Translate this page into:
A preliminary study of inherited thrombophilic risk factors in different clinical manifestations of venous thromboembolism in central Iran
Reprint requests: Dr Batoul Pourgheysari, Cellular and Molecular Research Center, Shahrekord University of Medical Sciences, Shahrekord, Iran e-mail: bat238@yahoo.com, pourgheysari@skums.ac.ir
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Inherited thrombophilia is known to be an important risk factor for developing venous thromboembolism. Whether such abnormalities may impact the development of deep vein thrombosis (DVT) and pulmonary embolism (PE) differently is not well defined. This preliminary study was undertaken to compare thrombophilic polymorphism in patients with DVT and PE.
Methods:
A total of 35 DVT, 23 DVT/PE, and 37 PE patients admitted to the Hajar Hospital, Shahrekord, Iran, between October 2009 and February 2011 were included in the study and 306 healthy volunteers matched by age and sex from the same geographical area with no history of venous or arterial diseases were included as control group. Factor V Leiden (FV 1691G/A, rs6025), prothrombin (FII 20210G/A), methylene tetrahydrofulate reductase (MTHFR 677C/T, rs1801133), and PLA2 polymorphisms of platelet glycoprotein IIb/IIIa (GpIIIa 1565T/C, rs5918) were investigated by polymerase chain reaction-restriction fragment length polymorphism.
Results:
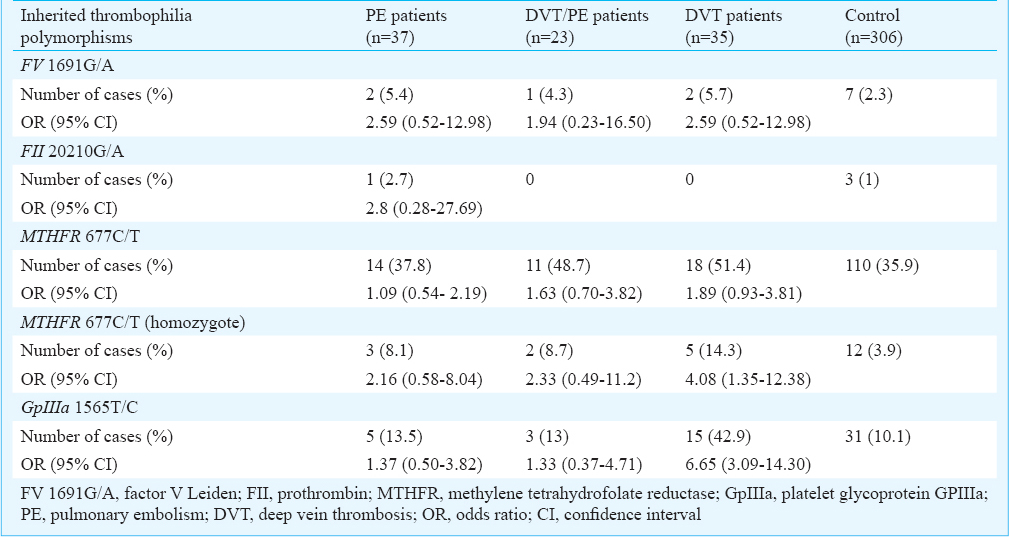
The number of patients with the investigated polymorphisms and homozygous carriers was significantly different among the groups (P<0.05). No significant difference was observed in the presence of FV 1691G/A and FII 20210G/A between any of the patients groups and the control group. GpIIIa 1565T/C and homozygous MTHFR 677C/T polymorphisms were higher in DVT patients compared with the control group (OR=6.65, 95% CI=3.09-14.30 and OR=4.08, 95% CI=1.35-12.38, respectively).
Interpretation & conclusions:
As none of the investigated polymorphisms were associated with PE, other thrombophilia polymorphisms may have a role in the pathogenesis of PE in these patients and should be investigated. Because of different prognostic risk factors among different types of patients, the treatment approach could be different.
