Translate this page into:
A study of hyperhomocysteinemia in cerebral venous sinus thrombosis
For correspondence: Dr Jayantee Kalita, Department of Neurology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Raebareli Road, Lucknow 226 014, Uttar Pradesh, India e-mail: jayanteek@yahoo.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Vegetarianism may result in low vitamin B12 and acquired hyperhomocysteinemia leading to thrombotic conditions such as cerebral venous sinus thrombosis (CVST). The clinico-radiological presentation and outcome of patients with hyperhomocysteinemia may be different from those without, but there is a paucity of information. This study was undertaken to find out the relationship of homocysteine (Hcy) with vitamin B12, folic acid and methyltetrahydrofolate reductase (MTHFR) mutation in the patients with CVST, and compare clinico-radiological severity and outcome of patients with and without hyperhomocysteinemia.
Methods:
Ninety-six CVST patients in whom Hcy level was measured, were included, and their risk factors and neurological, magnetic resonance (MR) imaging and MR venography findings were noted. They were evaluated for prothrombotic conditions including Hcy, vitamin B12, folic acid and MTHFR 677C→T mutation. Three month outcome was categorized as death, poor and good.
Results:
Seventy three per cent patients had risk factors; hyperhomocysteinemia in 52.1 per cent, protein S deficiency in 47.8 per cent, protein C deficiency in 19.4 per cent, MTHFR 677C→T mutation in 30.7 per cent, antinuclear antibody 11 per cent, and Factor V Leiden mutation in two per cent each. Thirty two per cent patients with hyperhomocysteinemia had no other thrombotic cause, and 22 per cent of them had either vitamin B12 and or folic acid deficiency only. The patients with hyperhomocysteinemia more frequently had vitamin B12 deficiency (70 vs. 13%), MTHFR 677C→T mutation (47.5 vs. 9.1%) and superior sagittal sinus thrombosis (78 vs. 56.5%) than normal Hcy group. The clinico-radiological severity and outcome were similar.
Interpretation & conclusions:
Hyperhomocysteinemia was an important correctable risk factor of CVST in patients from northern India, and majority of them had either low vitamin B12 level or MTHFR mutation.
Keywords
Cerebral venous thrombosis
folic acid
homocysteine
magnetic resonance venography
methyltetrahydrofolate reductase
risk factor
vitamin B12
Hyperhomocysteinemia is an emerging risk factor of stroke, coronary artery disease and dementia12. Homocysteine (Hcy) is an intermediate sulphydryl containing aminoacid derived from methionine, and proved to be toxic to neurons and vascular endothelium3. Hyperhomocysteinemia may be due to old age, smoking, chronic renal failure, and deficiency or defective metabolism of folic acid, vitamin B12 or pyridoxine. Vitamin B12, folic acid and pyridoxine are cofactors in remethylation and trans-sulphuration pathways of Hcy metabolism4. Impairment of Hcy metabolism may result in oxidative stress, endothelial dysfunction and prothrombotic state5.
Cerebral venous sinus thrombosis (CVST) is a rare disease accounting for 1-2 per cent of stroke, with a frequency of 3-4 million population, which increases to 7 million in children6. The frequency of puerperal CVST is higher in India (45/1000) compared to Western countries (12/100,000)7. The high incidence of CVST in developing countries compared to the developed may be due to genetic susceptibility compounded by the environmental factors such as dehydration, infection or trauma. In about 10-20 per cent patients with CVST, the underlying aetiology remains undiagnosed8. In India, vegetarianism is common, because of cultural and religious reasons. Folic acid deficiency and methyltetrahydrofolate reductase (MTHFR) 677C→T mutation have been reported as risk factors for hyperhomocysteinemia in CVST9. High frequency of vitamin B12 deficiency has been reported in rural and urban Indians which may result in hyperhomocysteinemia10. In a study on CVST from south India, low plasma folate level correlated with hyperhomocysteinemia but not with serum vitamin B12 level11. In a study from Italy, Hcy level in CVST was neither related to B12 nor to folic acid12. In the recent studies, hyperhomocysteinemia as a risk factor has been reported in 14-74 per cent patients. Males had higher frequency of hyperhomocysteinemia compared to females1314. In one study, Hcy and vitamin B12 levels were measured in 50 patients. Hcy level was high in 70 per cent and vitamin B12 low in 46 per cent, and had an inverse correlation15. None of the studies comprehensively evaluated relationship of Hcy with vitamin B12, folic acid, MTHFR mutation and clinico-radiological severity in CVST with adequate sample size. There are isolated case reports of CVST with homocysteinuria and vitamin B12 deficiency related hyperhomocysteinemia1617. Vegetarianism is a cultural practice among Indians, which may result in acquired hyperhomocysteinemia leading to thrombotic conditions such as CVST. This study reports the relationship of Hcy with vitamin B12, folic acid and MTFHR C677C→T polymorphism in the patients with CVST from northern India. The clinic-radiological severity and outcome of the patients with and without hyperhomocysteinemia were also compared.
