Translate this page into:
Acute effects on cardiovascular oscillations during controlled slow yogic breathing
Reprint requests: Dr. Kishore Kumar Deepak, Room No. 2008, Department of Physiology, Teaching Block, All India Institute of Medical Sciences, New Delhi 110 029, India e-mail: kkdeepak@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Breathing exercises are believed to modulate the cardiovascular oscillations in the body. To assess the validity of the assumption and understand the underlying mechanism, the key autonomic regulatory parameters such as heart rate variability (HRV), blood pressure variability (BPV) and baroreflex sensitivity (BRS) were recorded during controlled slow yogic breathing. Alternate nostril breathing (ANB) was selected as the yogic manoeuvre.
Methods:
Twelve healthy volunteers (age 30±3.8 yr) participated in the study. ANB was performed at a breathing frequency of 5 breaths per minute (bpm). In each participant, the electrocardiogram, respiratory movements, beat-to-beat BP and end-tidal carbon dioxide were recorded for five minutes each: before, during and after ANB. The records were analyzed for HRV, BPV and BRS.
Results:
During ANB, HRV analysis showed significant increase in the standard deviation of all NN intervals, low-frequency (LF) component, LF/HF (low frequency/high frequency) ratio and significant decrease in the HF component. BPV analysis showed a significant increase in total power in systolic BPV (SBPV), diastolic BPV (DBPV) and mean BPV. BRS analysis showed a significant increase in the total number of sequences in SBPV and DBPV and significant augmentation of α-LF and reduction in α-HF. The power spectrum showed a dominant peak in HRV at 0.08 Hz (LF component) similar to the respiratory frequency. The acute short-term change in circulatory control system declined immediately after the cessation of slow yogic breathing (ANB) and remained elevated in post-ANB stage as compared to the pre-ANB.
Interpretation & conclusions:
Significant increase in cardiovascular oscillations and baroreflex recruitments during-ANB suggested a dynamic interaction between respiratory and cardiovascular system. Enhanced phasic relationship with some delay indicated the complexity of the system. It indicated that respiratory and cardiovascular oscillations were coupled through multiple regulatory mechanisms, such as mechanical coupling, baroreflex and central cardiovascular control.
Keywords
Alternate nostril breathing
baroreflex sensitivity
blood pressure variability
heart rate variability
Voluntary breathing exercises with varying frequency1, depth and pauses2 are known to modulate cardiovascular sympathovagal oscillations. Respiration and cardiovascular systems are coupled oscillators, and a close non-linear coupling exists between them at different breathing frequencies1. Different breathing frequencies are capable of modifying the relationship between heart rate and systolic blood pressure (SBP) oscillations3. Respiratory frequency-mediated changes in heart rate variability (HRV) have been observed during simple mental and verbal activities4. Slow breathing at 0.1 Hz frequency (equivalent to Myer's wave) can acutely enhance the cardio-vagal baroreflex sensitivity (BRS)5. In patients with essential hypertension and chronic heart failure, the practice of slow breathing causes increase in vagal activity, BRS, oxygen saturation and ventilatory efficiency along with the reduction in chemoreflex and sympathetic activation67. These studies show that slow breathing can modulate cardiovascular parameters and enhance the BRS to maintain homeostasis.
In most studies the long- and short-term effects of slow breathing and alternate nostril breathing (ANB) have been evaluated. Regular practice of slow breathing exercise for three months improves autonomic functions8, and sympathetic tone reduction is observed with seven days’ practice of slow breathing9. A few studies conducted on the immediate effect of slow yogic breathing have reported that the right nostril breathing has sympathomimetic effect whereas the left nostril breathing has parasympathomimetic effect1011.
A significant increase in heart rate, low frequency (LF) power and LF/HF (low frequency/high frequency) ratio along with the reduction in HF power during ANB is reported in the study on participants with at least three months of extensive yoga training12. The same group has observed an increase in vagal activity during and after ANB in experienced yoga practitioners with no significant changes in frequency domain parameters in the study where breathing rate was not controlled during-ANB13. A very slow ANB (at 0.016 Hz i.e., 1 breaths per minute) can cause significant increase in respiratory sinus arrhythmia (RSA) and LF/HF ratio and can decrease the breathing frequency after ANB14.
In non-yoga practitioners, a significant increase in autonomic modulation of the heart without significant shift in the sympathovagal balance was noted immediately after ANB and paced breathing at 0.08 Hz respiratory frequency without respiratory pauses15. Only one study estimated the BRS in naive yoga practitioners during spontaneous, Ujjayi breathing, symmetric and asymmetric slow breathing16. The increase in BRS and decrease in BP were maximum in slow breathing with an equal duration of inspiration and expiration. Ujjayi breathing showed less improvement in BRS with no change in BP.
There is a paucity of literature on the acute effects of slow yogic breathing on HRV, blood pressure variability (BPV) and BRS to understand the underlying cardiovascular mechanism of pranayama practices, which can be studied. Hence, the present study was designed to observe the acute effects of slow yogic breathing on cardiovascular oscillations at 0.08 Hz respiratory frequency i.e., 5 bpm using HRV, BPV and BRS. ANB, the most common pranayama practice, was used as slow controlled yogic breathing. As pauses are an integral part of classical pranayama, we decided to include pauses in our slow yogic breathing protocol. In the present study, ANB was performed in 2:1:2:1 breathing ratio with pauses after inhalation and exhalation. Beneficial cardiovascular responses were also reported in trained yoga practitioners during Savitri pranayama with similar breathing ratio17.