Keywords
Deep vein thrombosis
GpIIIa 1565T/C polymorphism
pulmonary embolism
thrombophilia
thromophilia risk factors
venous thromboembolism
Venous thromboembolism (VTE) remains a serious medical condition in patients with known or unknown risk factors causing significant morbidity and mortality1. VTE manifests as deep vein thrombosis (DVT) and pulmonary embolism (PE). Thrombophilia is defined as a predisposition to increased risk of VTE. The importance of genetic thrombophilia factors in developing VTE has been increasingly recognized. Inherited gene disorders related to the haemostatic system have been documented as risk factors in different studies2345.
Factor V Leiden (FV 1691G/A, rs6025), prothrombin gene mutation G20210A (FII 20210G/A), the variant 677C/T of methylene tetrahydrofolate reductase (MTHFR 677C/T, rs1801133) and polymorphism A2 (PLA2) in platelet glycoprotein GPIIb/IIIa (GpIIIa 1565T/C, rs5918) are among main inherited risk factors. The prevalence of these polymorphisms is different according to ethnicity in normal population and VTE patients234. Whether these abnormalities may impact differently PE and DVT is not well defined, and the available data are controversial. FV 1691G/A is a mutation which makes factor V less susceptible to cleavage by activated protein C, FII 20210G/A polymorphism is a variant that leads to increased plasma prothrombin level5, 677C/T polymorphism of MTHFR leads to an elevation of homosysteine in plasma67, and GpIIIa 1565T/C polymorphism of GPIIb/IIIa increases the affinity of IIb/IIIa receptor to fibrinogen and makes the platelet more susceptible to aggregation8. Several studies demonstrated increased incidence of DVT than PE in carriers of FV 1691G/A91011, but others could not find such increased risk12. Such different clinical manifestations have not been reported for FII 20210G/A91011.
As there are limited data to compare PE and DVT in the context of thrombophilia in non-Caucasians, this preliminary study was conducted to compare thrombophilia polymorphisms in patients with PE and DVT and to evaluate whether such abnormalities impact these two types of patients in central Iran differently.
Material & Methods
Patients and study design: The study population comprised 35 DVT, 23 DVT/PE, and 37 PE patients who were admitted to the Hajar hospital, Shahrekord, Iran. The patients consecutively enrolled between October 2009 and February 2011 were included in the study. DVT was diagnosed by compression ultrasonography, Doppler ultrasonography, and D-dimer. PE was diagnosed by clinical presentation, chest X-ray, ventilation perfusion lung scan, electrocardiogram, and laboratory findings (blood gases and D-dimer). Patients entered into the study if they had one or more episodes of PE or DVT. Control group included 306 healthy volunteers (faculty and staff of the same hospital, matched by age and sex) from the same geographical area without history of venous or arterial diseases. Ethical approval for the study was obtained from Shahrekord University of Medical Sciences’ Ethics Committee. Written consent was obtained from all patients and control participants.
All patients and control participants underwent screening for investigated selected thrombophilia polymorphisms. Five ml of venous blood was collected from the anticubital vein without venous stasis. Genomic DNA was isolated from fresh blood or frozen samples. Polymerase chain reaction followed by restriction fragment length polymorphism was performed for the four polymorphisms (Thermocycler ACTEC, PC818, Japan). Primers and restriction enzymes (Tagc, Denmark and Fermentas, Russia) are shown in Table I.
