a cholera vaccine47.
A Kolkata-based modelling study done to assess the economic benefits of typhoid vaccine revealed that three typhoid-vaccination strategies (targeting only enrolled school children, targeting all children, and targeting adults and children) were ‘very cost-effective’48. On the other hand, in an urban slum in New Delhi, the mean total cost (patient and provider) of typhoid fever was US$ 126, with a hospitalized case costing much higher at US$ 63649. In a Kolkata-based study, the cost was US$ 72.7150. The benefits and cost of vaccination against typhoid were evident through such contrast. The study conducted by Severe Enteric Fever of India (SEFI) network at 18 sites across India (2017-2020), revealed that the mean cost of severe enteric fever was US$ 119.1. This study highlighted that the cost was likely to increase with emerging antimicrobial resistance, and emphasized the importance of preventive measures51. The safety, clinical acceptability and immunogenicity of typhoid conjugate vaccine (TCV) among adults was highlighted in another investgation52. Importantly, a study in South India is currently ongoing to determine the relative and absolute rate reduction of symptomatic, blood culture-confirmed S. Typhi infection with TCV53.
Diphtheria & tetanus; lack of protective immunity in adults
In the infectious disease hospital, Kolkata, 200 diphtheria patients were evaluated for demographic details, immunization status, clinical features, complications, and outcome. Adults ≥20 yr comprised 32 per cent of the total cases54. Another retrospective study was conducted on 241 confirmed diphtheria patients admitted to the same hospital, where the majority (50.6%) were ≥15 yr old. Only 27.4 per cent were fully immunized and the rest were either partially immunized (44.4%) or non-immunized (28.2%)55. The Delhi study56 conducted among 255 healthy adult participants in 2009 revealed that 53 per cent of adults were unprotected against diphtheria, and 47 per cent were susceptible to tetanus. Two more studies57,58 dealt with the prevalence of diphtheria among adult population in Jaipur, and Dibrugarh. Such findings underlined the needs for adult vaccination against these bacterial infections.
According to NFHS-5 report, 70 per cent mothers had antenatal check up in the first trimester, 58.1 per cent had at least four antenatal visits and 92 per cent of them were protected against neonatal tetanus in the last birth by adequate immunization11.
Vaccination in adults and awareness tools
We included maternal health and vaccination articles in the present review. Gandhi et al59, examined the NFHS-4 data and the Public Affairs Index to assess inequalities in the coverage of reproductive maternal new born and child health (RMNCH). It was found that there was erratic distribution of RMNCH coverage.
Another study from the State of Jammu and Kashmir60 in northern India used NFHS-4 data, the Census of India and Digest of Statistics to reveal a gap of 10.5 per cent for immunization and found a moderate negative correlation between the coverage gap and socioeconomic development60. Inadequate coverage of ‘quality ante-natal care’ was also indicated in other investigations61-65 using State or nationally representative data sources (NFHS-4 and NFHS-5).
Importantly, in a multi-centric (4 States) community-based study of older adults (>60 yr) in July 2018 on Influenza and respiratory syncytial virus (RSV) in India66, all four sites reported negligible influenza vaccination uptake (0.1-0.4%), low health insurance coverage (0.4-22%) and high tobacco use (19-52%). In another study on 72,250 adults (aged ≥45 yr), uptake of each of influenza, pneumococcal, typhoid and hepatitis B vaccine was less than two per cent67.
A few articles68,69 presented mobile messaging as a potential maternal and child healthcare tool. In a rural community-based study in 2013 in Vellore, Tamil Nadu68, 70 per cent of the individuals were willing to receive health information via text messages, and 98 per cent believed text messages could effectively spread health messages, including vaccination. However, reach, accessibility, and acceptance of different channels for health promotion, such as MobileApp (Saheli in Haryana)69, television, newspapers, health facilities, service providers, radio and other media (posters, pamphlets and various folk-art forms) were explored in large numbers during the COVID-19 pandemic70.
Vaccine hesitancy/acceptance: pre-COVID-19 & COVID-19 era
Articles dealing with vaccine hesitancy or acceptance in adults in India were published in considerable numbers following the COVID-19 pandemic. Among the 50 articles retrieved, only five were related to diseases other than COVID-19. While the majority of these studies were quantitative, qualitative investigations were few.
