Translate this page into:
Abundance & distribution of trombiculid mites & Orientia tsutsugamushi, the vectors & pathogen of scrub typhus in rodents & shrews collected from Puducherry & Tamil Nadu, India
Reprint requests: Dr Sadanandane Candasamy, ICMR - Vector Control Research Centre, Indira Nagar, Puducherry 605 006, India e-mail: cs_anandane@yahoo.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Human cases of scrub typhus are reported every year from Puducherry and adjoining areas in southern India. However, information on the presence of causative agent, Orientia tsutsugamushi, and its vectors is lacking. Hence, the objective of the study was to find out the vector as well as pathogen distribution in rodents and shrews present in the scrub typhus-reported areas in southern India.
Methods:
Trombiculid mites were collected by combing rats and shrews collected using Sherman traps and identified to species level following standard taxonomical keys. The serum samples of the animals were used for Weil–Felix test and the clots containing blood cells were used for DNA extraction and polymerase chain reaction (PCR).
Results:
A total of 181 animals comprising four rodent species and one shrew species were collected from 12 villages. High proportion of chiggers was collected from the shrew, Suncus murinus (79.1%) and Rattus rattus (47.6%). A total of 10,491 trombiculid mites belonging to nine species were collected. Leptotrombidium deliense, the known vector of scrub typhus pathogen, was the predominant species (71.0%) and the chigger (L. deliense) index was 41.1 per animal. Of the 50 animals screened for the pathogen, 28 showed agglutination against OX-K in Weil–Felix test indicating the presence of antibodies against O. tsutsugamushi, the causative agent of scrub typhus. PCR carried out with the DNA extracted from blood samples of two of the animals were positive for GroEl gene of O. tsutsugamushi.
Interpretation & conclusions:
L. deliense index was well above the critical limit of chigger load, indicating that all the villages were receptive for high risk of transmission of scrub typhus to human. Pathogen positivity was higher among animals collected from villages recorded for higher chigger indices due to active transmission between the chigger mites and reservoir host animals. The results are suggestive of routine vector/pathogen surveillance at hot spots to initiate timely preventive measures.
Keywords
Chigger index
Leptotrombidium deliense
Orientia tsutsugamushi
Puducherry
scrub typhus
trombiculid mites
Scrub typhus is an acute, febrile disease caused by infection with Orientia tsutsugamushi (Family: Rickettsiaceae). It is an obligate intracellular Gram-negative bacterium, transmitted among small animals and to humans by some species of larval trombiculid mites (chiggers). Mites carry the bacterium from larval stages to adults and to their progenies through transtadial and transovarial transmission. Small animals such as rodents and shrews act as natural or maintenance hosts1. Approximately one million cases of scrub typhus occur each year and more than a billion people are at risk worldwide2. Mortality rates in untreated patients range from 0 to 30 per cent depending on the geographical region. It is endemic in the Asia-Pacific region, known as the ‘tsutsugamushi triangle’34.
Scrub typhus is considered as a re-emerging infectious disease in India5. Recently, outbreaks of scrub typhus have been reported all over India including southern States67. It has emerged as an important cause of febrile illness in Puducherry and adjoining areas of Tamil Nadu, and confirmed cases have been reported every year8. However, no information is available on the occurrence of the causative agent of the disease, O. tsutsugamushi, on the host animals such as rodents and shrews and its vectors. Therefore, a preliminary study was undertaken to examine the abundance and distribution of trombiculid mite vectors through collection and identification of mites from the animals trapped from areas reported for human cases of scrub typhus.
Material & Methods
The study area was Puducherry district of the Union Territory of Puducherry and adjoining areas of Tamil Nadu, India. The district has plain land with dry and evergreen species of vegetation typical of tropical regions. Puducherry experiences tropical maritime climate with moderate variation in temperature and rainfall. The mean maximum temperature is 38.2°C and mean minimum temperature is 24°C. The average annual rainfall is about 126 cm and almost 68 per cent of it occurs from October to December.