Material & Methods
This was a retrospective study, and the data were obtained from a prospectively maintained CVST registry during January 2000 to December 2017. The study was conducted at Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India which is a tertiary care teaching institute. The study was approved by the Institute Ethics Committee (PGI/BE/774) and consent waiver also obtained (PGI/BE/599).
Inclusion and exclusion criteria: The patients with characteristic clinical features such as new onset headache, seizure, focal neurological deficit or altered sensorium evolving over a period of hours to days were suspected to have CVST. The diagnosis of CVST in these patients was confirmed on the basis of magnetic resonance venography (MRV). Only those patients were included in whom serum homocysteine level was measured and their clinical and radiological data were available. During the study period, 133 patients with CVST were admitted, 37 of them were excluded because of incomplete data. The analysis was done for 96 patients. The CVST patients with renal failure, liver failure, cancer, and vascular malformation or on vitamin supplementation therapy in last three months were excluded.
Evaluation: The demographic details, duration of illness and presenting symptoms such as headache, seizure, visual impairment, focal weakness or altered sensorium were noted. History of oral contraceptive use, pregnancy, puerperium, foetal loss, deep vein thrombosis, dehydration, infection, and evidence of systemic vasculitis (rash, arthritis, recurrent ulcer, or uveitis) were noted. Dietary habit, smoking and addiction history were noted. A detailed general and systemic examination was done. Evidence of raised intracranial pressure including fundus examination findings was noted. Consciousness was assessed by Glasgow Coma Scale (GCS)18. At the time of admission, Mini Mental State Examination (MMSE) scale19 was used in the patients with a GCS score of 15 (n=53), while in patients with altered sensorium (n=43), it was done at the time of discharge when they were conscious and co-operative. Presence of cranial nerve palsy was noted. Muscle power was assessed by Medical Research Council scale20, and muscle tone, tendon reflexes, sensations (touch, joint position and pin prick) and coordination were noted.
Investigations: Complete blood counts, haemoglobin, blood sugar, blood urea nitrogen, and serum creatinine, sodium, potassium, bilirubin, lactate dehydrogenase, transaminases, alkaline phosphatase, calcium and lipid profile were measured by auto analyzer (Architect i1000 SR, Abbott, USA). Erythrocyte sedimentation rate (ESR) at first hour was done by Westergren method21 and activated partial thromboplastin time and prothrombin time by, New Jersy photometric method22. Thyroid profile and HIV serology were done. Anti-phospholipids antibody (APLA) syndrome was diagnosed on the basis of International Consensus Statement23.
Patients were also screened for paroxysmal nocturnal haemoglobinuria. Factor V Leiden gene and MTHFR 677C→T gene mutations were done by PCR in the department of genetics of our institute24. Fasting serum vitamin B12 and folate, and plasma Hcy were measured by chemiluminescence immunoassay method using Chemiluminescent Immunoassay System (Immuilte-1000; Siemens Healthcare Diagnostics Technical Services, catalog number: LKVB1, UK). ELISA was used for APLA (Chorus kit, New Zealand) and anti-ds DNA antibody (Chorus kit, New Zealand), immunofluorescence (Euroimmun kit, Germany) for anti-nuclear antibody and nephlometry (Siemens kit, China) method for the measurement of C3 and C4. Protein C (STA-STACLOT PROTEIN C, Stago Diagnostics, New Jersey, USA), protein S (STA-STACLOT PROTEIN S, Stago Diagnostics, New Jersey, USA) and antithrombin III (STA Stachrom AT III) were assayed using analyzer (model no CC32019673, Stago Canada Ltd). Serum anti-parietal cell antibody was measured in the patients with low vitamin B12 level. Venous blood (5 ml) was collected for complete blood count, Hcy, MTHFR, HbA1c and paroxysmal nocturnal haemoglobinuria testing in EDTA vial; for prothrombin time, activated partial thromboplastin time, D-dimer, lupus anticoagulant, protein C and S, antithrombin III and fibrinogen levels in citrate buffer vial; and for serum chemistry, thyroid function tests, autoantibodies and vitamin B12 in plain vial.
Cranial magnetic resonance imaging (MRI) and MRV were done on a 1.5/3T MRI machine (Signa GE Medical system, Wisconsin, USA). The location and nature of parenchymal lesion were noted. On venography, the location of thrombosis, number of sinuses involved, and development of collaterals were noted.
Treatment: The patients were treated with low molecular weight heparin (enoxaparin, 100 unit/kg subcutaneously twice daily) for 10 days. Unfractionated heparin was used in those patients (n=26) who could not afford enoxaparin, in a dose of 5000 IU intravenously followed by 18 unit/kg/h infusion to keep activated partial thromboplastin time at 2.5 times of control. Oral anticoagulant [acenocoumarol (acitrom)] was prescribed after 10 days of heparin therapy and doses were titrated to keep INR between 2 and 3. The patients with hyperhomocysteinemia due to low vitamin B12 were treated with intramuscular vitamin B12 injection (1000 μg daily) for 10 days, followed by weekly for one month and thereafter monthly. Those with folic acid deficiency were treated with oral folic acid (5 mg daily). In the remaining patients, specific underlying cause was treated. Patients with raised intracranial pressure received intravenous mannitol and acetazolamide 250 mg thrice daily. Seizure was treated with sodium valproate, levetiracetam and/or clobazam. Patients with respiratory failure were treated with mechanical ventilation. Hemicraniectomy was done in three patients only and thrombectomy in none.