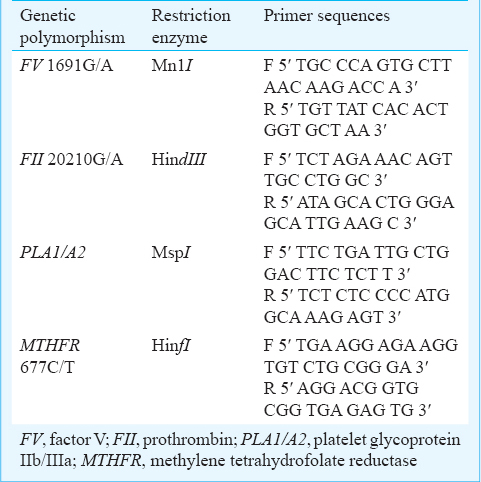
The MnI restriction enzyme digested the 267bp fragment of FV 1691G/A in to two fragments of 200bp and 67bp, whereas in wild type of FV, three fragments were produced (163, 67 and 37 bp). A 345bp fragment of prothrombin gene was amplified and digested with HindIII restriction enzyme for detection of FII 20210G/A polymorphism. There is no cleavage site in wild type whereas mutated allele produces two fragments of 322 and 23bp. A 264 bp fragment was amplified and digested to two fragments (222 and 42 bp) by MspI restriction enzyme in PLA1 polymorphism, whereas digestion of GpIIIa 1565T/C resulted in three fragments (173, 49, and 42bp). For identification of MTHFR 677C/T polymorphism a 198 bp fragment was amplified and digested by HinfI restriction enzyme in to two fragments of 175 and 23bp.
Statistical analysis: Statistical analysis was performed using SPSS (SPSS Inc., Chicago, USA). Descriptive analysis was used to describe patients’ characteristics. Odds ratio (OR) was used to describe the strength of association between genetic polymorphisms and PE or DVT. Fisher's exact test was used to make comparisons among the groups.
Results
A total of 95 patients (41 males and 54 females) with VTE entered in to the study. There was neither significant difference in the age among PE, DVT/PE, and DVT patients (53.78±18.69, 49.01±21.20, and 48.03±18.30 yr, respectively) nor between males and females. (35.1% of PE, 52.2% of DVT/PE, and 45.7% of DVT patients were male). Distal DVT was diagnosed in 31 patients and proximal DVT in 27. The most frequent transient risk factor prior to thrombosis in DVT was immobilization. In female patients with DVT, using contraceptive was the first acquired risk factor followed by immobilization. Surgery was the most frequent transient risk factor (47.4%) before PE episodes. No significant difference was seen in the family history among the three groups. Recurrent events were diagnosed in two (5.2%) of PE patients, six (26.1%) of PE/DVT, and 13 (37.1%) of DVT.
Investigated thrombophilia abnormalities: The presence of inherited thrombophilia abnormalities are shown in Table II. Overall, 22 polymorphisms were found in 37 PE patients and 15 polymorphisms in 23 DVT/PE patients compared to 35 polymorphisms in 35 DVT patients (1 per patient). The mean was 0.49 in the control group (P<0.05). Patients with DVT had more than four times of investigated genetic polymorphisms than control participants (OR: 4.52, 95% CI: 1.99-10.2). Such difference was not seen between the other two groups of patients and control group. There were more homozygous polymorphisms in DVT patients compared to PE and DVT/PE and the control group. Thirteen patients had coexistence of two polymorphisms.