The survey was carried out in 12 villages selected randomly from areas reported with human cases of scrub typhus. The data on human cases of scrub typhus were obtained from Puducherry Institute of Medical Sciences (PIMS), Puducherry. The diagnosis of scrub typhus was done by detection of the pathogen through immunoglobulin M enzyme-linked immunosorbent assay (ELISA), Weil–Felix test and nested polymerase chain reaction (PCR). Of the 12 villages, nine were from Puducherry district and three from adjoining Viluppuram district of Tamil Nadu. Month-wise collection of trombiculid mites was done in all the selected villages from November 2013 to October 2014.
Trapping and identification of rodents and shrews: Rodents and shrews were trapped using Sherman traps of the size of 3” × 3” × 10” (W × H × L), designed for live capture of rats. In each of the selected village, traps were set outdoors (peri-domestic areas) in pre-selected sites with scrubby vegetation and rodent burrows. Fifteen to 25 traps were placed in each of the villages selected on each day of sampling. The traps were baited with fried coconut and peanut butter. The traps were placed one hour before sun set (1700 h) and retrieved the next day morning (0600 h). The captured animals were anaesthetized and identified through morphological features9.
This study was conducted in the ICMR - Vector Control Research Centre, Puducherry. The study was approved by the institutional animal ethics committee.
Collection of ectoparasites and identification: The ectoparasites including chigger (larval) mites were collected by combing the animals against the fur over a white enamel tray. The snout, ears, limbs and axillary regions of individual animals were combed and the ectoparasites were preserved in 70 per cent ethanol until they were mounted on slides. Mites were mounted in Hoyer's medium10, examined under microscope and identified up to species level, following standard taxonomical keys11. Other ectoparasites collected were mounted in Hoyer's medium, examined under microscope and identified using the standard taxonomical keys12.
Detection of rickettsial pathogens in animals through serological assay and polymerase chain reaction (PCR): After collection of ectoparasites, blood samples were collected from the animals. A total of 50 rodents and shrews, selected randomly from four of the study areas, were used. The numbers of different species used depended on their availability from these places. From each animal, 1 ml of blood sample was taken through heart puncture without anticoagulant and serum separated. The serum samples were used for Weil–Felix test13 and the clots containing blood cells were used for DNA extraction and PCR. Weil–Felix test for antibody titre was done against three antigens, namely, OX-19 (Rickettsia typhi), OX-2 (Rickettsia conorii) and OX-K (O. tsutsugamushi)14 (Progen, Tulip diagnostics, Goa). The serum samples were diluted in physiological saline to get doubling dilutions of 1:10-1:160. An equal amount of the respective antigen was added to get final dilutions of 1:20-1:320. Tubes were incubated overnight at 37°C and the readings were taken. Matt formation in the tube due to antigen and antibody reaction was taken as positive. Button formation due to the absence of the reaction was counted as negative. Samples reacted with OX-19 antigen were considered to be positive for antibodies against murine typhus. Samples reacted with OX-2 antigen were attributed to be positive for antibodies against tick typhus and those reacted with OX-K antigen were considered to be positive for antibodies against scrub typhus. Against all the above antigens, matt formation at 1:80 dilution was taken as positive for the respective antibodies15.
Extraction of DNA from rat blood samples was done using GenElute Blood Genomic DNA kit (Sigma-Aldrich, USA). The extracted DNA was used as templates for amplification using primers. All the samples were processed for amplification of three different genes, namely, 56 kDa, GroEl and 16s rRNA of O. tsutsugamushi either through conventional or nested PCR. Detection of gene encoding 56 kDa, which amplifies 483 bp segments, was done through nested PCR, following the method described by Saisongkorh et al16. Primers used for the reaction were as follows: F’- 5′- TCAAGCTTATTGCTGAGTG CAATGTCTGC-3’; R’- 5’- AGGGATCCCTGCTGCTGTGCTTGCTGCG-3’ for the first round and F’- 5′-GATCAAGCTTCTC AGCCTACTATAATGCCC-3’; R’-5’-CTAGGGATCC CGACAGAGCACTATTAGGC-3’ for the second round. The PCR amplification mixture contained Green Master Mix (Promega, Madison, USA), each of 10 pmol of forward and reverse primers, and 1 μl of extracted DNA in a final volume of 25 μl. The cycling conditions were 95°C for 10 sec, 57°C for 30 sec and 72°C for 1 min, which was repeated 30 times, in a thermocycler (Eppendorf, Germany). The presence of diagnostic amplicons were visualized on a gel documentation system (GelDoc-It Imaging System, UVP, California, USA) after electrophoresis on 1.5 per cent agarose gel containing 0.5 μg/ml ethidium bromide.