The outcome was defined at three months using modified Rankin Scale (mRS)25 as death (mRS score 6), poor (mRS score 3-5) or good (mRS score ≤ 2)26.
Categorization of patients: The CVST patients were categorized into normal Hcy (<15 μg/ml) or hyperhomocysteinemia (≥15 μg/ml). Serum vitamin B12 level was considered low if the level was below 211 pg/ml and serum folic acid if below 3 ng/ml.
Statistical analysis: The demographic, clinical and laboratory findings of the patients with and without hyperhomocysteinemia were compared using Chi-square or Fisher exact test for categorical and student t or Mann–Whitney U test for continuous variables. If the data of continuous variable were not normally distributed, then Mann–Whitney U test was used for comparison between the two groups. The MRI and MRV findings were also compared between the two groups using Chi-square or Fisher exact test. The relationship between serum vitamin B12, folic acid, MTHFR gene mutation and outcome with hyperhomocysteinemia was compared using Chi-square test. The statistical analysis was done by SPSS (Statistical Package of Social Sciences, IBM, Chicago, IL, USA) version 16 software.
Results
There were 96 patients with CVST, and their median age was 28 (range 9-76) yr, two were below 15 yr of age, and 46 were females. The clinical presentation was acute (<48 h) in three, sub-acute (48 h to 30 days) in 77 and chronic (>30 days) in 16 patients. At the time of admission, raised intracranial pressure was present in 66 (68.7%), focal neurologic deficit in 47 (49%), seizures in 58 (60.4%) and isolated headache syndrome in 15 (15.6%) patients.
Risk factors of CVST: Risk factors could be detected in 73 per cent (n=70) patients; the commonest being hyperhomocysteinemia in 52 per cent (50/96) followed by protein S deficiency in 47.8 per cent (34/71), MTHFR mutation in 30.1 per cent (22/73), protein C deficiency in 19.4 per cent (14/72), antinuclear antibody in 12.1 per cent (11/91) and systemic lupus erythematosus and Factor V Leiden mutation in two per cent patients each. Antiphospholipid antibody was done in 13 suspected patients and was positive in 6. Female specific risk factors were present in 13 patients; puerperium in nine and case of oral contraceptives in four patients. The number of risk factors ranged between one and four. Multiplicity of risk factors was more commonly observed than the single risk factor. The commonest combination of risk factors was hyperhomocysteinemia with MTHFR mutation (19/40, 47.5%) and protein S (19/40; 47.5%) deficiency followed by and protein C (10/40; 25%) deficiency.
MRI and MRV findings: MRI revealed parenchymal lesion in 70 (72.9%) patients; haemorrhagic infarction in 53/70 (75.7%) and pale infarction in 17/70 (24.2%). On MRV, superficial venous system was involved in 82/96 (85.4%), deep in 4/96 (4.2%) and both in 10/96 (10.4%) patients (Figs 1 and 2). More than two sinuses were involved in 28 patients (median 2, range 1-4).
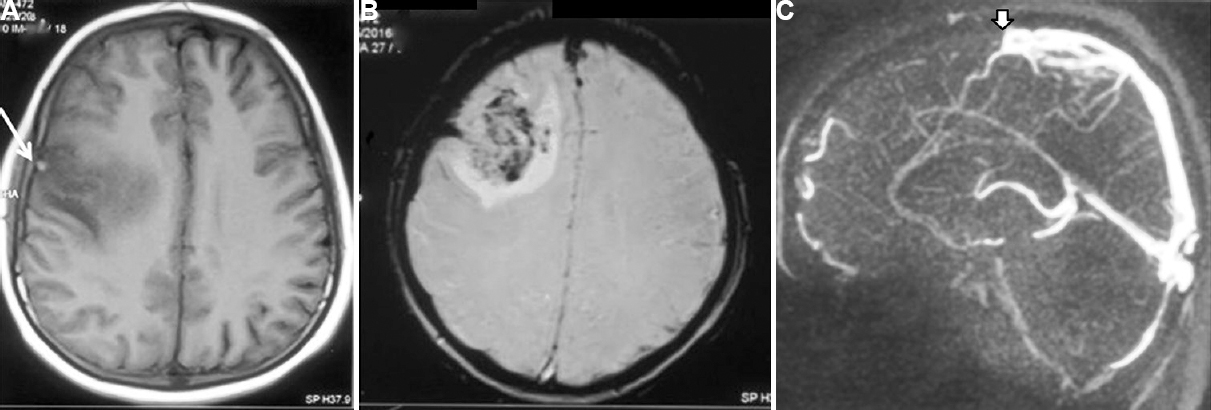
- Magnetic resonance imaging (MRI) and magnetic resonance venography (MRV) of a 21 years old male patient with cerebral venous sinus thrombosis who presented with headache, status epilepticus and altered sensorium for 10 days. (A) Haemorrhagic infarctions involving parietal areas (white arrow). (B) Gradient echo image showing blooming in the same area. (C) MRV shows thrombosis of superior sagittal sinus (white arrow). His serum vitamin B12 level was 83 pg/ml and homocysteine 50 μmol/l. He was treated with vitamin B12 (1000 μg) intramuscular injection, daily for 10 days followed by weekly for one month and then monthly thereafter. He improved completely at three months and his homocysteine level was normal (7.47 μg/ml).