Material & Methods
The study was conducted in April-September 2014 at Autonomic Function Lab, Department of Physiology, All India Institute of Medical Sciences, New Delhi, India. Twelve healthy volunteers participated in the study (average age, 30.0±3.8 yr). All the participants were normotensive with normal electrocardiogram and had no history of medical problem or prolonged medication. None of the participants were regular yoga practitioners. The volunteers with a history of long-term yoga practices (regular practice of more than six months) and chronic smokers were excluded from the study. The appeal was put on the notice board of the department, with objectives and experimental procedure details, to join the study. Students and staff willing to participate were included in the study. All participants were educated and were from a good socio-economic background. Written informed consent was obtained from them. The procedure for doing ANB was demonstrated by a yoga expert and the experimental protocol was explained to the participants. Ethical clearance was obtained from the Institute's Ethics Committee.
Participants were asked to come with light breakfast in the morning (at least two hours before the recordings) and were allowed to rest for 15 min before data acquisition. All recordings were done in sitting position and the total duration of recording was 15 min: five minutes baseline i.e., pre-ANB, five minutes during-ANB and five minutes post-ANB (Fig. 1). Keeping their eyes closed for five minutes, the volunteers did ANB in synchronization with the repetitive instructions announced continuously through an audio loop. Simultaneously, the sound was fed to the acquisition system as analogue input and recorded with other parameters to monitor the compliance of the participant.
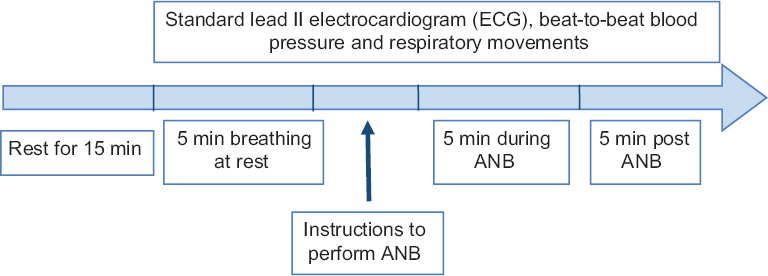
- Experimental protocol for the study of acute effect of alternate nostril breathing (ANB).
Procedure for alternate nostril breathing: Participants were instructed to breathe slowly using alternate nostril at a frequency of 0.08 Hz i.e., 5 bpm. Each breathing cycle comprised inspiration (four seconds), pause in end inspiration (two seconds), expiration (four seconds) and pause in end expiration (two seconds). The participants were instructed to sit up straight and follow the following protocol: (i) Close the right nostril and exhale; (ii) inhale deeply through the left nostril for four seconds and hold breath for two seconds; (iii) Close the left nostril and exhale through the right nostril for four seconds and hold for two seconds; (iv) Inhale deeply through the right nostril for four seconds and hold breath for two seconds; and (v) Close the right nostril and exhale through the left nostril for four seconds and hold for two seconds. Two cycles of inhalation and exhalation formed one round of ANB and it took 12 sec per ANB cycle.
Data collection and analysis: Biopac MP150 (BIOPAC Systems Inc., USA) with ECG100C amplifier was used for standard lead II ECG recording. Finometer (Finapres Medical Systems, The Netherlands) was used for non-invasive continuous beat-to-beat BP measurement. The cuff was placed on the middle phalanx of the middle finger and measurements were reconstructed to the brachial artery pressure and referenced to heart level through a built-in return-to-flow calibration and height correction system. Before recording, height correction and automatic calibration were done and physical (auto-calibration) was switched off for continuous recording. Respiratory movements were recorded using RSP100C amplifier (BIOPAC Systems Inc., USA). All signals were acquired using software ACQ version 4.0 (BIOPAC Systems Inc.) with sampling frequency of 1 kHz. Lab chart Pro 7 (ADInstruments, Australia) was used for the detection and measurement of beat-to-beat intervals from ECG and BP signal. End-tidal carbon dioxide was measured using Capnograph (L&T, India).
HRV and BPV were calculated offline from ECG and BP signal in time, frequency and time-frequency domain using HRVAS, a MATLAB-based software (Copyright© 2010, John T. Ramshur). In time domain, NN50 count divided by the total number of all NN intervals (pNN50), standard deviation of all NN intervals (SDNN), square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMSSD), and in frequency domain, the power was calculated in absolute and normalized units for LF, HF and their ratio. BRS was calculated by sequence and spectral method from Nevrokard™ BRS analysis/version 3.2.0 (Nevrokard Kiauta, Izola, Slovenia). In the sequence method, the BRS was estimated by identifying spontaneously occurring sequences of three or more consecutive heartbeats in which both the SBP and the subsequent RR intervals (RRI) changed in the same direction. These are called baroreflex sequences. The minimum criteria for change were 1 mmHg for SBP and five milliseconds for the RRIs. In spectral method, BP-to-RRI transfer function was computed. The amplitude of transfer function is analogous to the slope of RRI-BP relationship.
Statistical analysis: Data were screened for the assumption of normality using Shapiro–Wilks test. For parametric data, one-way repeated measures analysis of variance was applied, and for non-parametric data, Friedman test was applied to compare pre-ANB, during-ANB and post-ANB for the test of significance in GraphPad Prism 5.0 (GraphPad Software Inc. CA 92037 USA). Dunn's post hoc test was done for multiple comparisons.
Results
The mean heart rate, ΔRR (maximum-minimum RRI) and RSA increased significantly during-ANB and remained increased post-ANB. There was no significant change either during or after ANB in the end-tidal carbon dioxide and SBP, diastolic blood pressure (DBP) and mean arterial BP values (Table I).