The method involved in the detection of 16s rRNA through the amplification of a 220 bp segment was as described by Sonthayanon et al17. The primers used were as follows: F- 5’-CGAATTAATGCTGAGTTTGCTTAG-3’; R-5’-CTCTCAGACCAGCTAGAGATCACA-3’. The reaction mixture contained Green Master Mix, each of 10 pmol forward and reverse primers, and 4 μl of the DNA in a final volume of 30 μl. The thermal conditions were 35 cycles of 95°C for 1 min, 61°C for 1 min and 72°C for 30 sec in an Eppendorf thermocycler.
The primers used for GroEl gene PCR were as follows: F-5’-TTGCTGATGATGTAGACGGA-3’; R-5’-TGTTCACAACGAGAATTAACTT-3’ for amplification of 300 bp segment. Primers were designed from the conserved regions of GroEl gene sequences obtained from NCBI (https://www.ncbi.nlm.nih.gov/) using Primer 3 software (http://simgene.com/Primer3) and manufactured by Eurofins, Bangalore. The PCR reaction mixture contained Green Master Mix, 10 pmol each of forward and reverse primers, and 4 μl of the DNA in a final volume of 30 μl. Samples which showed amplification were sequenced using the respective forward primer and BigDye terminator (Applied Biosystems, USA). Sequences were obtained in an ABI automated Genetic analyzer 3130XL (Applied Biosystems, USA), edited using BioEdit 7.0.0 version (Ibis Biosciences, Carlsbad, USA), and analyzed using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistical analysis: The trap positivity rate and chigger infestation index (average number of chiggers per animal) were estimated. The difference in the chigger indices between villages/animal reservoirs was tested using one-way analysis of variance. The relationship between monthly chigger indices and incidence of human cases of scrub typhus was analyzed using Poisson regression analysis. The statistical analysis was carried out using the STATA SE 9.0 version, Stata Corp., Texas, USA.
Results
Village-wise number of traps placed, number of animals trapped and ectoparasites retrieved are given in Table I. During the study period, a total of 181 rodents and shrews were collected from 1422 traps set in the 12 villages. The trap positivity rate ranged from 3.6 to 21.8 per cent in different villages, and the overall trap positivity rate was 12.7 per cent.
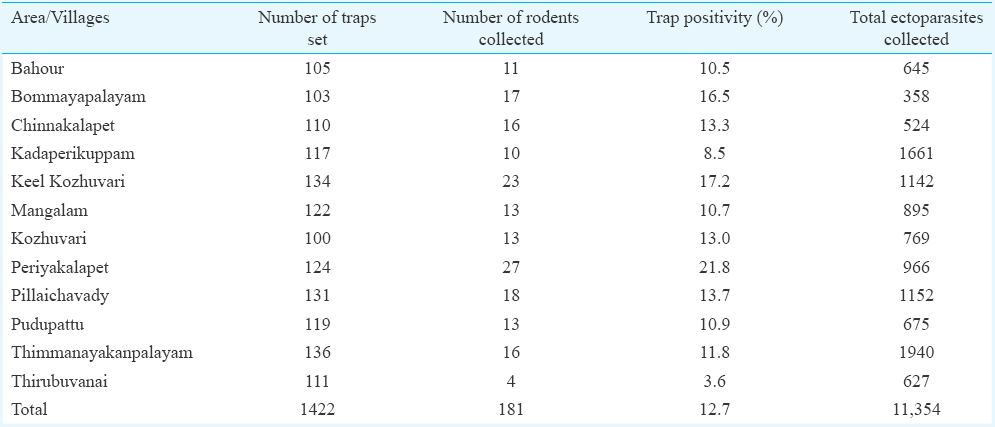
The animals trapped during the survey belonged to two orders, namely, Rodentia with four species and Soricomorpha with one species. The species of rodents were Rattus rattus (44.8%), Bandicota bengalensis (11.0%), Tatera indica (0.55%) and Mus musculus (0.55%). The species belonging to Soricomorpha collected was Suncus murinus (43.1%). Among these animals, 89 per cent were positive for ectoparasites such as mites, ticks, lice and/or fleas, and the total number collected was 11,354. The overall ectoparasite index was 62.7 per animal. More importantly, 83 per cent of the trapped animals were positive for trombiculid mites and 92.4 per cent of the total ectoparasite fauna sampled were trombiculid mites.