A cross-sectional study conducted from December 2020 to June 2021 in Mysuru, identified the factors associated with hesitancy and refusal of vaccines71. Although this was conducted during the pandemic time, the investigation focused on vaccines other than COVID-19. The reasons associated with vaccine hesitancy were as follows: (i) study participants did not think them as needed (46%); (ii) some considered vaccines unsafe (12%); and (iii) lack of awareness about places to go to and get vaccinated (7%). The study recorded that UIP vaccines were more acceptable among the public than non-UIP vaccines due to more ‘reliability and safety’ and their ‘worldwide acceptance’. Interestingly, prior to initiation of COVID-19 vaccination programme in India, a group of researchers explored vaccine hesitancy among the medical students and doctors and their immediate family members in Delhi72. The reasons for vaccine hesitancy were, (i) fear of side effects (51%); (ii) lack of awareness about the vaccines (49%); and (iii) the lack of national guidelines on adult vaccination (33%). The hesitancy for vaccine was highest for zoster (98%) and least for tetanus toxoid (58%). Significant hesitancy was also observed for pneumococcal, HPV, influenza and varicella-zoster vaccines. A qualitative study73 to understand the awareness, perceptions and choices while recommending the HPV vaccine to parents of adolescent girls, identified several barriers, faced by physicians: (i) lack of national-level guidance on the age, eligibility, and dosage; (ii) lack of opportunity to discuss the routine adolescent vaccines due to absence of practice-level contact with well or non-sick adolescents; and (iii) out-of-pocket expenditure and vaccine availability.
Similarly, the studies on COVID-19 vaccine hesitancy were cross-sectional in design. In the northern district of Rajasthan74, psychological antecedents and predictors of COVID-19 vaccine hesitancy among patients with chronic disease were studied. A study by Gupta et al75, described COVID-19 vaccine hesitancy among 80 per cent of pregnant women in Manipur. Another study76 conducted at a tertiary care centre in Kalyani, West Bengal, measured willingness to pay (WTP) for the COVID-19 vaccines among participants and their children. Although more than half of the adult respondents were unwilling to pay for vaccines for themselves, WTP for COVID-19 vaccination was higher for their children. The Indian Institute of Sciences, Bangalore, dealt with the role of leadership and incentive-based programmes in addressing vaccine hesitancy77. It concluded that political and community leaders had minimal role in encouraging COVID-19 vaccination. The role of timely, and accurate information, applications of telemedicine, and the need for optimal design of incentive-based vaccination programmes to get adequate coverage were highlighted. Community based studies were conducted in different urban and rural settings of India in Puducherry, Maharashtra (Pune), Tamil Nadu (Chengelput), and Uttar Pradesh (Kanpur). Respondents who had no acceptance for vaccine cited reasons like ‘fear of side effects’, ‘not effective’, ‘I am healthy’, and ‘I don’t need them’. Association of vaccine hesitancy with specific gender, age group, educational status or socio-economic position were not consistent including those involving health workers78-80. A few of the studies, explored trust in the government, source of information about the vaccine and political affiliation as explanatory variables81.
Among the few qualitative studies, the one conducted in Chennai, Tamil Nadu82, healthcare workers, religious leaders, community influencers, local administrators and representatives of marginalized communities were interviewed in-depth. The following five domains were explored (i) vaccine availability, (ii) trust in COVID-19 vaccines, (iii) vaccine-related concerns, (iv) health/risk balance and (v) vaccine prioritization. Eagerness to receive COVID-19 vaccines was linked with ‘freedom from fear’, ‘possible restoration of normalcy’, ‘protection of family’ and ‘ability to travel and work abroad’. Doubts surrounding safety and the fear of side effects of the COVID-19 vaccine were feeders to vaccine hesitancy. Despite such hesitancy, 70 per cent of the Indian adult population had been fully vaccinated and 93 per cent had received their first dose, just one yr after the launch of COVID-19 vaccination drive on January 16, 2022. Moreover, India delivered the highest single-day vaccinations of 25 million doses on September 17, 202183.
Adult vaccination guidelines
The Geriatric Society of India84, the Research Society for the study of Diabetes in India85, the Indian Society of Nephrology86, and the Federation of Obstetric and Gynaecological Societies of India87 issued adult vaccination guidelines at various time points. Importantly, the Association of Physicians of India (API), in collaboration with the other associations and ‘Societies of Subject Experts’ in the country, brought out an exhaustive adult immunization consensus guideline in 2024. ‘Bridging the childhood vaccines’ in adult healthcare has been underlined in this consensus guideline. Vaccines like rotavirus and BCG (Bacillus Calmette-Guérin), which are conventionally administered in the pediatric age group, may find their role in the adult population in some particular circumstances like immunocompromised conditions and among HCWs, etc. Box highlights the consensus recommendation for vaccines in the elderly population belonging to the selected age group in India88.
Although the measles and rubella (MR) vaccine is included in the national immunization schedule for children in India, mumps is not included. However, MMR (measles, mumps, rubella) vaccination has been recommended for adults by different medical societies like API, FOGSI, etc88. As there is not enough evidence to suggest that mumps is a disease of public health importance89, MR vaccine was incorporated in EPI in 2017-193.