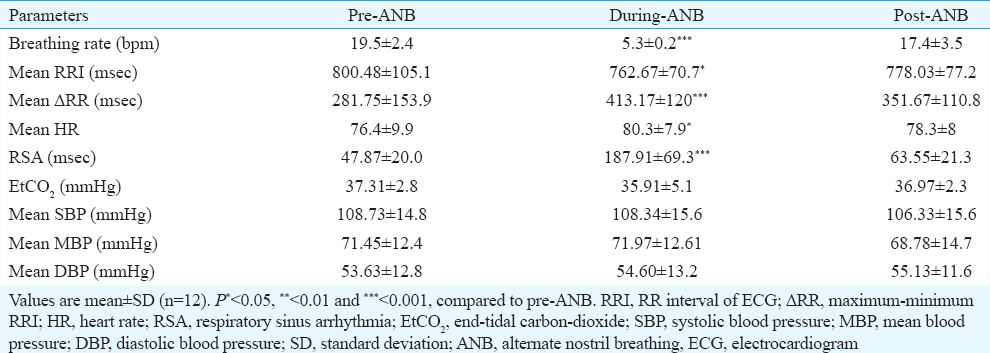
Heart rate variability: The time domain analysis showed that SDNN increased significantly (P< 0.01) from 47.1±18.5 (pre-ANB) to 82.2±23.3 (during-ANB) and remained elevated at 57.71±15.4 in post-ANB. pNN50 and RMSSD increased non-significant by during-ANB and decreased significantly (P< 0.05) post-ANB as compared to during-ANB (Table II).
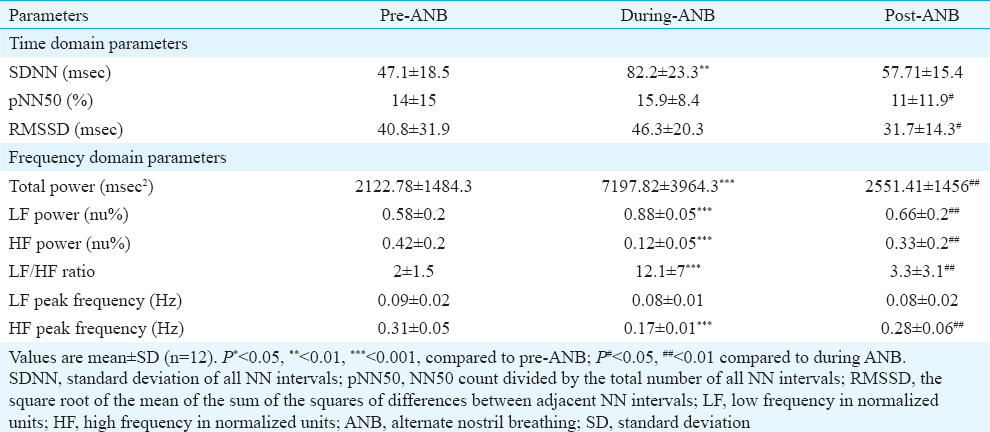
The frequency domain analysis showed that the total power of HRV (msec2) increased significantly during-ANB from 2122.78±1484.3 to 7197.82±3964.3 as compared to pre-ANB values and decreased to 2551.41±1456 in post-ANB. LF power (nu%) also significantly increased from 0.58±0.2 to 0.88±0.05 and decreased to 0.66±0.2 in post-ANB. HF power (nu%) reduced significantly from 0.42±0.2 to 0.12±0.05 during-ANB and decreased to 0.33±0.2 in post-ANB. LF/HF ratio increased significantly from 2±1.5 to 12.1±7 during-ANB and decreased to 3.3±3.1 in post-ANB (Table II).
The analysis of dominant peak in power spectrum showed that during-ANB the power of LF was found to peak at 0.08 Hz, similar to the respiratory frequency (Fig. 2).
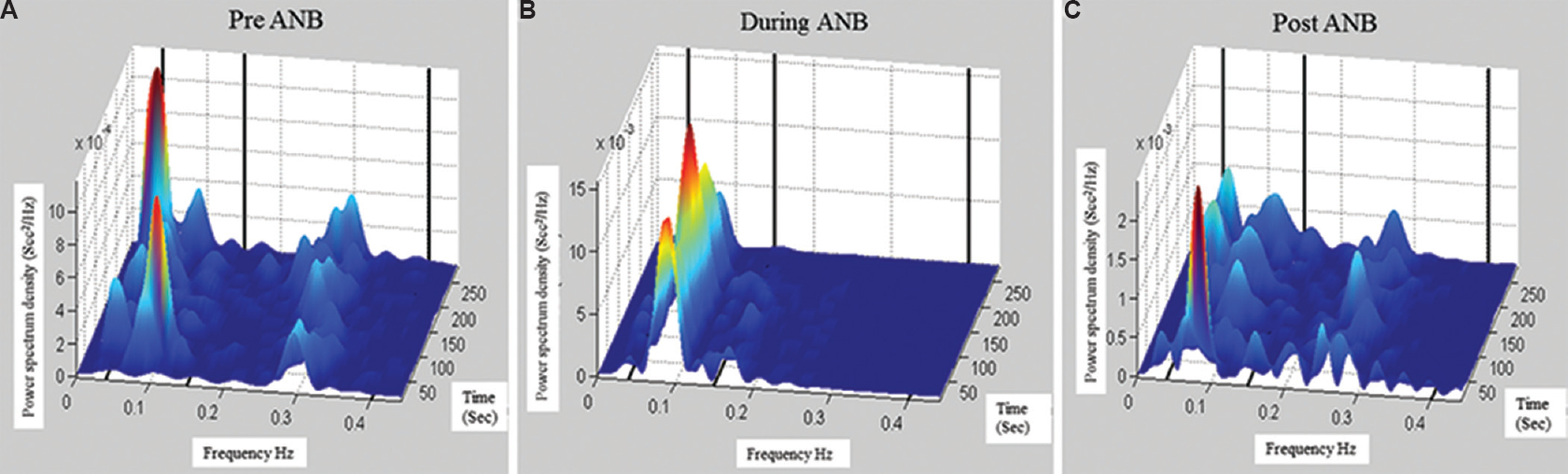
- Frequency distribution in power spectrum of RR intervals in time-frequency domains. (A) Pre-alternate nostril breathing (B) during-alternate nostril breathing and (C) post-alternate nostril breathing. In during-ANB, maximum power is at 0.08 Hz, similar to respiratory frequency. It explains that the increase of low frequency power during-alternate nostril breathing is the supplementary because of respiration.