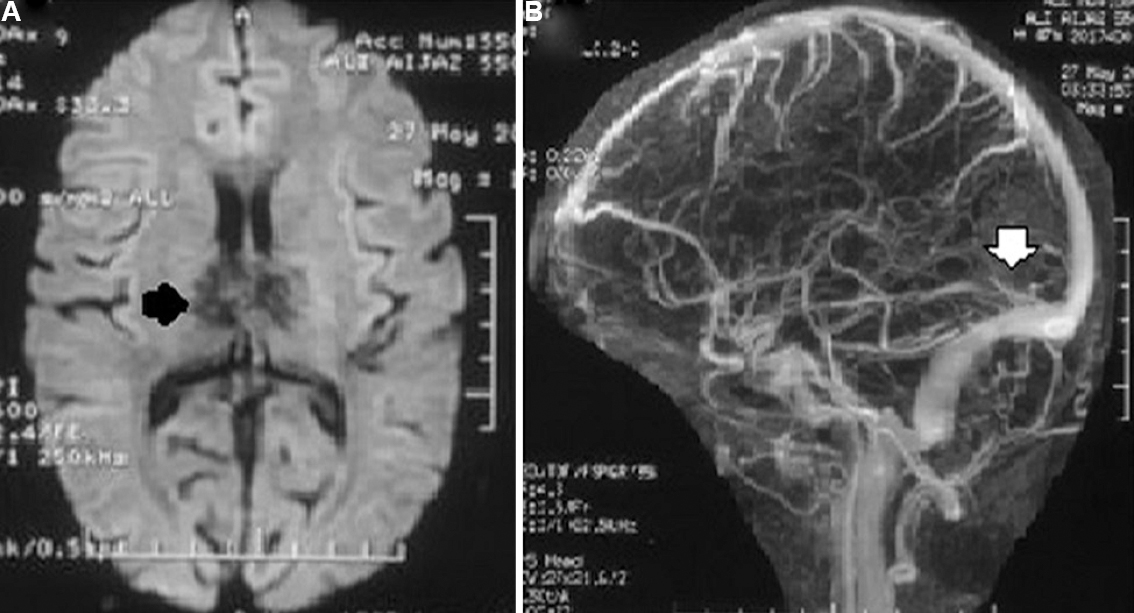
- Magnetic resonance imaging (MRI) and magnetic resonance venography (MRV) of a patient with cerebral venous sins thrombosis who presented with headache, forgetfulness, behavioural abnormality and parkinsonian features. (A) Diffusion MRI reveals haemorrhagic infarction of anterior thalamus (black arrow). (B) MRV reveals deep cerebral vein thrombosis (white arrow). He had low serum folate (2 ng/ml) and high homocysteine (30 μg/ml). He was treated with anticoagulant and folic acid (5 mg daily, per oral), and improved completely at three months. His repeat serum folate (15 ng/ml) and homocysteine (9.3 μg/ml) at three months were normal.
CVST with and without hyperhomocysteinemia: Hyperhomocysteinemia was present in 50 (52.1%) patients; mild (15-30 μg/ml) in 11, moderate (30-50 μg/ml) in 33 and severe (>50 μg/ml) in 6 patients. The age, duration of illness, smoking and hematological and biochemical parameters were not significantly different between the CVST patients with and without hyperhomocysteinemia. The underlying risk factors such as prothrombotic conditions, vasculitis, APLA syndrome and female specific risk factors were also similar between the two groups (Tables I and II). On MRV, the patients with hyperhomocysteinemia had higher frequency of superior sagittal sinus thrombosis compared to normal Hcy group (78 vs. 56.5%; P<0.05). CVST patients with hyperhomocysteinemia also had insignificantly higher frequency of two or more sinus involvement (34% vs. 23.9%; P=0.27). The details are presented in Table III.