Indian Consensus Guideline on adult immunization recommends at least one dose of Tdap (Tetanus, diphtheria, acellular pertussis) vaccine during each pregnancy, preferably between 27 and 36 wk of gestation88.
Two vaccines for dengue are now prequalified by WHO90; however, those vaccines are yet to be licensed in India. A phase 3 trial of India’s first indigenous tetravalent dengue vaccine is currently underway91. Likewise, efforts are ongoing to prevent infections such as HIV, malaria, tuberculosis and leprosy88 through adult immunization.
Discussion
This rapid review has synthesized available in-country evidence around gaps in adult vaccination in India and identified programme and policy needs. Researchers all across the country generated information on viral and bacterial infections and their long-term impact, which led to subsequent programmatic discussions and helped develop adult immunization guidelines. For example, globally 27 per cent of the cervical cancer cases are contributed by India. The current estimates indicate that approximately 1,00,000 new cases were diagnosed and 60,000 deaths occurred annually in the country, accounting nearly 1/3rd of the global cervical cancer deaths92. HPV vaccination has a huge potential to change this scenario. Noticeably HPV vaccine constitutes a key component of the WHO strategy for worldwide elimination of cervical cancer12. However, several barriers, such as high costs and low public awareness, prohibit the introduction of prophylactic vaccines in countries like India, Nepal, Bangladesh, and Srilanka93.
Noticeably, influenza viral infection has immediate relevance to adults with or without comorbidities. There are indications for influenza vaccines in adults belonging to the high-risk groups in South Asian countries94. The National Centre for Disease Control (NCDC) has reported 6351 cases of influenza, with 132 deaths in India as of 202495. In this context, it is important to note that influenza vaccination has recently gained due attention and has found home in recommendations in the national guidelines in South Asian countries, including India94. However, the present uptake is less than two per cent among adults aged ≥45 yr20, which is of great concern.
Similarly, it is important to recognize that respiratory syncytial virus (RSV) is one of the important viral pathogens identified in older adults with acute respiratory tract infection96. Though we could not find any Indian data on the same, globally, vaccine trials are ongoing against RSV among older adults97. Other than respiratory tract infection (RTI) causing viruses, other viral infections in adults, which are of public health importance are JE, MR and Hepatitis B (Box).
In Japan, the Republic of Korea, and Taiwan (China), the introduction of the JE vaccine in routine childhood vaccination programmes began about 50 yr ago. Combined with this, increased urbanization and improved agricultural practices resulted in the elimination of JE from these countries98. JE vaccine was introduced in UIP-India, for children only in the year 2013. On the other hand, occurrence of JE in adults in parts of India are matter of concern, as cited above. The latest consensus guideline addresses this concern (Box).
In contrast, measles vaccine is presently a part of UIP and the consensus guideline recommends it for adults in the age group of 18-49 yr88. Similarly, the Hepatitis B vaccine was included in UIP for children. However, the consensus guideline now recommends it for adults, with and without co-morbidities like chronic kidney diseases, chronic liver diseases, etc88. Further, HCWs constitute a group, which is vulnerable to HBV and rubella due to occupational exposure. Notably, 37% of Hepatitis B Virus (HBV) infections among Health Care Workers (HCWs) are due to percutaneous occupational exposure to body fluids99. According to WHO, the estimates of Hepatitis B vaccine, among HCWs vary from 18 per cent in Africa to 77 per cent in Australia and New Zealand40.
Among bacterial diseases in adults, those caused by pneumococcal infection provides opportunities for prevention through vaccination. In the population aged <2 and ≥ 65 yr, the highest rates of invasive pneumococcal diseases (IPDs), have been reported. The mortality rates in these conditions are very high in individuals ≥ 65 yr of age due to depleting immune mechanisms and associated co-morbid conditions45. This highlights the importance of investigations from different regions of India44-46, which examined the extent of protective coverage offered by existing pneumococcal vaccines in the country, vis- a-vis circulating serotypes.
Another bacterial infection in adults of considerable public health importance is typhoid. The estimated economic benefits of providing typhoid vaccines in low- and middle-income settings in India have been captured in this rapid review. The World Health Organization (WHO) recommended introducing typhoid conjugate vaccine (TCV) in lower- and middle- income countries (LMICs). However, this requires substantial investment. With only 1.15 per cent of gross domestic product public spending towards health care, India’s vaccination programme has been slow to introduce new vaccines and relies heavily on out-of-pocket payments for treatment100.
The lack of protective immunity against diphtheria and tetanus in adults, as highlighted in this review, requires urgent attention. The only existing adult vaccination initiative in India caters to pregnant women. Noticeably, tetanus toxoid (TT) vaccine was replaced by tetanus diphtheria (Td) in UIP-India schedule. As per NHFS-5, there was increased antenatal check-up, antenatal visits, and past protection against neonatal tetanus as compared to NFHS-411, which led the country to validate for maternal and neonatal tetanus elimination by mid-April 2015 with the help of multilateral development partners such as WHO, UNICEF and others101.