Blood pressure variability: Time domain parameters of BPV showed that SDNN and RMSSD values were significantly higher during-ANB than pre-ANB in systolic BPV (SBPV), diastolic BPV (DBPV) and mean BPV (MBPV). In frequency domain, the total power of BPV increased significantly from 4103±2717 to 7849±3713, 1372±94 to 2781±1371 and 1653±1087 to 3824±1838 in SBPV, DBPV and MBPV, respectively (Table III). In SBPV spectrum, the LF power was higher during-ANB as compared to the pre-ANB power. It significantly decreased post-ANB as compared to the during-ANB. No change in LF power of DBPV and MBPV was observed.
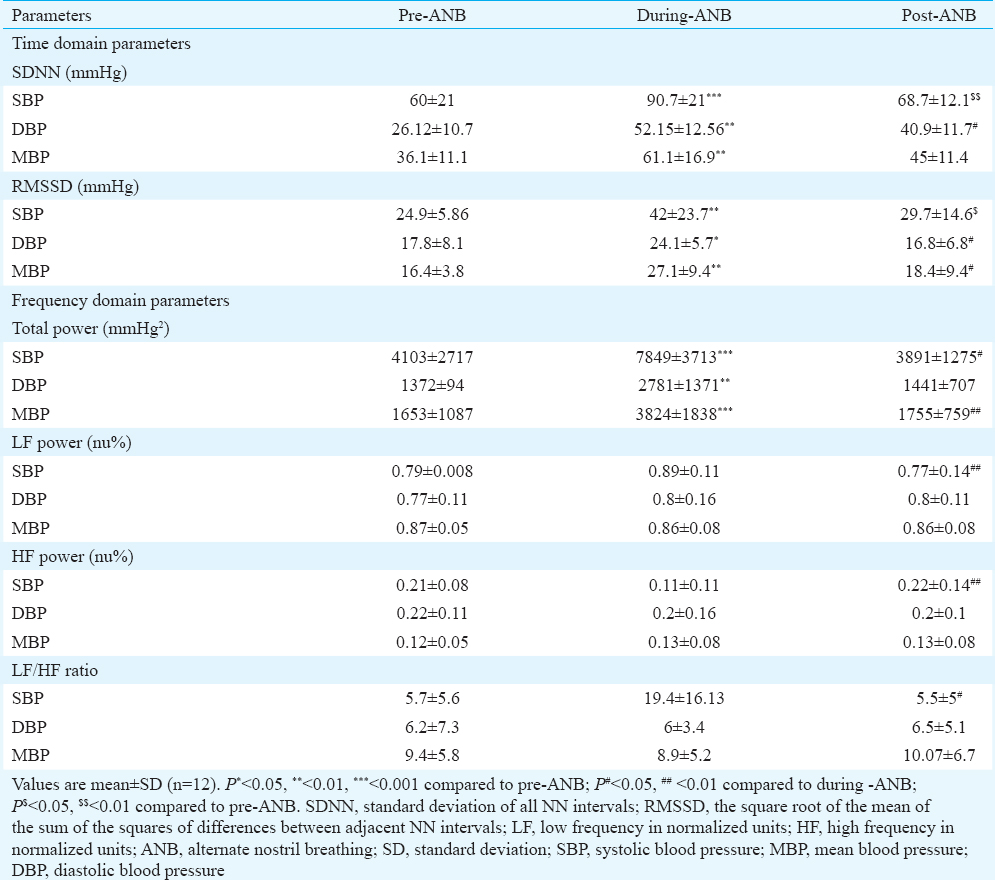
In SBPV spectrum, the HF power was decreased during-ANB as compared to the pre-ANB power. It significantly increased post-ANB as compared to the during-ANB. No change in HF power of DBPV and MBPV was observed. LF/HF ratio in SBPV was increased during-ANB and decreased significantly in post-ANB. The LF/HF ratio in DBPV and MBPV did not change (Table III).