Species diversity and spatial distribution of trombiculid mites: Species diversity of trombiculid mites collected from different study villages is given in Table II. A total of 10,491 trombiculid mites belonging to nine species were retrieved from the trapped animals and Leptotrombidium deliense was the predominant species (71.0%) followed by L. insigne (18.7%) and Schoengastiella sp. (5.5%). The other trombiculid mite species collected were Trombicula hypodermata, Microtrombicula sp., Helenicula sp., Schoengastia sp., Walchia sp. and Schoutedenichia sp. (Table II). Village-wise analysis showed predominance of L. deliense in 10 out of 12 villages. The percentage of L. deliense ranged from 36.7 to 97.7 per cent in different villages surveyed.
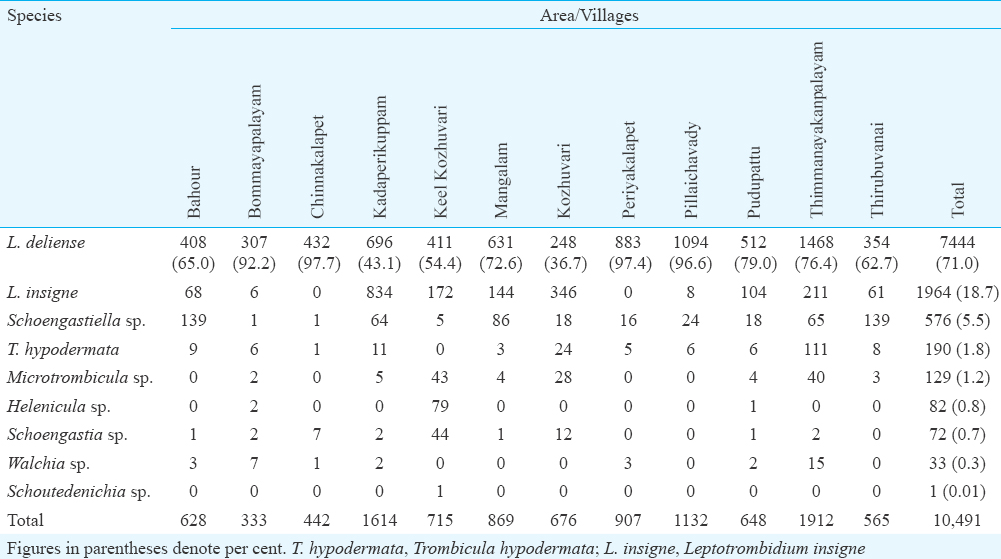
Chigger infestation index: Majority of the trombiculid mites (58.8%) was collected from the known index animal of scrub typhus, i.e. S. murinus followed by R. rattus (36.9%), B. bengalensis (4.2%) and T. indica (0.10%). The overall chigger (all trombiculid mites) index was the highest of 79.1 for S. murinus, and it was, respectively, 47.6, 22.0 and 11.0 for R. rattus, B. bengalensis and T. indica (Table III). The chigger index for S. murinus was significantly higher than that of R. rattus and B. bengalensis (P<0.05). The L. deliense index was also the highest for S. murinus (57.8) as compared to R. rattus (32.1), B. bengalensis (16.6) and T. indica (5.0) (P<0.01).