| Parameters | Normal homocysteine (n=46), n (%) | Raised homocysteine (n=50), n (%) |
|---|---|---|
| Age (mean±SD) yr | 35.20±15.05 | 31±4.02 |
| Male | 20 (43.4) | 30 (60) |
| Vegetarian | 29 (63) | 29 (58) |
| Smoker | 4 (8) | 2 (4) |
| Alcoholic | 3 (6.5) | 5 (10) |
| Illness duration (days) | 21.36±28.07 | 38.30±108.07 |
| <2 | 0 (0) | 3 (6) |
| 2-30 | 40 (86.9) | 37 (74) |
| >30 | 6 (13) | 10 (20) |
| Motor deficit | 19 (41.3) | 28 (56) |
| Raised ICP | 29 (63) | 37 (74) |
| Seizure | 28 (60.8) | 30 (60) |
| Status epilepticus | 7 (15.2) | 9 (18) |
| CN involvement | 12 (26) | 21 (42) |
| Papilloedema | 15 (32.6) | 18 (36) |
| Mechanical ventilator | 4 (8 ) | 2 (4) |
| GCS | ||
| ≥9 | 35 (76) | 44 (88) |
| <9 | 11 (24) | 6 (12) |
| mRS at three months | ||
| 0-2 | 40 (87) | 47 (94) |
| 3-5 | 5 (11) | 1 (2) |
| 6 | 1 (2) | 2 (4) |
CN, cranial nerve; ICP, intracranial pressure; mRS, modified Rankin Scale, SD, standard deviation, GCS, Glasgow Coma Scale
| Parameters | Reference range (units) | Normal homocysteine (46), n (%) | Raised homocysteine (50), n (%) |
|---|---|---|---|
| Fibrinogen | 160-450 (mg/dl) | 284±92.31 | 312±109 |
| MCV | 92±9 (fl/cell) | 87.87±8.19 | 86.77±10.46 |
| MCH | 29.5±2.5 (pg/cell) | 29.59±10.81 | 27.55±5.09 |
| Hemoglobin | 12-16 (mg/dl) | 12.26±2.26 | 11.82±2.83 |
| Hematocrit | (45-52% for men) (37-48% for women) | 38.28±6.18 | 37.53±8.26 |
| HDL | ≥60 (mg/dl) | 37.48±9.54 | 37.10±9.79 |
| Triglyceride | <150 (mg/dl) | 155.88±65.34 | 196.26±125.90 |
| Total cholesterol | <200 (mg/dl) | 181.05±37.81 | 182.30±51.73 |
| LDL | <100 (mg/dl) | 107.08±28.64 | 103.84±34.71 |
| ESR mm at one hour | 0-20 (mm/h) | 32.54±19.45 | 31.84±21.83 |
| Platelet | 1.65-4.15 (×106/µl) | 209782.60±84886 | 208800±81789 |
| Antinuclear antibody (n=91) | 2/44 (4.5) | 9/47 (19.1)* | |
| Antiphospholipid antibody | |||
| beta2-GP1- | 3-18 (au/ml) | 6 (13.04) | 7 (14) |
| IgG ACLA- | 3-18 (gpl/ml) | ||
| IgM ACLA- | 3-18 (mpl/ml) | ||
| Protein C (n=72) | 70-130% | 4/32 (12.5) | 10/40 (25) |
| Protein S-(n=71) | 65-140% | 15/31 (48.4) | 19/40 (47.5) |
| Antithrombin III (n=44) | 80-120% | 1/19 (5.2) | 3/25 (12) |
| Folic acid | |||
| <3.5 | >3.5 (ng/ml) | 12 (26) | 21 (42) |
| 3.5-5.5 | 17 (40) | 13 (26) | |
| >5.5 | 17 (40) | 16 (32) | |
| Vitamin B12 | |||
| <200 | >200 (pg/ml) | 6 (13.04) | 35 (70)** |
| 200-500 | 21 (45.6) | 8 (16)** | |
| >500 | 19 (41.3) | 7 (14)** | |
| MTHFR (n=73) | |||
| CC | 30/33 (91) | 21/40 (52.5)** | |
| CT | 3/33 (9) | 12/40 (30)** | |
| TT | 0 | 7/40 (17.5)** |
P *<0.05 **<0.01 compared to normal homocysteine group; MCV, mean corpuscular volume, MCH, mean corpuscular haemoglobin; HDL, high density lipoprotein; LDL, low density lipoprotein; ESR, erythrocyte sedimentation rate; beta2-GP1, Beta-2 glycoprotein 1; ACLA, anticardiolipin antibodies; MTHFR, methyltetrahydrofolate reductase
| Parameters | Normal homocysteine (n=46), n (%) | Raised homocysteine (n=50), n (%) |
|---|---|---|
| MRI findings | ||
| Parenchyma involved | 30 (65.2) | 40 (80) |
| Type of lesion | ||
| Ischemic | 5 (17) | 12 (30) |
| Hemorrhagic | 25 (83) | 28 (70) |
| Bilateral | 7 (15.2) | 11 (22) |
| MRV findings | ||
| One sinus involved | 19 (41.3) | 17 (34) |
| Two sinuses involved | 16 (34.8) | 16 (32) |
| More than two sinuses | 11 (23.9) | 17 (34) |
| Superficial | 41 (89) | 41 (82) |
| Deep | 1 (2) | 3 (6) |
| Both | 4 (9) | 6 (12) |
| Superior sagittal | 26 (56.5) | 39 (78)* |
| Inferior sagittal | 0 (0) | 3 (6) |
| Transverse | 29 (63) | 31 (60.2) |
| Sigmoid | 22 (47.8) | 20 (40) |
| Straight | 7 (15.2) | 8 (16) |
| Vein of Galen | 0 (0) | 3 (6) |
| Cortical vein thrombosis | 2 (4.3) | 5 (10) |
*P<0.05 compared to normal homocysteine group
Severity of hyperhomocysteinemia and clinico-radiological association: The severity of hyperhomocysteinemia was not associated with rapidity of onset, GCS score, focal deficit, raised ICP, papilloedema, number of sinuses involved and parenchymal lesion (Table IV).