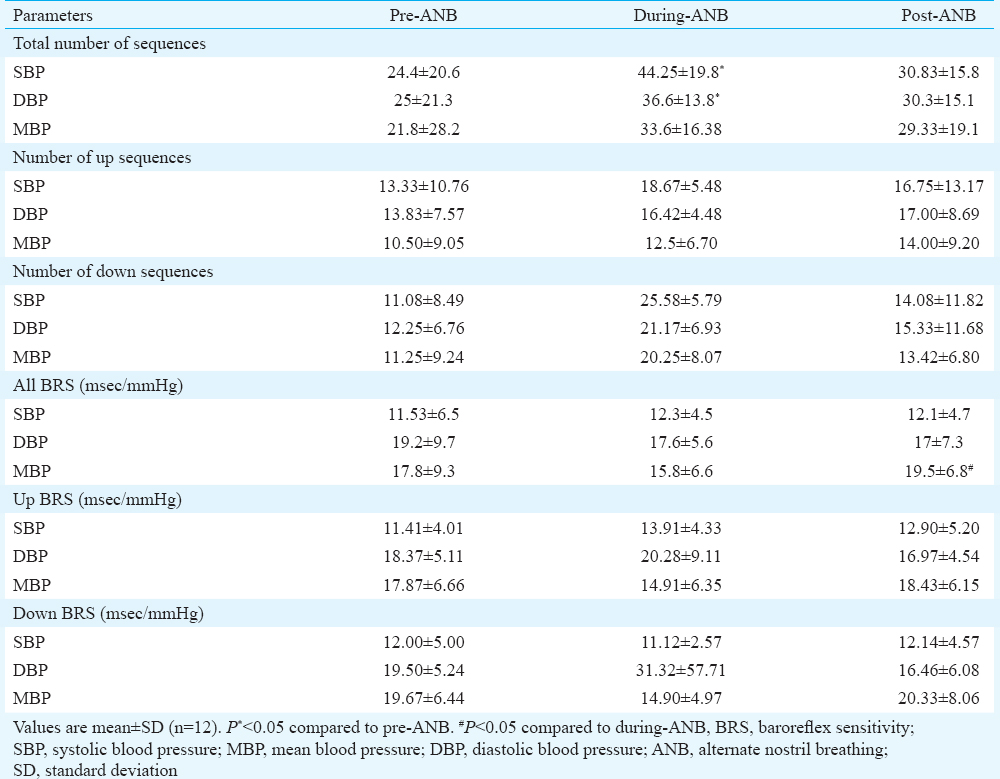
Baroreflex sensitivity: Significant increase in the total number of baroreflex sequences in both SBP and DBP was found during-ANB (Table IV and Fig. 3) in sequence analysis. Increase in the All-BRS of mean blood pressure (MBP) was observed post-ANB. No significant change was observed in up and down BRS in SBP, DBP and MBP.
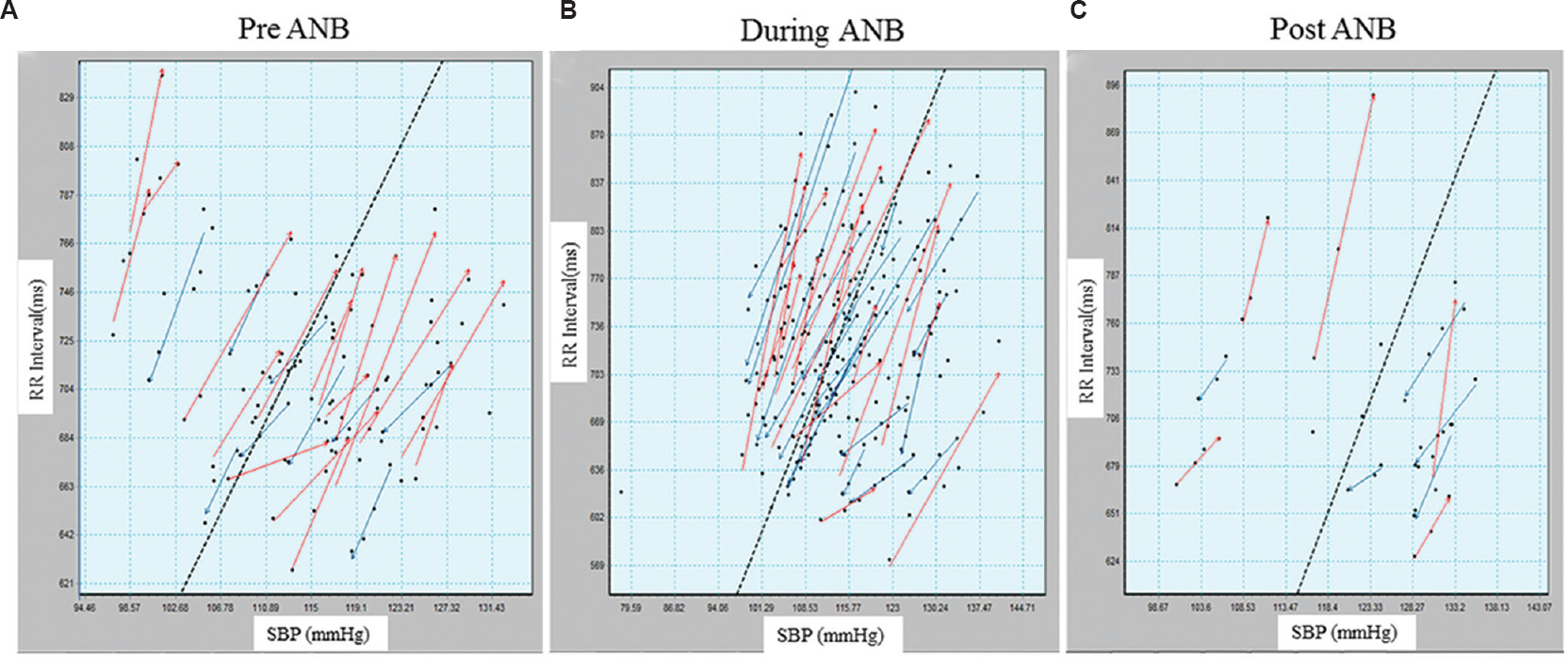
- Baroreflex sensitivity by sequence method, up sequences in red arrows and down sequences in blue arrows, (A) pre-alternate nostril breathing, (B) during-alternate nostril breathing and (C) post-alternate nostril breathing. In during-alternate nostril breathing, number of up and down sequences significantly increased.