Preventing tuberculosis in adults through vaccines poses the greatest hurdle, as an efficacious vaccine is yet to be available for different ages102-111. Although the retrospective data analysis of a community-based trial at Chingleput revealed that BCG revaccination in a community could offer modest protection against the development of TB disease at the end of 15 yr112, researchers have suggested that benefit might be obtained by re-vaccinating adults in the age group of 14-25 yr with BCG102,103.
In 2015, the WHO Strategic Advisory Group of Experts on Immunization defined vaccine hesitancy as a delay in acceptance or refusal of vaccination despite the availability of vaccination services, which can vary across time, place, and vaccines and is influenced by factors such as complacency, convenience, and confidence. WHO identified it as one of the top ten global health threats in 2019113. In recent times the COVID-19 pandemic and vaccination against COVID-19 brought such issues again to the fore. The concerns leading to vaccine hesitancy among the recipients generally constitute safety, science, efficacy, side effects, availability and a belief that they have sufficient immunity to combat. Also, in some studies age, sex, education and religion played important roles114-119. Addressing such issues during adult vaccination programmes thus appears paramount.
The lack of an adequate number of studies focussing on the effects of adult vaccination on reducing their morbidity and mortality, as well as hospital admissions following vaccine-preventable infections, did not allow us to present detailed critical insight on the topic.
In conclusion, the implementation of adult vaccination in India would require: (i) increasing awareness about the seriousness of vaccine-preventable diseases in the community and HCWs, (ii) engaging healthcare systems including government and community-based organizations effectively, and (iii) conducting research around development and production of affordable, safe, and effective vaccines in Indian context. Considering the changing epidemiology of the diseases7, developing appropriate communication strategies and tools120 will be the key for reaping the benefits of such interventions. The COVID-19 pandemic has also underlined the vulnerabilities of the elderly and adults with comorbidities to emerging and re-emerging microbial pathogens, which further highlights the importance of improving the situation of the poor uptake of adult immunizations across the country.
Financial support & sponsorship
None.
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- 50th anniversary of the Expanded Programme on Immunization (EPI). Available from: https://www.who.int/news-room/events/detail/2024/01/01/default-calendar/50th-anniversary-of-the-expanded-programme-on-immunization-(epi), accessed on July 19, 2024.
- Synthesizing evidences for policy translation: A public health discourse on rotavirus vaccine in India. Vaccine. 2014;32:A162-70.
- [Google Scholar]
- Expanded programme on Immunization (EPI) factsheet 2023: India. Available from: https://www.who.int/publications/i/item/India-EPI-factsheet-2023, accessed on July 19, 2024.
- Population pyramids of the world from 1950 to 2100. Available from: https://www.populationpyramid.net/india/2024, accessed on August 25, 2024.
- Ageing and health. Available from: https://www.who.int/india/health-topics/ageing#:∼:text=According%20to%20Census%202011%2C%20India,seniors%20into%20loneliness%20and%20neglect, accessed on August 25, 2024.
- The need for vaccination in adults with chronic (noncommunicable) diseases in India - lessons from around the world. Hum Vaccin Immunother. 2022;18:2052544.
- [Google Scholar]
- HPV Genotypes distribution in Indian women with and without cervical carcinoma: Implication for HPV vaccination program in Odisha, Eastern India. BMC Infect Dis. 2017;17:30.
- [Google Scholar]
- Prevalence and distribution of high-risk human papilloma virus (HPV) types in invasive squamous cell carcinoma of the cervix and in normal women in Andhra Pradesh, India. BMC Infect Dis. 2005;5:116.
- [Google Scholar]
- Human papillomavirus type distribution in cervical cancer in Delhi, India. Int J Gynecol Pathol. 2006;25:398-402.
- [Google Scholar]
- National Family Health Survey (NFHS-5), 2019-21. Available from: https://dhsprogram.com/pubs/pdf/FR375/FR375.pdf, accessed on August 25, 2024.
- Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine. 2018;36:4783-91.
- [Google Scholar]
- Evidence-based impact projections of single-dose human papillomavirus vaccination in India: A modelling study. Lancet Oncol. 2022;23:1419-29.
- [Google Scholar]
- Health and economic effects of introducing single-dose or two-dose human papillomavirus vaccination in India. BMJ Glob Health. 2023;8:e012580.
- [Google Scholar]
- Cervical cancer elimination in Indian context: Moving from barriers to facilitators. Cancer. 2022;128:4041-6.