| Parameter | Mild HHcy (n=11) | Moderate HHcy (n=33) | Severe HHcy (n=6) |
|---|---|---|---|
| Illness duration (days) | |||
| <2 | 1 | 2 | 0 |
| 2-30 | 7 | 24 | 6 |
| >30 | 11 | 7 | 0 |
| GCS score | |||
| <9 | 0 | 6 | 0 |
| ≥9 | 11 | 27 | 6 |
| Raised ICP | 8 | 25 | 4 |
| Motor deficit | 5 | 20 | 3 |
| Cranial nerve involvement | 8 | 11 | 2 |
| Seizure | 6 | 21 | 3 |
| Status epilepticus | 1 | 8 | 0 |
| Papilloedema | 3 | 14 | 1 |
| Mechanical ventilator | 0 | 2 | 0 |
| MTHFR (n=40) | |||
| CC | 6 | 13 | 2 |
| CT | 4 | 7 | 1 |
| TT | 0 | 5 | 2 |
| Sinus (types) involvement | |||
| Superficial | 10 | 25 | 6 |
| Deep | 1 | 2 | 0 |
| Both | 0 | 6 | 0 |
| Sinus (number) involvement | |||
| >2 | 7 | 12 | 1 |
| ≤2 | 4 | 21 | 5 |
| Parenchyma involved | 9 | 26 | 5 |
| mRS at three months | |||
| 0-2 | 10 | 31 | 6 |
| 3-5 | 1 | 0 | 0 |
| 6 | 0 | 2 | 0 |
| Outcome | |||
| Good | 10 | 31 | 6 |
| Poor | 1 | 2 | 0 |
GCS, Glasgow Coma Scale; ICP, intracranial pressure; MTHFR, methyltetrahydrofolate reductase; mRS, modified Rankin Scale
Association of homocysteine with folic acid, vitamin B12 and MTHFR 677C→T mutation: Hyperhomocysteinemia was associated with vitamin B12 deficiency in 35 of 41, folic acid deficiency in 21 of 33 and MTHFR 677C→T mutation in 19 of 23 patients (Table II). Of the 35 patients with vitamin B12 deficiency only, 10 per cent had antiparietal cell antibody. Of the 50 patients with hyperhomocysteinemia, 34 had additional conventional risk factors of CVST. In the remaining 16 patients, seven had vitamin B12 deficiency, four had both vitamin B12 and folic acid deficiency and five had isolated hyperhomocysteinemia. Hyperhomocysteinemia was not related to folic acid, but vitamin B12 was significantly lower in the patients with hyperhomocysteinemia compared to those with normal Hcy level (35 vs. 6; P<0.01). MTHFR 677C→T gene polymorphism was studied in 73 patients; seven (9.7%) patients had homozygous (TT genotype) and 15 (20.5%) had heterozygous (CT genotype) mutation. All the patients with TT genotypes and 12 of 15 (80%) patients with CT genotype had hyperhomocysteinemia whereas only 21 of 51 (41%) CC genotype had hyperhomocysteinemia (P<0.001). The severity of hyperhomocysteinemia was not associated with the types of MTHFR mutation. The relationship of Hcy with vitamin B12, folic acid and other prothrombotic conditions is presented in Table V. A repeat Hcy and vitamin B12 level after three months revealed normalization of vitamin B12 level in all, and Hcy in 76 per cent patients.
| Categories | Patient with ↑Hcy (n=50), n (%) |
|---|---|
| ↑Hcy+↓vitamin B12 | 7 (14) |
| ↑Hcy+↓FA | 0 |
| ↑Hcy+↓vitamin B12+↓FA + no prothrombotic condition | 4 (8) |
| ↑Hcy+↓vitamin B12/FA + other risk factors | 29 (58) |
| ↑Hcy+other prothrombotic condition | 5 (10) |
| Only↑Hcy | 5 (10) |
Hcy, homocysteine; FA, folic acid
Outcome: Four (4.2%) patients died during the hospital stay due to severe raised intracranial pressure. At six months, four (4.3%) patients had poor, five (5.4%) good and 83 (90.2%) had complete recovery. Follow up mean MMSE was 27.39±3.41 and 28.52±2.22 (P=0.06) in patients with normal Hcy and hyperhomocysteinemia groups, respectively. There was recurrence of seizure in four patients on tapering of antiepileptic drugs. The good outcome however, was not different between the patients with and without hyperhomocysteinemia (94 vs. 87%; P=0.18) as well as grade of hyperhomocysteinemia (mild vs. moderate vs. severe hyperhomocysteinemia- 91 vs. 94 vs. 100%; P=0.75). Three patients had hemicraniectomy, one died and two had good recovery at three months.
Discussion
Hyperhomocysteinemia was present in 52 per cent patients with CVST which was significantly associated with vitamin B12 deficiency and MTHFR mutation but not with serum folic acid. The extent, severity and outcomes of CVST did not differ in the patients with hyperhomocysteinemia from those with normal Hcy level.
