Translate this page into:
A possible need for routine screening for Strongyloides stercoralis infection in Indian haemophilia patients
*For correspondence: shrimatishetty@yahoo.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Strongyloidiasis caused by Strongyloides stercoralis, a human pathogenic parasite, is one of the most common and globally distributed but still one of the most neglected parasitic infections of clinical importance. There are limited reports from India on the prevalence of this parasite, and most are limited to hospital-based data1234. The route of transmission may be through faecal-oral or through penetration of Strongyloides filariform larvae through skin. The most eccentric characteristic of this parasite is its ability to persist within the host and replicate for years with no or minimal symptoms such as intermittent gastrointestinal problems. However, in immunocompromised patients it has the capability of being life-threatening, leading to a spectrum of clinical complications5.
India harbours the second highest number of patients with haemophilia globally, majority belonging to the poverty-stricken section6. The haemophilia patients are known to suffer from immunosuppression due to repeated transfusion with plasma-derived products, often with corticosteroid treatment in those who are positive for inhibitors7. Routine testing of HIV, hepatitis C virus (HCV) and HBV is regularly done in these patients as their prevalence in haemophilia patients is substantially high due to multiple transfusions8. However, the prevalence of other infections in this population is less well known.
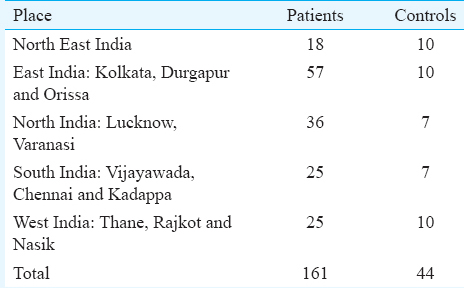
The objective of this preliminary study was to determine the percentage of haemophilia patients from different regions in India infected with S. stercoralis. Such a study would help us determine whether screening haemophilia patients for this infection should be done routinely for the better care of haemophilia patients in regions where this infection is common. Stool examinations including direct faecal smear, formalin-ethyl acetate concentration, Harada-Mori filter paper culture, and agar plate culture are usually used for routine screening of S. stercoralis infection9. However, as plasma samples were transported appropriately from different regions, we detected the S. stercoralis IgG antibodies. From December 2013 to December 2015, a total of 161 congenital haemophilia patients along with age-matched 44 controls [a few (n=16) were patient relatives not affected with haemophilia (staying in the same locality) and others were volunteers (n=28) were from the institute] were screened by an indirect ELISA for S. stercoralis IgG antibodies (Diagnostic Automation, United States) (Table I) at the ICMR-National Institute of Immunohaematology (NIIH), Mumbai, India. The study was approved by the ethics committee of the institute. A written informed consent was obtained from all patients. Of these, 139 were haemophilia A (112 were severe haemophilia and 27 were moderate) and 22 were haemophilia B. Only 12 cases were positive for inhibitor against factor VIII. Both patients and controls were ≥15 yr of age; the age range being from 15 to 48 yr, median age: 24 yr.
The coagulation tests and ELISA for S. stercoralis of all the patients were performed. The diagnosis of haemophilia was confirmed. Patients who were transfused with factor replacement products at least five days before were included in the study. Of these, patients whose prothrombin time and thrombin time were deranged were excluded. The place for sample collection for the patients of different regions varied. The patients of West India were referred to KEM Hospital, Mumbai, for treatment and sample collection was done at NIIH, Mumbai. In all the other places, haemophilia patients were called at the regional hospitals and citrated blood was collected and centrifuged and plasma samples were transported appropriately. For transport, the aliquots were stored at −20°C temporarily and transported in dry ice.
The statistical analysis was conducted using the GraphPad Software Prism 5 (GraphPad Software Inc., CA, USA). All P values were calculated with two-tailed Fisher's exact test.