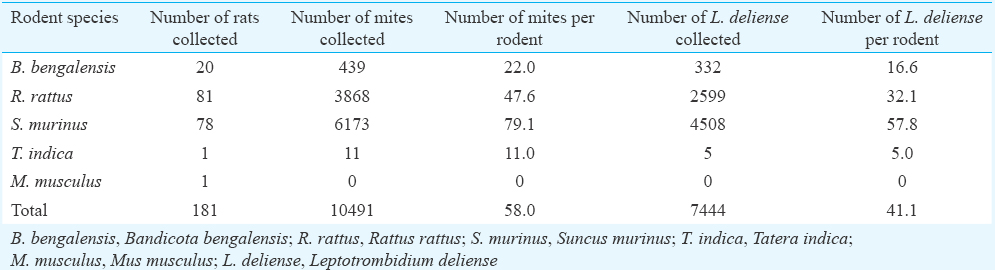
The chigger (all trombiculid mites) index estimated from different villages ranged from 19.5 to 161.4 and the overall index was 58.0 per animal. The index of the known vector mite, L. deliense, ranged from 17.9 to 91.8 in different villages surveyed, and the overall index was 41.1 per animal. The index for Schoengastiella sp., the suspected vector of scrub typhus pathogen, ranged from 0.06 to 34.8 in different villages and the overall index was 3.2 per animal.
Seasonal distribution of scrub typhus cases and chigger mites: The data on human cases of scrub typhus reported during the study period (November 2013-October 2014) were obtained from PIMS, Puducherry, and analyzed. Month-wise reported human cases of scrub typhus and estimated chigger indices are presented in Fig. 1. Scrub typhus cases were reported in all the months, except in May. Most of the cases occurred in the relatively cooler months (October-January) and the peak was in December. Very few cases were recorded from April to August. Month-wise analysis of chigger index showed that it was higher during the cooler months (October-December) and was at its peak in November. The number of human cases of scrub typhus reported in different months showed a significant association with that of the chigger index (P<0.05).
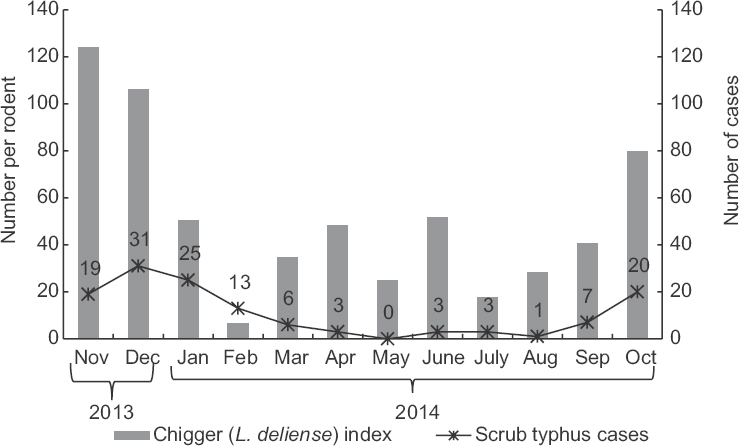
- Month-wise reported cases of scrub typhus and estimated chigger (Leptotrombidium deliense) index.
Ectoparasites other than trombiculid mites: A total of 863 ectoparasites, other than trombiculid mites, were retrieved from the animals. Of these, the fur mite, Demodex sp. (Order: Trombidiformes) formed 56.0 per cent of the total other ectoparasites collected. The tropical rat mite Ornithonyssus bacoti (Order: Mesostigmata) was the other species of mite collected (0.58%). Brown dog ticks Rhipicephalus sanguineus (29.2%) and spined rat lice Polyplax spinulosa (13.3%) were also retrieved from the trapped animals. Flea species Xenopsylla cheopis formed 0.9 per cent of the total other ectoparasites collected and the overall flea index was 0.04 per animal.
Prevalence of rickettsial pathogens in animals: Of the 50 blood samples collected from rodents and shrews, 28 showed agglutination against OX-K in Weil–Felix test indicating the presence of antibodies against O. tsutsugamushi, the causative agent of scrub typhus (Table IV). Among the 28 rodent serum samples, 20 showed matt formation at 1:80 and eight showed matt formation at 1:160. R. rattus showed the highest number of positivity followed by S. murinus and B. bengalensis. Apart from this, 18 of the animals screened were positive for antibodies of murine/tick typhus pathogen. Among those positive for O. tsutsugamushi antibodies, 11 samples reacted for the antibodies of murine/tick typhus pathogen.