This study highlights the role of vitamin B12 deficiency related hyperhomocysteinemia as a risk factor of CVST. The earlier studies have highlighted the importance of folic acid and MTHFR gene mutations as a risk factor of CVST911. In a study, Hcy, vitamin B12, folic acid and MTHFR gene mutations have been reported in 45 patients with CVST, compared with 90 controls. The risk of developing CVST in patients with hyperhomocysteinemia was 4.6 and with low folate 3.5; however, MTHFR mutation was not related to the risk of CVST. The patients with MTHFR mutation and low folate level had high Hcy level9. A study from India also reported independent association of Hcy with CVST with an odds ratio of 10.8 after adjustment of vitamin B12 and folate levels. Vitamin B12 and folate levels were not associated with puerperal CVST, and MTHFR mutations in the CVST were not different from the controls11. Lack of association of Hcy with folate and vitamin B12 in CVST has also been reported in another study as well27. Elevated Hcy although has been reported in smoker28 but we did not find association of smoking and hyperhomocysteinemia. This may be due to very few smokers in our study. Homocysteine metabolism is dependent on folic acid, vitamin B12 and pyridoxine. The deficiency of these vitamins results in hyperhomocysteinemia through remethylation or trans-sulphuration pathways4. The non-vegetarian diet is the main source of vitamin B12. On the other hand, the vegetarian food has adequate supply of folic acid and pyridoxine. In the present study, 80 per cent of patients with hyperhomocysteinemia either had vitamin B12, folic acid or both deficiencies, and 70 per cent of patients with hyperhomocysteinemia had low vitamin level which was mainly due to dietary deficiency, and autoimmune cause was found in 10 per cent patients only. We have studied MTHFR 677C→T gene polymorphism in 73 patients, and CT (30 vs. 9%) and TT (17.5 vs. 0%) genotypes were more frequently associated with hyperhomocysteinemia. This may suggest the role of MTHFR in hyperhomocysteinemia especially in those who had normal vitamin B12 level. In north Indian population, heterozygous MTHFR gene polymorphism (CT) has been reported in 15.5 per cent, and homozygous (TT) in 3.5 per cent29. There are reports of CVST in children with hyperhomocysteinemia and homocysteinuria due to cystathione β synthase mutation, but these patients usually present with early onset of clinical manifestations30. A population-based case-control study including 4375 patients with a first deep vein thrombosis of the leg or pulmonary embolism and 4856 controls did not find an association of either homozygous or heterozygous MTHFR 677C→T mutation with venous thromboembolism31. In a meta-analysis32, the effects of Factor V Leiden, prothrombin 20210A and MTHFR 677C→T were evaluated in 11,000 patients with venous thromboembolism and 21,000 controls. This large pooled analysis revealed no effect for MTHFR 677C→T mutation on venous thromboembolism. Factor V Leiden and PT20210A were confirmed to be moderate risk factors32.
Hyperhomocysteinemia is associated with thrombotic process through several mechanisms including platelet aggregation, increased activation of Factor V Leiden mutation, prothrombin activation, inhibition of protein C activation, and inhibition of tissue plasminogen activator binding to endothelial cells33. Hyperhomocysteinemia irrespective of aetiology (nutritional, genetic or immune mediated) is a risk factor of thrombotic events. Correction of dietary deficiency of vitamin B12 may be an easy and cost effective measure to prevent hyperhomocysteinemia related CVST. Vitamin B12 deficiency may be multifactorial (nutritional, genetic, immune mediated, etc.). Vitamin B12 deficiency has been reported to be common in north India34. If the association of Hcy with vitamin B12 deficiency in CVST is confirmed, it may provide a basis for prevention of CVST in these patients. In the present study, we have equal proportion of male and female patients although CVST has a female preponderance735. Higher frequency of hyperhomocysteinemia has been reported in males compared to females in earlier studies1314, but we did not find significant gender difference of hyperhomocysteinemia in our study.
This study was limited by inadequate evaluation of underlying vitamin B12 deficiency. Different genetic causes of vitamin B12 deficiency were also not evaluated.
In conclusion, our findings showed that hyperhomocysteinemia was associated in 52 per cent of patients with CVST in northern India, and was mainly related to dietary deficiency of vitamin B12 and MTHFR mutation. Hyperhomocysteinemia was not related to clinico-radiological severity and outcome. Further population based studies are needed to evaluate the role of dietary vitamin B12 deficiency in producing hyperhomocysteinemia leading to vascular complications.
Acknowledgment
Authors thank Dr S K Mandal, Center of Bio Medical Research, Lucknow, for statistical analysis and Shri Shakti Kumar for secretarial help.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Homocysteine, folic acid and coronary artery disease: Possible impact on prognosis and therapy. Indian J Chest Dis Allied Sci. 2008;50:39-48.
- [Google Scholar]
- Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6.
- [Google Scholar]
- Hyperhomocysteinemia as a risk factor for the neuronal system disorders. J Physiol Pharmacol. 2014;65:15-23.
- [Google Scholar]
- The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci. 2016;17:1733.