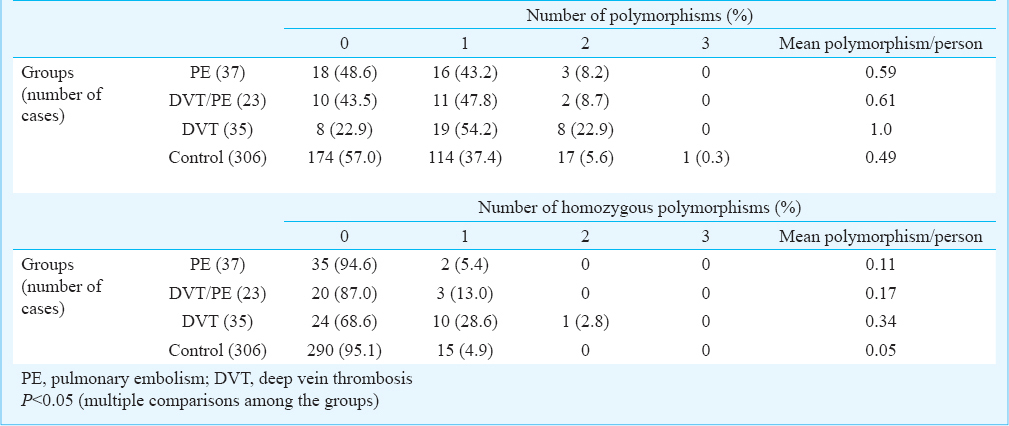
Table III compares the presence of each polymorphism among the four groups. No significant difference was found in the occurrence of FV1691GA, FII 20210G/A, and MTHFR 677C/T among the four groups, but a significant difference was seen in GpIIIa 1565T/C (P<0.001). In comparison between patients, GpIIIa 1565T/C polymorphism was found to be more than four times higher in DVT patients compared to PE and DVT/PE (OR: 4.80, 95% CI: 1.51-15.25 and OR: 5.00, 95% CI: 1.250-19.99, respectively). Such difference was not seen in other polymorphisms between PE and DVT/PE patients, PE and DVT, and DVT/PE and DVT (data not shown in Table).
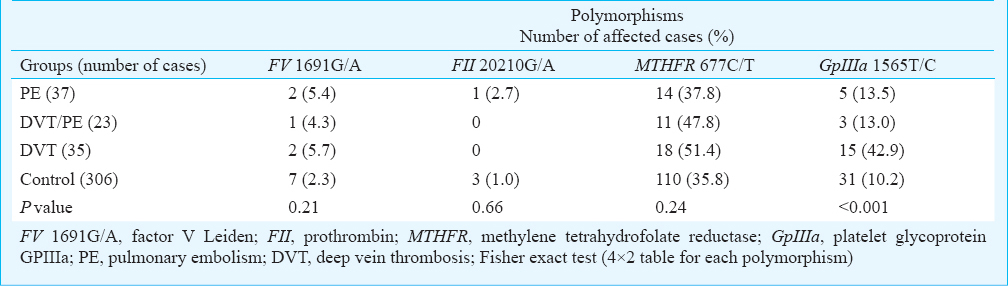
Table IV shows comparison of different polymorphisms individually between the three groups of patients and the control group. No significant difference was observed between any group of patients and the control group in the frequency of FV 1691G/A. Only one case of FII 20210G/A mutation was seen in PE patients, but not in DVT and DVT/PE, and three in the control group, with no significant difference. The frequency of MTHFR 677C/T polymorphism in patients subgroups was not significantly different from the control group. The prevalence of homozygosity of the polymorphism was significantly higher in DVT patients but not PE and DVT/PE, compared with the control group (P=0.02, 0.22, and 0.25, respectively). Of the 35 DVT patients, 15 carried GpIIIa 1565T/C polymorphism, significantly higher compared with the control group (P<0.001, OR: 6.65 and 95% CI; 3.09-14.30). No difference was seen in the frequency of this polymorphism between the other two groups and the control group.
Discussion
DVT and PE are usually considered to be similar in the context of thrombophilia. However, data on some of these polymorphisms are not consistent. In this study, we compared these two clinical manifestations of VTE and demonstrated some differences. Some of the investigated polymorphisms were associated with DVT but not with DVT/PE or PE alone. Our data demonstrated that the majority of patients showed single or multiple thrombophilic defects. Patients with DVT had significantly higher number of investigated thrombophilic polymorphisms compared with the control and the other two groups. This finding may support the hypothesis that thrombophilia leads to more stable clots which are less likely to detach and to make embolism. Previous data on the risk of PE or DVT associated with the presence of FVG1691A mutation are controversial. Rahimi et al13 have reported the association between FV1691G/A mutation and DVT in Kurdish population in western Iran. Such divergence may be related to different backgrounds of Kurdish patients and ours. Gohil et al14 reported a prevalence of 23.5 per cent in PE patients while Biswas et al15 from India reported 10.3 per cent prevalence in DVT patients, both significantly higher compared to the control group. The homozygous FV 1691G/A has been found with more severe thrombotic phenotype, but the heterozygous with lower thrombotic risk16. Grifoni et al17 found a higher prevalence in DVT compared to the control group, but not a significant difference between PE patients and the control group. The prevalence was significantly higher in DVT and PE patients in Turkey1819, but not in Chinese/Thai patients14. Although we found the frequency of the polymorphism two times higher in our DVT patients, it did not reach to a significant level, which could be related to the lower number of patients or different ethnic background. Our data did not support the hypothesis of FV1691G/A paradox20 as we did not find any association between DVT and FV 1691G/A.