- [Google Scholar]
- Centre urges States to create awareness and take steps for prevention of cervical cancer among girl students. Available from: https://pib.gov.in/PressReleasePage.aspx?PRID=1885597, accessed on August 25, 2024.
- FOGSI GCPR Screening and management of preinvasive lesions of cervix and HPV vaccination. Available from: https://www.fogsi.org/wp-content/uploads/2018/03/FOGSI-GCPR-March-2018-final.pdf, accessed on August 25, 2024.
- Human papillomavirus Q & A for parents. Available from: https://iapindia.org/pdf/vaccine-information/HPV-VACCINE.pdf, accessed on August 25, 2024.
- Poor vaccine effectiveness against influenza B-related severe acute respiratory infection in a temperate north Indian State (2019-2020): A call for further data for possible vaccines with closer match. Vaccines (Basel). 2021;9:1094.
- [Google Scholar]
- Factors associated with 2009 pandemic influenza A (H1N1) vaccination acceptance among university students from India during the post-pandemic phase. BMC Infect Dis. 2011;11:205.
- [Google Scholar]
- Community awareness, use and preference for pandemic influenza vaccines in Pune, India. Hum Vaccin Immunother. 2015;11:2376-88.
- [Google Scholar]
- Antenatal influenza vaccination in urban Pune, India: Clinician and community stakeholders’ awareness, priorities, and practices. Hum Vaccin Immunother. 2021;17:1211-22.
- [Google Scholar]
- Knowledge, attitude, and practices about the seasonal influenza vaccination among healthcare workers in Srinagar, India. Influenza Other Respir Viruses. 2013;7:540-5.
- [Google Scholar]
- Coping with influenza A/H1N1 in India: empathy is associated with increased vaccination and health precautions. Int J Health Promot Educ. 2016;54:283-94.
- [Google Scholar]
- The biggest barrier to influenza vaccination in pregnant females in India: Poor sensitization of the care providers. Vaccine. 2018;36:3569-70.
- [Google Scholar]
- Vaccination rates for pandemic influenza among pregnant women: An early observation from Chennai, South India. Lung India. 2012;29:232-5.
- [Google Scholar]
- Effectiveness of a single dose of Japanese encephalitis vaccine among adults, Assam, India, 2012-2018. Vaccine. 2021;39:4973-8.
- [Google Scholar]
- Japanese encephalitis outbreak in Assam, Northeast India, January to August 2022. Global Biosecurity. 2023;5
- [Google Scholar]
- Envelope protein gene based molecular characterization of Japanese encephalitis virus clinical isolates from West Bengal, India: A comparative approach with respect to SA14-14-2 live attenuated vaccine strain. BMC Infect Dis. 2013;13:368.
- [Google Scholar]
- Arexvy: A comprehensive review of the respiratory syncytial virus vaccine for revolutionary protection. Viral Immunol. 2024;37:12-15.
- [Google Scholar]
- Japanese encephalitis: Strategies for prevention and control in India. Indian J Med Spec. 2019;10:12-7.
- [Google Scholar]
- Measles outbreak in adults: A changing epidemiological pattern. Med J Dr DY Patil Vidyapeeth. 2017;10:447-52.
- [Google Scholar]
- Measles seroprevalence in persons over one year of age in Chandigarh, India. Hum Vaccin Immunother. 2022;18:2136453.
- [Google Scholar]
- Measles, mumps, and rubella: A cross-sectional study of susceptibility to vaccine-preventable diseases among young people in India. Med J Armed Forces India. 2019;75:70-3.
- [Google Scholar]
- Case based measles surveillance in Pune: Evidence to guide current and future measles control and elimination efforts in India. PLoS One. 2014;9:e108786.
- [Google Scholar]
- Immune status of health care personnel & post vaccination analysis of immunity against rubella in an eye hospital. Indian J Med Res. 2006;124:553-8.
- [Google Scholar]
- Human papillomavirus screening in north Indian women. Asian Pac J Cancer Prev. 2012;13:2643-6.
- [Google Scholar]
- Burden of Congenital Rubella Syndrome (CRS) in India: A systematic review. Indian Pediatr. 2012;49:377-99.
- [Google Scholar]
- Hepatitis B in health care workers: Indian scenario. J Lab Physicians. 2009;1:41-8.
- [Google Scholar]
- Low levels of awareness, vaccine coverage, and the need for boosters among health care workers in tertiary care hospitals in India. J Gastroenterol Hepatol. 2008;23:1710-5.
- [Google Scholar]
- Incidence of needlestick injury among healthcare workers in western India. Indian J Med Res. 2023;158:552-8.
- [Google Scholar]
- Streptococcus pneumoniae as a cause of community-acquired pneumonia in Indian adolescents and adults: A systematic review and meta-analysis. Clin Med Insights Circ Respir Pulm Med. 2019;13:1179548419862790.