The increase in the total number of sequences indicated that slow yogic breathing augmented central reflex globally using baroreflex loop. To further examine this, relationship between RRI (seconds) and SBP (mmHg) was plotted against time (Fig. 4). The RRI and SBP (mmHg) plots during-ANB created a sine wave-like curve of peaks and valleys, and the pattern became simple and sinusoidal similar to the respiratory signal. The RRI showed phasic synchronicity with SBP. Based on the sinusoidal changes in RRI, the vertical bars (continuous) were plotted between RRI and SBP in pre-ANB, during ANB and post-ANB, to depict the time correspondence. The simultaneous rise and fall of BP and RRI suggested 0° phase relationship between RRI and SBP, which was more evident in pre- and post-ANB (represented by solid vertical bars). This phenomenon was observed during the first 4-5 beats of ’during-ANB’. Thereafter, the rise in SBP preceded changes in RRI, suggesting a phasic delay between RRI and SBP during-ANB (represented by dotted vertical bars). This lasted a little beyond the cessation of ANB. A small number of dotted lines also appeared post-ANB.
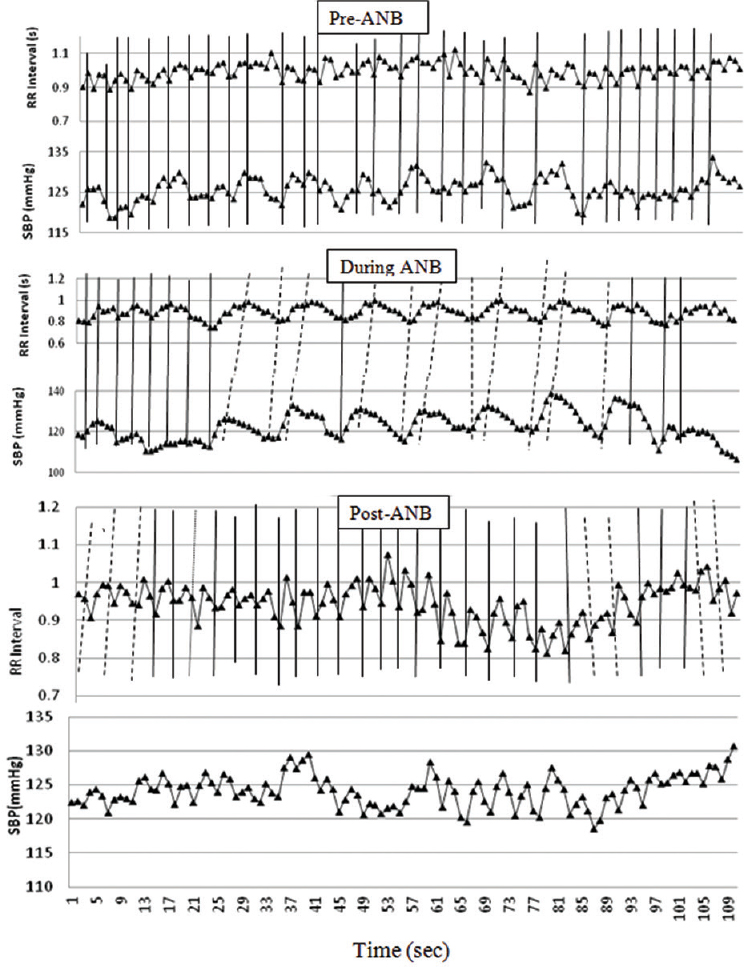
- RR interval and systolic blood pressure plotted in time series for pre-, during- and post-alternate nostril breathing (ANB). Based on the sign changes in RR interval, vertical bars (continuous) have been plotted to depict time correspondence of RR interval and systolic blood pressure. Solid vertical bars indicate that both events are happening simultaneously without any phase lag. Dotted vertical bars indicate the phase lag between RR interval and systolic blood pressure. Simultaneous rise and fall of blood pressure and RR interval and 0° phase relationship were evident in pre- and post-ANB and in the initial part of during-ANB. Systolic blood pressure preceded changes in RR interval during-ANB, indicating alteration in phasic relationship, which lasted little over in post-ANB.
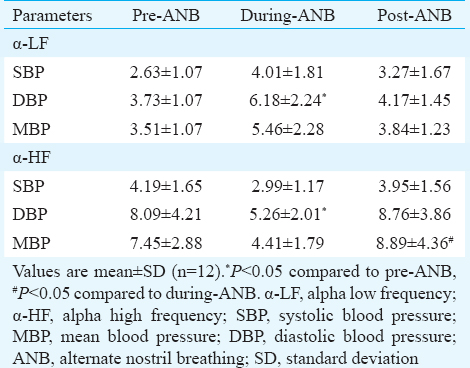
Spectral analysis of RRI versus DBP showed significant attenuation of α-LF (pre-ANB 3.73 vs. during-ANB 6.18) and augmentation of α-HF (pre-ANB 8.09 vs. during-ANB 5.26) during-ANB as compared to pre-ANB values (Table V).
Discussion
The study revealed that the voluntary respiratory modulation at breathing frequency of 5 bpm significantly increased the inherent cardiac and vascular oscillation. This was evident by the increased overall variability observed in HRV and BPV analysis. The magnitude of RSA increased significantly during the manoeuvre. It also augmented global central reflex using baroreflex loop as indicated in BRS analysis. The acute short-term excitation of circulatory control system declined immediately after the cessation of slow yogic breathing (ANB) and remained elevated in post-ANB stage as compared to pre-ANB.
