Translate this page into:
A report on the presence of GES-5 extended spectrum beta-lactamase producing Pseudomonas aeruginosa associated with urinary tract infection from north-east India
* For correspondence: ab0404@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Guiana extended spectrum (GES) beta lactamase belongs to molecular class A1 frequently found in Gram-negative rods by and large in Pseudomonas aeruginosa in addition to other members of Enterobacteriacae2. Till date, only a few studies on epidemiology and environmental burden of GES-type ESBLs have been published12.
We present the occurrence of blaGES-5 harbouring Pseudomonas aeruginosa isolated from human urine specimen from north-east India.
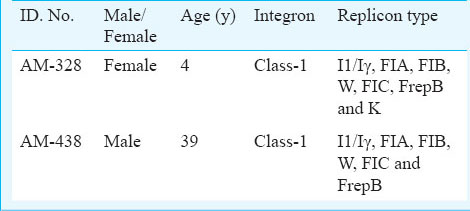
The study was conducted in the department of Microbiology, Assam University, Silchar, Assam, India, from January to December 2012. The first isolate (AM 328) was obtained from the urine sample of a 4 month old female in May 2012 and the second isolate (AM 438) was recovered from the urine of a 39-year-old male in July 2012 (Table). These patients attended the Out Patient Department of Silchar Medical College and Hospital, Silchar, Assam and diagnosed with urinary tract infection. The selection of the samples was based on the initial screening of isolates for the presence of ESBL3. Antimicrobial susceptibility was determined by Kirby Bauer disc diffusion method on Muller-Hinton agar plates3. The following antibiotics were used for antimicrobial susceptibility: cefopodoxime (10 μg), amikacin (30 μg), gentamicin (10 μg), ciprofloxacin (30 μg), trimithoprim/dulphamethoxazole (1.25/23.75 μg), tigecycline (15 μg), cefepime (30 μg), imipenem (10 μg), meropenem (10 μg), ceftriaxone (30 μg), aztreonam (30 μg) and cefoxitin (30 μg). Minimum inhibitory concentrations (MIC) of various antibiotics [cefotaxime, ceftazidime, Ceftriazone, cefepime, imipenem, meropenem, ertapenem and aztreonam (Hi-Media, Mumbai, India] were determined on Muller Hinton agar plates containing 2,4, 8, 16, 32, 64, 128, 256 mg/l of antibiotics, by agar dilution method according to Clinical and Laboratory Standards Institute (CLSI) guidelines3.
For amplification and characterization of blaESBL genes, a set of six primers were used: blaTEM, blaCTX-M, blaSHV, blaOXA-2, blaOXA-10 and blaGES as described previously1. Reactions were run under the following conditions: initial denaturation 94°C for 5 min, 33 cycles at 94°C for 35 sec, 51°C for one min, 72°C for one min and final extension at 72°C for seven min. PCR product was purified by Gene Jet PCR purification Kit (Thermo Scientific, Lithuania); 30 μl of purified products were used for sequencing along with blaGES. Efflux pump activity of the isolates was phenotypically detected by double disc synergy test4 using imipenem (10 μg), ertapenem (10 μg) and CCCP (100mM) (carbonyl cyanide m-chlorophenylhydrazone) (Hi-Media, Mumbai, India) as described earlier4. MIC reduction assay was performed using imipenem and ertapenem alone and in combination with CCCP at a concentration 20 μg/ml5. For detection of class 1 and class 2 integron, integrase gene PCR were performed as described previously6. For detection of association of gene cassette with blaESBL gene, two PCR reactions were carried out, one with HS287 and blaGES-1-B, another with HS286 and blaGES-1-B17. The amplified products were further sequenced. Transformation was carried out using Escherichia coli JM107 as recipient. Transformants were selected on Luria-Bertani (L-B) agar (Hi-Media, Mumbai, India) plates containing 0.5 mg/l cefotaxime. L-B agar with and without cefotaxime 0.5 mg/l control plate was used. Conjugation experiments were performed between clinical isolates as donors and a streptomycin resistant E. coli recipient strain B (Genei, Banglore). Overnight culture of the bacteria was diluted in L-B broth and was grown at 37 °C till the optical density (O.D.) of the reciepient and donor culture reached 0.8-0.9 at A600 absorbance. Donor and recipient cells were mixed at 1:5 donor-to-recipient ratios and transconjugants were selected on cefotaxime (0.5 mg/l) and streptomycin (800 mg/l) agar plates; 1.0 μl of each sample was used for plasmid profiling and analysed by agarose gel electrophoresis (1% agarose, Hi-Media, Mumbai, India). For the detection of incompatibility group type of plasmid in all blaGES-5 producing strains, PCR based replicon typing was carried out targeting 18 different replicon types, to perform five multiplex and three simplex PCRs to amplify the FIA, FIB, FIC, HI1, HI2, I1/Iγ, L/M, N, P, W, T, A/C, K, B/O, X, Y, F and FIIA replicons as described previously8. Typing of blaOXA-2 harbouring isolates was done by Enterobacterial repetitive intergenic consensus sequences PCR9.