Pertaining to FII 20210G/A and its incidence in patients with DVT or PE, the data are controversial and higher or similar prevalence has been reported compared to control group13142122. The increased risk of PE has been found with FII 20210G/A mutation, but decreased risk with FV 1691G/A in Italy20. No significantly difference was seen in the mutations in our patients and control groups and the frequency was lower compared with that previously reported in Caucasians and some other ethnics51014. Our data were consistent with the studies which found no relationship between DVT or PE and this polymorphism1322. The thrombophilia risk factors which were significantly associated with DVT but not PE or DVT/PE in our study were GpIIIa 1565T/C polymophism of platelet glycoprotein IIb/IIIa and homozygous MTHFR 677C/T. We have previously reported that patients with VTE have significantly higher prevalence of coinheritance of more than one polymorphism, which is reflected more clearly in MTHFR 677C/T/GpIIIa 1565T/C23. Here we found the highest frequency of MTHFR 677C/T and GpIIIa 1565T/C in our DVT patients. GpIIIa 1565T/C polymorphism increases the affinity of IIb/IIIa platelet receptor to fibrinogen and makes more aggregation as a baseline for thrombosis8. In this case the clots may be more stable and adherent to the vessel wall and are less likely to detach, resulting in PE. Our data were not in agreement with Ivanov et al22 who found GpIIIa 1565T/C polymorphism higher in PE patients than in control group. Such discrepancy may be related to the ethnicity as well as risk factors and environment which equally influence the carriers and non-carriers. The association of polymorphism with arterial thrombosis has been reported in some studies2425.
The important finding of the present study was the prevalence of homozygous MTHFR 677C/T in DVT patients. The polymorphism results in a modest increase in homocysteine in plasma which may have a pathogenic significance in thrombosis2627. Hyperhomocysteinemia may not be a direct cause of thrombosis but a marker of systemic or endothelial stress and platelet activation28.
We only studied four thrombophilia polymorphisms which could be considered as a limitation of our study. The other thrombophilia polymorphisms may have a role in PE pathogenesis. Inherited additional unknown or known risk factors may contribute to thrombosis in PE patients. In addition, limited number of patients was another limitation of our study.
In conclusion, our data indicated that FV 1691G/A and FII 20210G/A polymorphisms were not associated with PE, DVT/PE, and DVT in Central Iran. The GpIIIa 1565T/C polymorphism of GP IIb/IIIa and homozygous MTHFR 677C/T were associated with increased risk of DVT, but not PE and DVT/PE. Other thrombophilia risk factors may have a role in PE pathogenesis and should be investigated in this population. Because of different prognostic risk factors in PE and DVT, the treatment approach could be different.
Acknowledgment
This work was supported by grant no. 884 by Deputy for Research and Technology of Shahrekord University of Medical Sciences, Shahrekord, Iran.
References
- Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(Suppl 4):S495-501.
- [Google Scholar]
- Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121:458-63.
- [Google Scholar]
- Is the prevalence of the factor V Leiden mutation in patients with pulmonary embolism and deep vein thrombosis really different? Thromb Haemost. 1999;81:345-8.
- [Google Scholar]
- A common genetic variation in the 3’-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88:3698-703.