- [Google Scholar]
- Roles of homocysteine in cell metabolism: Old and new functions. Eur J Biochem. 2001;268:3871-82.
- [Google Scholar]
- The post-thrombotic syndrome: Risk factors and impact on the course of thrombotic disease. J Thromb Haemost. 2005;3:2671-6.
- [Google Scholar]
- Cerebral venous and arterial thrombosis in pregnancy and puerperium. A study of 135 patients. Angiology. 1983;34:731-46.
- [Google Scholar]
- Diagnosis and management of cerebral venous thrombosis: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158-92.
- [Google Scholar]
- Hyperhomocysteinemia, low folate and vitamin B12 concentrations, and methylene tetrahydrofolate reductase mutation in cerebral venous thrombosis. Stroke. 2004;35:1790-4.
- [Google Scholar]
- Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J Assoc Physicians India. 2006;54:775-82.
- [Google Scholar]
- Homocysteine, folate and vitamin B(12) in puerperal cerebral venous thrombosis. J Neurol Sci. 2008;272:43-7.
- [Google Scholar]
- Hyperhomocysteinemia and other thrombophilic risk factors in 26 patients with cerebral venous thrombosis. Eur J Neurol. 2004;11:405-9.
- [Google Scholar]
- Clinical profile, risk factors and outcomes in patients with cerebral venous sinus thrombosis: A study from Western India. J Assoc Physicians India. 2019;67:49-53.
- [Google Scholar]
- Risk factors and predictors of outcomes in 243 Chinese patients with cerebral venous sinus thrombosis: A retrospective analysis. Clin Neurol Neurosurg. 2019;183:105384.
- [Google Scholar]
- Co-relation of cerebral venous sinus thrombosis with Vitamin B12 and homocysteine levels in a tertiary care centre. J Assoc Physicians India. 2019;67:34-7.
- [Google Scholar]
- An uncommon presentation of hyperhomocysteinemia and vitamin B12 deficiency: A case report. J Med Case Rep. 2019;13:36.
- [Google Scholar]
- A case of homocystinuria due to CBS gene mutations revealed by cerebral venous thrombosis. J Neurol Sci. 2014;336:257-9.
- [Google Scholar]
- Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81-4. doi: 10.1016/s0140-6736(74)91639-0. PMID: 4136544
- [Google Scholar]
- (1975). ””Mini-mental status”. A practical method for grading the cognitive state of patients for the clinician”. J Psychiatric Res. 1975;12(3):189-98.
- [Google Scholar]
- 1976. Medical Research Council. Aids to examination of the peripheral nervous system. Memorandum no. 45. London: Her Majesty's Stationary Office; Available from: https://mrc.ukri.org/documents/pdf/aids-to-the-examination-of-the-peripheralnervous-system-mrc-memorandum-no-45-superseding-warmemorandum-no-7/
- Diagnostic tests: the erythrocyte sedimentation rate range and limitations of the technique. Triangle. 1957;3(1):20-5. PMID: 13455726
- [Google Scholar]
- Development of a photometric assay for activated partial thromboplastin time and its application to the Cobas Bio centrifugal analyzer. Thromb Res. 1985;40(6):721-30. doi: 10.1016/0049-3848(85)90310-x. PMID: 4089837
- [Google Scholar]
- International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295-306.
- [Google Scholar]
- Evaluation of MTHFR C677T polymorphism in ischemic and hemorrhagic stroke patients. A case-control study in a Northern Indian population. J Neurol Sci. 2011;304:67-70.
- [Google Scholar]
- Modification of Rankin Scale: Recovery of motor function after stroke. Stroke. 1988;19:1497-1500.
- [Google Scholar]
- Do the risk factors determine the severity and outcome of cerebral venous sinus thrombosis? Transl Stroke Res. 2018;9:575-81.
- [Google Scholar]
- MTHFR gene polymorphism and its relationship with plasma homocysteine and folate in a North Indian population. Biochem Genet. 2010;48:229-35.
- [Google Scholar]
- Vitamin B12 deficiency causing hyperhomocysteinaemia and cerebral venous sinus thrombosis. Intern Med J. 2012;42:601-3.
- [Google Scholar]
- No association between the common MTHFR 677C->T polymorphism and venous thrombosis: Results from the MEGA study. Arch Intern Med. 2007;167:497-501.
- [Google Scholar]
- Risk of venous thromboembolism associated with single and combined effects of Factor V Leiden, Prothrombin 20210 A and Methylenetethraydrofolate reductase C677T: A meta-analysis involving over 11,000 cases and 21,000 controls. Eur J Epidemiol. 2013;28:621-47.
- [Google Scholar]
- Cerebral vein thrombosis and mild hyperhomocysteinemia: Three new cases. Neurol Sci. 2002;23:225-7.
- [Google Scholar]
- A study of homocysteine level in North Indian subjects with special reference to their dietary habit. E Spen Eur E J Clin Nutr Metab. 2007;2:E116-9.
- [Google Scholar]
- Prognosis of cerebral vein and dural sinus thrombosis: Results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35:664-70.
- [Google Scholar]