When characterized genotypically, the first isolate AM 328 possessed multiple beta lactamase genes, harboured CTX-M, SHV and GES while the second isolate AM 438 harboured only single blaGES gene. Sequencing of the PCR product with the GES primers revealed that both the isolates harboured a blaGES-5 variant gene. Efflux pump mediated carbapenem resistance was also noticed in both isolates. A sharp reduction in MIC was observed against ertapenem when CCCP was added at a fixed concentration of 20 μg/ml. Class 1 integron was found in both isolates (Table). Sequencing results confirmed that blaGES-5 was class 1 integron borne. Both isolates were carrying multiple plasmids. A plasmid of ~ 40 Kb was common in both. Incompatibility typing of the first isolate showed that there was diverse Incompatibility groups Inc groups: I1/Iγ, FIA, FIB, W, FIC, FrepB and K while in the second isolate group I1/Iγ, FIA, FIB, W, FIC and FrepB were found (Table). Transformation was successful with AM-438 where it was found that blaGES-5 was located within W Inc type plasmid. However, these were not conjugatively transferable to E. coli. The first isolate was found to be susceptible to imipenem and cefoxitin and the second isolate showed suceptibility against imipenem, meropenem and gentamicin. While the first isolate showed MIC for all tested antibiotics as >256 mg/l, the second isolate showed 64 mg/l to carbapenems and monobactam and 32 mg/l to cephalosporins. ERIC PCR result showed that both isolates belonged to diverse clonal types.
The presence of GES-5 gene has been frequently detected in E. coli1011, Klesbsiella pneumoniae12 and P. aeruginosa1314.
In agreement with the current study, integron mediated GES-5 has been earlier reported from other parts of the Asia11. However, in our study, presence of blaGES-5 was traced from the community isolate which emphasized the need for epidemiological investigation, origin and evolution of this resistant determinant from this geographical location. Unlike the previous concept15, the studied isolates were phenotypically susceptible to carbapenem, whereas presence of efflux pump activity was noticed with ertapenem.
In conclusion, the presence of blaGES-5 in P. aeruginosa is perhaps the first report from this part of India. Presence of this rare type ESBL in community and their presence within plasmid require further investigation for potential transmission dynamics and proper therapeutic alternatives.
References
- Prevalence of Ambler class A and D β- lactamases among clinical isolates of Pseudomonas aeruginosa in Korea. J Antimicrob Chemother. 2005;56:122-7.
- [Google Scholar]
- Extended-spectrum beta-lactamases in Pseudomonas aeruginosa. J Antimicrob Chemother. 1998;42:128-31.
- [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 21st Informational Supplement S21. M100, Wayne, PA, USA: CLSI; 2011.
- [Google Scholar]
- Molecular epidemiology and mechanisms of carbapenem resistance in Acinetobacter baumannii endemic in New York City. Clin Infect Dis. 2003;37:214-20.
- [Google Scholar]
- Use of an efflux pump inhibitor to determine the prevalence of efflux pump-mediated fluoroquinolone resistance and multidrug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:565-70.
- [Google Scholar]
- Identification of epidemic strains of Acinetobacter baumannii by integrase gene PCR. J Clin Microbiol. 2001;39:8-13.
- [Google Scholar]
- Gene cassette PCR: Sequence-independent recovery of entire genes from environmental DNA. Appl Environ Microbiol. 2001;67:5240-6.
- [Google Scholar]
- Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63:219-28.
- [Google Scholar]
- Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acid Res. 1991;19:6823-31.
- [Google Scholar]
- Novel GES/IBC extended spectrum beta-lactamase variants with carbapenemase activity in clinical enterobacteria. FEMS Microbiol Lett. 2004;234:209-13.
- [Google Scholar]
- Emergence of Escherichia coli sequence type ST131 carrying both the blaGES-5 and blaCTX-M-15 Genes. Antimicrob Agents Chemother. 2011;55:2974-5.
- [Google Scholar]
- First outbreak of Klebsiella pneumoniae clinical isolates producing GES-5 and SHV-12 extended spectrum beta-lactamases in Korea. Antimicrob Agents Chemother. 2005;49:4809-10.
- [Google Scholar]
- A Pseudomonas aeruginosa isolate producing the GES-5 extended-spectrum beta-lactamase. J Antimicrob Chemother. 2006;57:1261-2.
- [Google Scholar]
- Detection of P. aeruginosa harboring blaCTX-M-2, blaGES-1 and blaGES-5, blaIMP-1 and blaSPM-1 causing infections in Brazilian tertiary-care hospital. BMC Infect Dis. 2012;12:176.
- [Google Scholar]