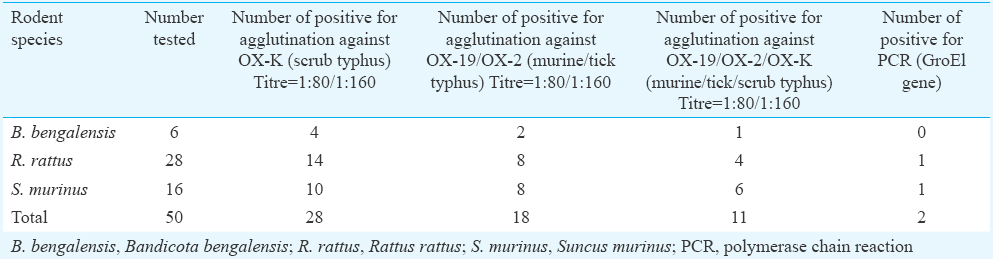
PCR carried out with the blood samples for detection of O. tsutsugamushi showed that of the 50 rats tested, two were positive for GroEl gene (Fig. 2). Subsequently, the amplified 300 bp product was sequenced and confirmed as that of O. tsutsugamushi. These two samples were positive for antibodies also. PCR for 16s RNA (220 bp) and 56 kDa (483 bp) did not detect the respective genes.
![Agarose gel showing polymerase chain reaction product of GroEl gene of Orientia tsutsugamushi blood samples of animals [Lane 1: Positive control; Lane 6: Negative control; Lanes 2 & 4: animal samples (Negative); Lane 3: animal sample no. 23 (Positive); Lane 5: animal sample no. 46 (Positive)].](/content/175/2016/144/6/img/IJMR-144-893-g006.png)
- Agarose gel showing polymerase chain reaction product of GroEl gene of Orientia tsutsugamushi blood samples of animals [Lane 1: Positive control; Lane 6: Negative control; Lanes 2 & 4: animal samples (Negative); Lane 3: animal sample no. 23 (Positive); Lane 5: animal sample no. 46 (Positive)].
Number of small animals with antibodies against scrub typhus pathogen was more in Periyakalapet (10 of 24), Bommayapalayam (11 of 16) and Pillaichavady (all the six rodents) villages, which showed higher chigger indices. The two animals which were PCR positive for GroEl gene were collected from Bommayapalayam, the area with highest number of seropositivity for scrub typhus in animals as well as one of the areas with higher chigger index.
Discussion
Scrub typhus has been reported as a re-emerging disease in Puducherry and adjoining areas of Tamil Nadu in India7. However, information on the vector mites involved or natural existence of the causative rickettsial pathogen in small animals was lacking. A total of 181 animals were collected using rat traps and the trap positivity rate was significantly greater than that reported for the scrub typhus-affected areas of Himachal Pradesh and Kolkata1819. However, it was lower than that observed in West Bengal, Meghalaya and Kerala32021 which could be due to the different ecological situations.
L. deliense was the most abundant species among the nine species of trombiculid mites collected during the present study. This species was present at substantial number in all villages surveyed. Being the main vector of the scrub typhus pathogen, O. tsutsugamushi, such predominance of L. deliense, has been reported during the disease outbreaks in many places in India18 as well as in Northern Thailand22.
L. deliense is the established vector of scrub typhus in most of the endemic countries, including India. A total of 204 trombiculid mite species have been recorded from different ecological settings in India. Of these, four species, namely, L. deliense, Leptotrombidium dihumerale, Leptotrombidium subintermedium and Schoengastiella ligula, have been implicated as vectors of scrub typhus1123. However, most of the outbreaks have been attributed only to the abundance of L. deliense as the vector species3. In the present study, pertaining to the recent outbreak of scrub typhus in Puducherry, L. deliense was the most prevalent and abundant vector species. Apart from this, Schoengastiella sp., and R. sanguineus, the incriminated tick vector of Indian tick typhus pathogen24 have also been documented.