- [Google Scholar]
- Prevalence of moderate hyperhomocysteinemia in patients with early-onset venous and arterial occlusive disease. Ann Intern Med. 1995;123:747-53.
- [Google Scholar]
- No association between the common MTHFR 677C -> T polymorphism and venous thrombosis - results from the MEGA study. Arch Intern Med. 2007;167:497-501.
- [Google Scholar]
- Increased platelet aggregability associated with platelet GPIII alpha Pl(A2) polymorphism - The Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 1999;19:1142-7.
- [Google Scholar]
- Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism - pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. (Study Group for Polled - Analysis in Venous Thromboembolism) Thromb Haemost. 2001;86:809-16.
- [Google Scholar]
- Type and location of venous thromboembolism in patients with factor V Leiden or prothrombin G20210A and in those with no thrombophilia. J Thromb Haemost. 2007;5:98-101.
- [Google Scholar]
- Prevalence of factor V Leiden and prothrombin G20210A mutations in unselected patients with venous thromboembolism. Brt J Haematol. 2000;110:125-9.
- [Google Scholar]
- Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med. 2004;140:330-7.
- [Google Scholar]
- Deep venous thrombosis and thrombophilic mutations in western Iran: association with factor V Leiden. Blood Coagul Fibrinolysis. 2010;21:385-8.
- [Google Scholar]
- The genetics of venous thromboembolism A meta-analysis involving approximately 120,000 cases and 180,000 controls. Thromb Haemost. 2009;102:360-70.
- [Google Scholar]
- Factor V Leiden: Is it the chief contributor to activated protein C resistance in Asian-Indian patients with deep vein thrombosis? Clin Chim Acta. 2008;392:21-4.
- [Google Scholar]
- Inherited thrombophilia: Implications for prevention and treatment of venous thromboembolism. Semin Thromb Hemost. 2009;35:683-94.
- [Google Scholar]
- The thrombophilic pattern of different clinical manifestations of venous thromboembolism. a survey of 443 cases of venous thromboembolism. Semin Thromb Hemost. 2012;38:230-4.
- [Google Scholar]
- Thrombophilia in young patients with acute myocardial infarction. Saudi Med J. 2008;29:48-54.
- [Google Scholar]
- Genetic mutations in Turkish population with pulmonary embolism and deep benous thrombosis. Clin Appl Thromb-Hemost. 2011;17:E87-94.
- [Google Scholar]
- The risk of symptomatic pulmonary embolism due to proximal deep venous thrombosis differs in patients with different types of inherited thrombophilia. Thromb Haemost. 2008;99:1030-4.
- [Google Scholar]
- Impact of thrombophilic genetic factors on pulmonary embolism: early onset and recurrent incidences. Lung. 2008;186:27-36.
- [Google Scholar]
- PLA2 polymorphism of platelet glycoprotein IIb/IIIa but not Factor V Leiden and prothrombin G20210A polymorphisms is associated with venous thromboembolism and more recurrent events in central Iran. Blood Coagul Fibrinolysis. 2013;24:471-6.
- [Google Scholar]
- Polymorphisms of the human platelet alloantigens HPA-1, HPA-2, HPA-3, and HPA-4 in ischemic stroke. Am J Hematol. 2008;83:570-3.
- [Google Scholar]
- Hyperhomocysteinemia prevalence among patients with venous thromboembolism. Clin Appl Thromb Hemost. 2011;17:487-93.
- [Google Scholar]
- A cross-sectional study to detect the prevalence of hyperhomocysteinemia in cases of deep vein thrombosis. Indian J Surg. 2010;72:323-6.
- [Google Scholar]
- Hypothesis: hyperhomocysteinemia is an indicator of oxidant stress. Med Hypotheses. 2011;77:1088-93.
- [Google Scholar]
- The prevalence of inherited thrombophilic polymorphisms in an asymptomatic Australian antenatal population. Aust N Z J Obstet Gynaecol. 2008;48:536-41.
- [Google Scholar]