Thirty three (20.4%) haemophilia patients were found to be positive for the parasitic S. stercoralis infection; the maximum percentage positivity, i.e. 25 per cent seen in patients of North India region (Table II). By the indirect ELISA, positive patients were found to have an IgG concentration ranging from 4 to 64 μg/ml plasma against Strongyloides antigen. No association was seen between Strongyloides infection and inhibitor positivity in patients, although the number of inhibitor positive samples was small. Samples with Optical density (OD) greater than 0.7 were considered strong positive and those with OD of 0.3-0.6 were considered moderate positive. Among controls, n=6 (13.6%) were found positive for the infection. When compared to controls (13.6%), overall (n=33) 20.4 per cent haemophilia patients were positive for this parasitic infection (P<0.05).

Limitations of this study were that only the ELISA test was performed and the possible cross-reactivity of S. stercoralis and Wuchereria bancrofti was not excluded along with other helminth infections, such as Ascaris lumbricoides infection, hookworm infection and schistosomiasis9. However, of the 33 positive patients, complete blood count and other details could be acquired only for 15 patients; five patients had a high eosinophil count (>600/μl) and few of them (n=8) suffered from minor gastrointestinal problems.
Strongyloidiasis is one of the most clinically important but ignored infections because of its asymptomatic behaviour. However, in conditions where immunity is suppressed, the infective larvae penetrate the mucosa, enter the bloodstream and further spread to multiple organs; sometimes leading to meningitis, sepsis and mainly hyperinfection or dissemination10. Humid and warm temperature in combination with poor sanitation and lower socio-economic status have been found associated with an increased prevalence of strongyloidiasis in the tropics11, due to faecal contamination of soil.
The prevalence of this infection in general population in other countries has been reported earlier; in Peninsular Malaysia (1.2-1.7%), Thailand (2.3-28.9%), Cambodia (10.3-21%), China (11.7%) and Japan (5-10%)12. Although our data were preliminary with a low sample size, yet a high percentage positivity for S. stercoralis infection was seen in both controls and haemophilia patients. Haemophilia patients with this infection, who already suffer from internal bleeds, joint swelling, etc., may be at risk of hyperinfection and dissemination. The treatment of strongyloidiasis if detected early before the patient is immunocompromised is not difficult. For strongyloidiasis, a single dose of ivermectin, for one or two days or two courses of albendazole administered 10 days apart, is generally prescribed13. However, in immunocompromised patients with infection who develop hyperinfection or dissemination, the treatment may require repeating doses of ivermectin. Our preliminary results indicate multi-transfused haemophilia patients may be screened by the routine stool test for this not so uncommon infection specifically in tropical countries like India. However, a more comprehensive study including the stool tests and a larger sample size is required to confirm these findings.
Financial support & sponsorship: The authors thank the Indian Council of Medical Research, New Delhi, India, for funding the study
Conflicts of Interest: None.
References
- Risk factors for acquiring Strongyloides stercoralis infection among patients attending a tertiary hospital in South India. Indian J Med Microbiol. 2011;29:147-51.
- [Google Scholar]
- Strongyloidiasis in Assam, India: A community-based study. Trop Parasitol. 2011;1:30-2.
- [Google Scholar]
- Strongyloides stercoralis: Global distribution and risk factors. PLoS Negl Trop Dis. 2013;7:e2288.
- [Google Scholar]
- Chronic diarrhoea in HIV patients: Prevalence of coccidian parasites. Indian J Med Microbiol. 2008;26:172-5.
- [Google Scholar]
- Strongyloidiasis - the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103:967-72.
- [Google Scholar]
- Epidemiology & social costs of haemophilia in India. Indian J Med Res. 2014;140:19-31.
- [Google Scholar]
- Mechanisms of transfusion-associated immunosuppression. Curr Opin Hematol. 1994;1:457-61.
- [Google Scholar]
- Intestinal strongyloidiasis and hyperinfection syndrome. Clin Mol Allergy. 2006;4:8.
- [Google Scholar]
- Intestinal strongyloidiasis: A diagnosis frequently missed in the tropics. Trans R Soc Trop Med Hyg. 2009;103:242-6.
- [Google Scholar]
- Epidemiological characteristics of strongyloidiasis in inhabitants of indigenous communities in Borneo Island, Malaysia. Korean J Parasitol. 2016;54:673-8.
- [Google Scholar]
- Manifestations, diagnosis, and treatment of Strongyloides stercoralis infection. Ann Pharmacother. 2007;41:1992-2001.
- [Google Scholar]