It has already been reported that low breathing frequency increases the amplitude of RSA18. RSA is modulated by both vagal and sympathetic modulations1920. Heart rate fluctuations due to respiration are mediated by vagal modulation and during slow breathing, it is reflected in LF component of HRV. Our data also supported sympathetic (increase in LF power) and parasympathetic enhancement (increase in RSA) during-ANB.
The values of frequency domain parameters of HRV gave an impression of increased sympathetic and decreased parasympathetic influence on autonomic tone during-ANB. Major contribution to this increase came from LF power. Similar results were reported during Suryanadi pranayama at 6 bpm without breath holding10. Changes in breathing frequency can override the haemodynamic fluctuations in both time and frequency domain parameters. Power of respiration and cardiovascular variability increase sharply and non-linearly at a respiratory frequency of 0.07-0.09 Hz1. In agreement with this study, the analysis of dominant peak in power spectrum in our study showed that during-ANB the power of LF was found to peak at 0.08 Hz, exactly similar to the respiratory frequency. Slow breathing <0.15 Hz might increase LF power in HRV and mimic false sympathetic activation4. Hence, the increased LF power during-ANB may be due to the mechanical effect of breathing rate which gives additional or false rise of sympathetic tone. McCraty and Shaffer21 have advocated that above 0.1 Hz rhythm, sympathetic nervous system does not appear to be involved, whereas heart rhythms <0.05 Hz can be affected by parasympathetic system21. During slow breathing below 8.5 bpm, vagal activity can generate oscillations in LF band. In agreement to this, significant increase in RSA amplitude and increased LF power during-ANB is a respiration-related vagal efferent-mediated influence through central mechanism i.e., by non-baroreflex mechanism22. The LF/HF ratio increased significantly during-ANB, but pre- and post-ANB changes were insignificant as found in earlier study13. In the present study, LF, HF power and their ratios were significantly different during-ANB versus post-ANB, indicating the lasting effect of slow yogic breathing manoeuvre on cardiovascular autonomic system.
The possible underlying mechanism could be, firstly, decreased vagal tone with increased heart rate, and sympathovagal balance during-ANB may be due to conscious effort to meet the challenges in pacing with audio instructions for breathing in fixed inhalation and exhalation ratio. Similar to the present study, a large increase in LF/HF ratio and decrease in HF component have been observed during controlled breathing when compared with spontaneous breathing at the same breathing frequency23. Second, ANB is a combination of right and left nostril breathing. It has been reported that short-term practice of left nostril breathing improves vagal tone and right nostril breathing increases sympathetic tone8924. Third, breath holding leads to both cardiac sympathetic and parasympathetic activation simultaneously25, and in the present study, breathing ratio during ANB was 4:2:4:2 (end inspiration breath holding and end expiration breath holding of two milliseconds). BPV was increased during ANB. The variability in the vascular tone remained increased after the cessation of slow yogic breathing. This high BPV could probably be due to central or mechanical coupling. There could be two possible interactions during slow breathing (ANB). First is the mechanical effect of respiration leading to change in BP which in turn leads to change in heart rate, and second, changes in heart rate leading to changes in BP.
BRS calculation by sequence method showed that the total number of sequences in SBPV and DBPV was significantly higher during ANB. It indicates that baroreflex-mediated cardiovascular fluctuations are due to respiratory frequency26. The detailed analysis of baroreflex recruitment for up and down sequences did not show significant change though an increased trend in both sequences was observed during and after the cessation of slow yogic breathing. Thus, slow yogic breathing such as ANB may induce altered phasic relationship between heart rate and BP as shown in our study. These findings can be confirmed with high-order computational mathematical analysis such as cross-spectrum analysis and transfer function analysis.
The augmentation of BRS was also calculated by the spectral method at respiratory frequency. The DBPV showed a significant increase in α-LF and a significant decrease in α-HF. An earlier study showed that LF RRI fluctuations were baroreflex dependent27. Along with the increase in α-LF index, both up and down sequences were increased significantly during-ANB. It indicates that these fluctuations are mediated by the baroreflex loop and that ANB augments baroreflex globally.
Centres controlling respiratory and cardiovascular systems are situated very close to each other in the medulla and therefore, they influence each other. Hence, it is important to know how oscillations in one system affect the other system. In healthy individuals, the oscillations in RRI and BP are synchronous with respiratory frequency. Vaschillo et al28 proposed two closed loop models of the baroreflex system for controlling BP. Respiratory oscillations produce heart rate oscillations and thereby, BP oscillations. Baroreceptors sense and regulate the BP and modulate brain centres that control the heart rate and vascular tone. In agreement with the above model, the present study showed an increased LF power of HRV during-ANB, which might be indicative of the mechanical effect of respiration. Simultaneously, the increased time domain parameters and decreased HF power generate transient, rapid excitation of cardiovascular autonomic centres due to respiratory modulation. These may be vagally mediated and predominantly caused by central non-baroreflex mechanisms. Increased BPV, baroreflex recruitment, α-LF index and altered phasic relationship with some delay in heart rate and BP may indicate the involvement of baroreflex mechanism. These results suggest that slow yogic breathing, like ANB at 5 bpm with fixed inspiratory and expiratory pauses, induces multiple regulatory mechanisms in cardiovascular system.
In conclusion, our study shows that the teaching/training of individuals to breathe slowly may have beneficial effects on cardiovascular autonomic regulation in health and in various cardiovascular diseases. Transient and rapid excitation of cardiovascular system during and after ANB suggests that slow yogic breathing may serve as a physiologic method to draw upon cardio-vagal reserve. One such application could be the strengthening of BRS during postural challenges. Slow yogic breathing using ANB suggests that cardiovascular fluctuations are mediated through multiple regulatory mechanisms including the baroreceptor mechanism but mainly through mechanical coupling with breathing i.e., by non-baroreflex and central mechanisms. Cross-spectrum analysis and non-linear method of HRV estimation will facilitate the understanding of the underlying mechanism of slow yogic breathing as the future scope of study.