- [Google Scholar]
- Invasive pneumococcal infections in Vellore, India: Clinical characteristics and distribution of serotypes. BMC Infect Dis. 2013;13:532.
- [Google Scholar]
- Prevalence of pneumococcal serotypes in adults ≥50 years of age. Indian J Med Microbiol. 2017;35:95-100.
- [Google Scholar]
- Invasive pneumococcal disease in Indian adults: 11 years’ experience. J Microbiol Immunol Infect. 2019;52:736-42.
- [Google Scholar]
- Rethinking cholera and typhoid vaccination policies for the poor: private demand in Kolkata, India. World Dev.. 2009;37:399-409.
- [Google Scholar]
- Evaluating investments in typhoid vaccines in two slums in Kolkata, India. J Health Popul Nutr. 2009;27:711-24.
- [Google Scholar]
- Costs of illness due to typhoid fever in an Indian urban slum community: Implications for vaccination policy. J Health Popul Nutr. 2004;22:304-10.
- [Google Scholar]
- Treatment cost for typhoid fever at two hospitals in Kolkata, India. J Health Popul Nutr. 2009;27:725-32.
- [Google Scholar]
- Cost of illness due to severe enteric fever in India. J Infect Dis. 2021;224:S540-7.
- [Google Scholar]
- Safety and immunogenicity of a Vi polysaccharide-tetanus toxoid conjugate vaccine (Typbar-TCV) in healthy infants, children, and adults in typhoid endemic areas: A multicenter, 2-cohort, open-label, double-blind, randomized controlled phase 3 study. Clin Infect Dis. 2015;61:393-402.
- [Google Scholar]
- An observer-blinded, cluster randomised trial of a typhoid conjugate vaccine in an urban South Indian cohort. Trials. 2023;24:492.
- [Google Scholar]
- Outcomes of respiratory diphtheria in a tertiary referral infectious disease hospital. Indian J Med Sci. 2010;64:373-7.
- [Google Scholar]
- Trend, morbidity profile and immunization status of diphtheria admitted cases: A 5-years review from a sentinel centre in Kolkata. Indian J Public Health. 2021;65:60-3.
- [Google Scholar]
- Serological immunity to diphtheria and tetanus in healthy adults in Delhi, India. Trop Doct. 2009;39:160-3.
- [Google Scholar]
- Diphtheria: It is still prevalent!!! Int J Pediatr Otorhinolaryngol. 2016;86:68-71.
- [Google Scholar]
- Recent outbreaks of diphtheria in Dibrugarh district, Assam, India. J Clin Diagn Res. 2016;10:DR01-3.
- [Google Scholar]
- Level of inequality and the role of governance indicators in the coverage of reproductive maternal and child healthcare services: Findings from India. PLoS One. 2021;16:e0258244.
- [Google Scholar]
- Analyzing the disparities in the coverage of maternal and child health services: A district-level cross-sectional analysis of Jammu and Kashmir. Indian J Public Health. 2020;64:130-4.
- [Google Scholar]
- Level of completion along continuum of care for maternal, newborn and child health services and factors associated with it among women in India: A population-based cross-sectional study. BMC Pregnancy Childbirth. 2021;21:731.
- [Google Scholar]
- Utilisation, equity and determinants of full antenatal care in India: Analysis from the National Family Health Survey 4. BMC Pregnancy Childbirth. 2019;19:327.
- [Google Scholar]
- Utilization and determinants of adequate quality antenatal care services in India: Evidence from the National Family Health Survey (NFHS-5) (2019-21) BMC Pregnancy Childbirth. 2023;23:800.
- [Google Scholar]
- Wealth and education-related inequalities in the utilisation of reproductive, maternal, newborn, and child health interventions within scheduled tribes in India: An analysis of Odisha and Jharkhand. BMC Public Health. 2024;24:1605.
- [Google Scholar]
- Geographic Inequities in coverage of maternal and child health services in Haryana State of India. Matern Child Health J. 2019;23:1025-35.
- [Google Scholar]
- Cohort profile: Indian network of population-based surveillance platforms for influenza and other respiratory viruses among the elderly (INSPIRE) BMJ Open. 2021;11:e052473.
- [Google Scholar]
- Vaccination coverage among older adults: A population-based study in India. Bull World Health Organ. 2022;100:375-84.
- [Google Scholar]
- A study to assess the feasibility of text messaging service in delivering maternal and child healthcare messages in a rural area of Tamil Nadu, India. Australas Med J. 2014;7:175-80.
- [Google Scholar]
- Feasibility and acceptability of Saheli, a WhatsApp chatbot, on COVID-19 vaccination among pregnant and breastfeeding women in rural North India. BMJ Innovations. 2023;9:195-206.