The chigger index recorded in the present study was significantly higher than that reported in other eco-epidemiological settings such as Meghalaya20, Kerala21 and Kolkata19. The highest chigger index was observed with S. murinus. This species is emerged as the most preferred host for the vector mites present in the study villages. Previous study in Darjeeling3 also reported higher chigger index for S. murinus.
Seasonal occurrence of scrub typhus varies with climate change in different geographical regions, and in the southern part of India, it occurs more frequently during the cooler months (October-January)5. This seasonal difference could be the reason for the reported higher number of cases during the cooler months in Puducherry and it coincided with the peak prevalence of trombiculid mites. As a result, a significant association was observed between the monthly index of L. deliense and the number of human cases of scrub typhus.
Although occurrence of scrub typhus pathogen in small animals has been reported from many countries such as Malaysia and Thailand2526, no reports are available on its presence in animals considered as natural hosts of this rickettsial pathogen in India. In the present study, more than 50 per cent of animals tested were positive for antibodies against the scrub typhus pathogen, indicating its high prevalence in the villages of Puducherry. Three species of animals tested were positive for antibodies against the pathogen and PCR showed presence of O. tsutsugamushi in two animals (R. rattus and S. murinus). Antibody positivity was higher among animals collected from villages recorded for higher chigger indices, indicating active transmission between the chigger mites and reservoir/maintenance animals.
Only a few animals were positive for murine-borne (endemic typhus) and tick-borne (epidemic typhus) rickettsials, indicating the risk of their transmission to human. Endemic and epidemic typhus caused by different species of rickettsial pathogens have already been reported from many places in India2728.
The present study had a few limitations. Rodents were examined for rickettsial antibodies by the non-specific Weil–Felix test. However, to confirm O. tsutsugamushi infection, rodent samples were subjected to PCR and two of the samples were positive for GroEl gene of O. tsutsugamushi. These two samples were positive in Weil–Felix test also. Most of the 12 villages were along the East Coast road. A larger area comprising other parts of Puducherry district and surrounding districts of Tamil Nadu could have identified more scrub typhus foci and ‘mite islands’.
Scrub typhus has been reported from diverse ecological settings such as mountainous regions, rainforests, semi-arid deserts, sea shores, river banks and terrain undergoing secondary growth29. The terrain features of Puducherry with secondary scrub and bushy vegetation growth and tropical maritime climate are congenial for the survival of rodents and shrews as well as trombiculid mite vectors. The L. deliense index was well above the critical level of chigger load, i.e. 0.69 per animal30 in all the villages surveyed. The presence of scrub typhus pathogen in the reservoir/maintenance animals along with abundance of the vector population above the critical level, as observed in this preliminary study, was indicative of the facts that these villages were at the risk of transmission of scrub typhus. However, an extensive study is required to identify the ecological hot spots for routine and regular small animal and mite surveillance, which will help initiate timely preventive measures.
Acknowledgment
The technical assistance rendered by Sarvashri A. Ravi, V. Padmanathan and M. Balasubramanian of the Vector Biology and Control division of the Centre is gratefully acknowledged.
Conflicts of Interest: None.
References
- Scrub typhus. In: Warrell DA, Cox TM, Fifth JD, eds. Oxford textbook of medicine (5th ed). USA: Oxford University Press; 2010. p. :919-24.
- [Google Scholar]
- Emergence of Schoengastiella ligula as the vector of scrub typhus outbreak in Darjeeling: has Leptotrombidium deliense been replaced? Indian J Public Health. 2011;55:92-9.
- [Google Scholar]
- A review of scrub typhus management in 2000-2001 and implications for soldiers. J Rural Remote Environ Health. 2003;2:14-20.
- [Google Scholar]
- Geographical distribution, effect of season & life cycle of scrub typhus. JK Sci. 2010;12:63-4.
- [Google Scholar]
- Re-emergence of scrub typhus in Northeast India. Int J Infect Dis. 2012;16:e889-90.
- [Google Scholar]
- Scrub typhus meningitis in South India - a retrospective study. PLoS One. 2013;8:e66595.