Our study had some limitations. The breath rate was kept controlled at 0.08 Hz during intervention. A new experimental design with fixed reduction in the spontaneous breathing rate (such as 50%) may revoke the effects of slow controlled breathing. Post-ANB data were collected only for five minutes, which might be a persistence of the sympathetic effect of respiration. Post-ANB monitoring should be done for at least 20 min in four periods of five minutes each. This will help us to understand the precise immediate effect of ANB on the cardiovascular system, which subsequently may create a platform for understanding the underlying mechanism involved in the long duration of ANB practice.
Acknowledgment
The authors acknowledge the Ministry of AYUSH for financial assistance and to the students and staff for participating in the study. The authors thank Dr Sabyasachi Sircar for helping in editing of the final manuscript.
Conflicts of Interest: None.
References
- Influence of respiration on heart rate and blood pressure fluctuations. J Appl Physiol (1985). 1993;74:617-26.
- [Google Scholar]
- Impact of acute respiratory stress on cardiac autonomic control in young healthy subjects explored by time and frequency domain methods. Chin J Physiol. 2009;52:299-305.
- [Google Scholar]
- Effect of respiratory rate on the relationships between RR interval and systolic blood pressure fluctuations: A frequency-dependent phenomenon. Cardiovasc Res. 1998;38:332-9.
- [Google Scholar]
- Effects of controlled breathing, mental activity and mental stress with or without verbalization on heart rate variability. J Am Coll Cardiol. 2000;35:1462-9.
- [Google Scholar]
- Variability of phase shift between blood pressure and heart rate fluctuations: A marker of short-term circulation control. Circulation. 2003;108:292-7.
- [Google Scholar]
- Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation. 2002;105:143-5.
- [Google Scholar]
- Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005;46:714-8.
- [Google Scholar]
- Effect of short-term practice of breathing exercises on autonomic functions in normal human volunteers. Indian J Med Res. 2004;120:115-21.
- [Google Scholar]
- Effects of slow breathing exercise on cardiovascular functions, pulmonary functions and galvanic skin resistance in healthy human volunteers - A pilot study. Indian J Med Res. 2013;137:916-21.
- [Google Scholar]
- Immediate effect of different pranayam on short term heart rate variability in health care students – A preliminary study. Int J Physiol. 2014;2:39-43.
- [Google Scholar]
- Differential effects of uninostril and alternate nostril pranayamas on cardiovascular parameters and reaction time. Int J Yoga. 2014;7:60-5.
- [Google Scholar]
- Immediate effect of specific nostril manipulating yoga breathing practices on autonomic and respiratory variables. Appl Psychophysiol Biofeedback. 2008;33:65-75.
- [Google Scholar]
- Blood pressure and heart rate variability during yoga-based alternate nostril breathing practice and breath awareness. Med Sci Monit Basic Res. 2014;20:184-93.
- [Google Scholar]
- On spectral analysis of heart rate variability during very slow yogic breathing. Conf Proc IEEE Eng Med Biol Soc. 2005;3:2467-70.
- [Google Scholar]
- Influence of alternate nostril breathing on heart rate variability in non-practitioners of yogic breathing. Int J Yoga. 2012;5:66-9.
- [Google Scholar]
- Cardiovascular and respiratory effect of yogic slow breathing in the yoga beginner: What is the best approach? Evid Based Complement Alternat Med. 2013;2013:743504.
- [Google Scholar]
- Immediate cardiovascular effects of Savitri pranayama in sitting and supine positions in female volunteers. Yoga Mimamsa. 2012;44:101-12.
- [Google Scholar]
- Effect of breathing pattern on blood pressure and heart rate oscillations in humans. Clin Exp Pharmacol Physiol. 1993;20:619-26.
- [Google Scholar]
- Nervous mechanisms of spontaneous oscillations of systolic blood pressure and heart rate. Arch Mal Coeur Vaiss. 1990;83:1065-8.
- [Google Scholar]
- Heart rate variability biofeedback: How and why does it work? Front Psychol. 2014;5:756.
- [Google Scholar]
- Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob Adv Health Med. 2015;4:46-61.
- [Google Scholar]
- Comments on point: Counterpoint: Respiratory sinus arrhythmia is due to a central mechanism vs.respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol (1985). 2009;106:1745-9.
- [Google Scholar]
- Consciously controlled breathing decreases the high-frequency component of heart rate variability by inhibiting cardiac parasympathetic nerve activity. Tohoku J Exp Med. 2014;233:155-63.
- [Google Scholar]
- Slow yogic breathing through right and left nostril influences sympathovagal balance, heart rate variability, and cardiovascular risks in young adults. N Am J Med Sci. 2014;6:145-51.
- [Google Scholar]
- Effect of repetitive end-inspiration breath holding on very short-term heart rate variability in healthy humans. Physiol Meas. 2014;35:2429-45.
- [Google Scholar]
- Respiratory modulation of cardiovagal baroreflex sensitivity. J Appl Physiol (1985). 2009;107:718-24.
- [Google Scholar]
- Effect of sinoaortic denervation on frequency-domain estimates of baroreflex sensitivity in conscious cats. Am J Physiol. 1999;276(6 Pt 2):H1987-93.
- [Google Scholar]
- Heart rate variability biofeedback as a method for assessing baroreflex function: A preliminary study of resonance in the cardiovascular system. Appl Psychophysiol Biofeedback. 2002;27:1-27.
- [Google Scholar]