- [Google Scholar]
- Newspapers online portals in India: Coverage of COVID-19 vaccination awareness. Int J Media Inf Lit. 2022;7:221-32.
- [Google Scholar]
- Factors influencing the hesitancy and refusal of vaccines in India: A study-using tool developed by WHO SAGE Working Group. Trends Immunother. 2024;8
- [Google Scholar]
- Hesitancy for adult vaccines among healthcare providers and their family members in Delhi, India: A cross-sectional study. Dialogues Health. 2022;1:100044.
- [Google Scholar]
- Awareness, perceptions, and choices of physicians pertaining to human papillomavirus (HPV) vaccination in India: A formative research study. Vaccine X. 2022;12:100228.
- [Google Scholar]
- Identifying psychological antecedents and predictors of vaccine hesitancy through machine learning: A cross sectional study among chronic disease patients of deprived urban neighbourhood, India. Monaldi Arch Chest Dis. 2022;92
- [Google Scholar]
- COVID-19 Vaccine hesitancy among pregnant women: A facility-based cross-sectional study in Imphal, Manipur. Indian J Public Health. 2022;66:98-103.
- [Google Scholar]
- Willingness to pay for a COVID-19 vaccine for oneself and one’s child among individuals attending a tertiary care centre in West Bengal, India. Niger Postgrad Med J. 2022;29:296-302.
- [Google Scholar]
- Role of leadership and incentive-based programs in addressing vaccine hesitancy in India. Vaccine X. 2023;15:100346.
- [Google Scholar]
- Vaccine hesitancy among the nursing officers working in a tertiary care hospital, Puducherry - A mixed-method study. Clin Epidemiol Glob Health. 2023;22:101300.
- [Google Scholar]
- COVID-19 vaccination intention and hesitancy: Mistrust on COVID-19 vaccine benefit a major driver for vaccine hesitancy among healthcare workers; a cross-sectional study in North India. J Prev Med Hyg. 2022;63:E219-30.
- [Google Scholar]
- Attitude and acceptance of Covid-19 vaccine amongst medical and dental fraternity - a questionnaire survey. Rocz Panstw Zakl Hig. 2021;72:185-91.
- [Google Scholar]
- A cross-sectional study on COVID-19 vaccine hesitancy in peri-urban areas in Kanpur, Uttar Pradesh, India. Monaldi Arch Chest Dis. 2023;94
- [Google Scholar]
- Community voices around COVID-19 vaccine in Chennai, India: A qualitative exploration during early phase of vaccine rollout. Indian J Med Res. 2022;155:451-60.
- [Google Scholar]
- India marks one year of COVID vaccination. Available from: https://www.who.int/india/news/feature-stories/detail/india-marks-one-year-of-covid-vaccination, accessed on August 25, 2024.
- Indian guidelines for vaccination in older adults. Available from: https://www.geriatricindia.com/indian_vaccination_guidelines.html, accessed on August 25, 2024.
- RSSDI Clinical practice recommendations for the management of type 2 diabetes mellitus 2022. Int J Diabetes Dev Ctries. 2022;42:1-143.
- [Google Scholar]
- Guidelines for vaccination in patients with chronic kidney disease. Indian J Nephrol. 2016;26:S15-8.
- [Google Scholar]
- Vaccination in women. Available from: https://www.fogsi.org/wp-content/uploads/2015/11/vaccination_women.pdf, accessed on August 25, 2024.
- Indian consensus guideline on adult immunization. Available from: https://apiindia.org/reader/Indian%20Consensus%20Guideline%20on%20Adult%20Immunization, accessed on Aug 27, 2024.
- Measles & rubella vaccination campaign handbook for frontline healthcare workers. Frequently asked questions on MR vaccination-campaign. Available from: https://www.slideshare.net/slideshow/faqs-on-measles-rubella-vaccination-campaign-including-routine-immunization/77294378, accessed on August 25, 2024.
- WHO prequalifies new dengue vaccine. Available from: https://www.who.int/news/item/15-05-2024-who-prequalifies-new-dengue-vaccine, accessed on August 25, 2024.
- ICMR and Panacea Biotec initiate the first dengue vaccine phase 3 clinical trial in India with indigenous dengue vaccine, Dengi All. Available from: https://pib.gov.in/PressReleasePage.aspx?PRID=2045090, accessed on August 25, 2024.
- HPV vaccination of girl child in India: Intervention for primary prevention of cervical cancer. Asian Pac J Cancer Prev. 2018;19:2357-8.
- [Google Scholar]
- Human papillomavirus infection and cervical cancer prevention in India, Bangladesh, Sri Lanka and Nepal. Vaccine. 2008;26:M43-52.