- [Google Scholar]
- Outbreaks of scrub typhus in Puducherry & Tamil Nadu during cooler months. Indian J Med Res. 2015;142:591-7.
- [Google Scholar]
- Agarwal VC. Taxonomic Studies on Indian Muridae and Hystricidae (Mammalia: Rodentia). Records of the Zoological Survey of India, Occasional Paper No. 180. Zoological Survey of India, Calcutta 2000:1-177.
- A manual of Acarology (3rd ed). Lubbock (Texas): Texas Tech University Press; 2009.
- Studies on the Trombiculid Mite Fauna of India. Records of the Zoological Survey of India, Occasional paper No.212. Zoological Survey of India, Kolkata 2003:1-539.
- Pictoral keys to arthropods, reptiles, birds and mammals of public health significane, Department of Health and Human Service, Centre for Disease Control and Prevention (CDC) Atlanta, Georgia. Available from: http://www.cdc.gov/nceh/ehs/publications/pictorial_keys.htm
- Relevance of Weil–Felix test in diagnosis of scrub typhus in India. J Assoc Physic India. 2006;54:619-21.
- [Google Scholar]
- Scrub typhus and other Rickettsiosis. CD Alert (National Centre for Disease Control (NCDC) Newsletter). 2009;13:1-8.
- [Google Scholar]
- Serological evidence for wide distribution of spotted fevers & typhus fever in Tamil Nadu. Indian J Med Res. 2007;126:128-30.
- [Google Scholar]
- Evaluation of nested PCR for the diagnosis of scrub typhus among patients with acute pyrexia of unknown origin. Trans R Soc Trop Med Hyg. 2004;98:360-6.
- [Google Scholar]
- Rapid diagnosis of scrub typhus in rural Thailand using polymerase chain reaction. Am J Trop Med Hyg. 2006;75:1099-102.
- [Google Scholar]
- Outbreak investigation of scrub Typhus in Himachal Pradesh (India) J Commun Dis. 2004;36:277-83.
- [Google Scholar]
- Entomological surveillance for small mammal and their ectoparasites with special reference to potential of scrub typhus at Kolkata Port Trust (KPT), Kolkata (India) J Paramed Sci. 2014;5:1-6.
- [Google Scholar]
- Entomological surveillance for small mammal and their ectoparasite in scrub typhus affected areas of Meghalaya, (India) J Entomol Zool Stud. 2013;1:27-9.
- [Google Scholar]
- Eco-entomological investigation in scrub typhus affected area of Thiruvananthapuram, Kerala (India) and their control/containment measures. Int J Curr Microbiol Appl Sci. 2013;2:43-9.
- [Google Scholar]
- Scrub typhus outbreak, Northern Thailand, 2006-2007. Emerg Infect Dis. 2013;19:774-7.
- [Google Scholar]
- Scrub typhus. United States Army Medical Research Unit, Institute for Medical Research, Malaysia, Bulletin No. 21 1983:1-107.
- Rickettsial infections. In: Parthasarthy A, ed. Textbook of pediatric infectious diseases, st ed (1st ed). New Delhi, India: Jaybee Brothers Medical Publishers; 2013. p. :376-85.
- [Google Scholar]
- Involvement of small mammals in the transmission of scrub typhus in Malaysia: isolation and serological evidence. Trans R Soc Trop Med Hyg. 1973;67:838-45.
- [Google Scholar]
- Occurrences of Orientia tsutsugamushi in small animals from Thailand. Am J Trop Med Hyg. 2003;69:519-24.
- [Google Scholar]
- Serological evidence for the continued presence of human rickettsioses in Southern India. Ann Trop Med Parasitol. 2001;95:395-8.
- [Google Scholar]
- The past and present threat of rickettsial diseases to military medicine and international public health. Clin Infect Dis. 2002;34(Suppl 4):S145-69.
- [Google Scholar]
- Population indices of chiggers (Leptotrombidium deliense) and incidence of scrub typhus in Chinese military personnel, Pescadores Islands of Taiwan, 1976-1977. Trans R Soc Trop Med Hyg. 1982;76:85-8.
- [Google Scholar]