- [Google Scholar]
- Recommendations for vaccination against seasonal influenza in adult high risk groups: South Asian recommendations. J Assoc Physicians India. 2016;64:3-11.
- [Google Scholar]
- Seasonal influenza A (H1N1): State/UT- wise number of cases & deaths from 2018 to 2024* (As on 31.05.2024). Available from: https://ncdc.mohfw.gov.in/wp-content/uploads/2024/07/Seasonal-Influenza-A-upto-31.05.2024.pdf, accessed on August 25, 2024.
- Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: A systematic review and meta-analysis. J Infect Dis. 2020;222:S577-83.
- [Google Scholar]
- Respiratory syncytial virus prevention within reach: The vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23:e2-e21.
- [Google Scholar]
- Estimated global incidence of Japanese encephalitis: A systematic review. Bull World Health Organ. 2011;89:766-74. 774A-774E
- [Google Scholar]
- Seroprevalence of hepatitis B virus infection and associated factors among health care workers in Southern Ghana. IJID Reg. 2023;6:84-9.
- [Google Scholar]
- Cost of illness due to severe enteric fever in India. J Infect Dis. 2021;224:S540-7.
- [Google Scholar]
- India achieves the goal of maternal and neonatal tetanus (MNT) elimination. Available from: https://www.who.int/india/footer/quick-links/media/india-achieves-the-goal-of-maternal-and-neonatal-tetanus-elimination-(mnte), accessed on August 25, 2024.
- Revaccination with Bacille Calmette-Guérin: Some issues to consider. Indian J Med Res. 2023;157:160-2.
- [Google Scholar]
- Aqueous extract of rhubarb promotes hepatotoxicity via facilitating PKM2-mediated aerobic glycolysis in a rat model of diethylnitrosamine-induced liver cancer. Drug Des Devel Ther. 2024;18:4497-510.
- [Google Scholar]
- New tuberculosis vaccines in India: Modelling the potential health and economic impacts of adolescent/adult vaccination with M72/AS01E and BCG-revaccination. BMC Med. 2023;21:288.
- [Google Scholar]
- Epitope promiscuity and population coverage of Mycobacterium tuberculosis protein antigens in current subunit vaccines under development. Infect Genet Evol. 2020;80:104186.
- [Google Scholar]
- Potential impact of spatially targeted adult tuberculosis vaccine in Gujarat, India. J R Soc Interface. 2016;13:20151016.
- [Google Scholar]
- Modelling the global burden of drug-resistant tuberculosis avertable by a post-exposure vaccine. Nat Commun. 2021;12:424.
- [Google Scholar]
- The potential impact of novel tuberculosis vaccine introduction on economic growth in low- and middle-income countries: A modeling study. PLoS Med. 2023;20:e1004252.
- [Google Scholar]
- Influence of sex, age & nontuberculous infection at intake on the efficacy of BCG: Re-analysis of 15-year data from a double-blind randomized control trial in South India. Indian J Med Res. 2006;123:119-24.
- [Google Scholar]
- Accelerating research and development of new vaccines against tuberculosis: A global roadmap. Lancet Infect Dis. 2022;22:e108-20.
- [Google Scholar]
- Affordability of adult tuberculosis vaccination in India and China: A dynamic transmission model-based analysis. Vaccines (Basel). 2021;9:245.
- [Google Scholar]
- Revisiting the Chingleput BCG vaccination trial for the impact of BCG revaccination on the incidence of tuberculosis disease. Indian J Med Res. 2023;157:152-9.
- [Google Scholar]
- COVID vaccine hesitancy among the tribal population and its determinants: A community-based study at Berhampore block of Murshidabad District, West Bengal. Indian J Public Health. 2023;67:21-7.
- [Google Scholar]
- COVID-19 vaccination intention and vaccine characteristics influencing vaccination acceptance: A global survey of 17 countries. Infect Dis Poverty. 2021;10:122.
- [Google Scholar]
- COVID-19 vaccine acceptance in South Asia: A multi-country study. Int J Infect Dis. 2022;114:1-10.
- [Google Scholar]
- Hesitant or not? The association of age, gender, and education with potential acceptance of a COVID-19 vaccine: a country-level analysis. J Health Commun. 2020;25:799-807.
- [Google Scholar]
- Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat Commun. 2022;13:3801.
- [Google Scholar]
- A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat Med. 2023;29:366-75.
- [Google Scholar]
- Perceived COVID-19 vaccine effectiveness, acceptance, and drivers of vaccination decision-making among the general adult population: A global survey of 20 countries. PLoS Negl Trop Dis. 2022;16:e0010103.
- [Google Scholar]
- Technologies for strengthening immunization coverage in India: A systematic review. Lancet Reg Health Southeast Asia. 2023;23:100251.
- [Google Scholar]






