Translate this page into:
A novel approach for characterizing variations in serum peptides in rheumatic heart disease
Reprint requests: Dr Yingbin Xiao, Department of Cardiovascular Surgery, Xinqiao Hospital, The Third Military Medical University, Chongqing 400 037, China e-mail: xiaoybmedsci@163.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Acute rheumatic fever and rheumatic heart disease (RHD) are important public health problems in developing countries. In this study, peptidomic analyses on RHD patients and healthy individuals were performed to characterize variations in serum peptide levels using label-free quantitation approaches.
Methods:
Blood samples were obtained from 160 healthy controls and 160 RHD patients. Of the 448 identified peptides, 272 were analyzed by two label-free mass spectrometry methods, the spectral count and spectral index.
Results:
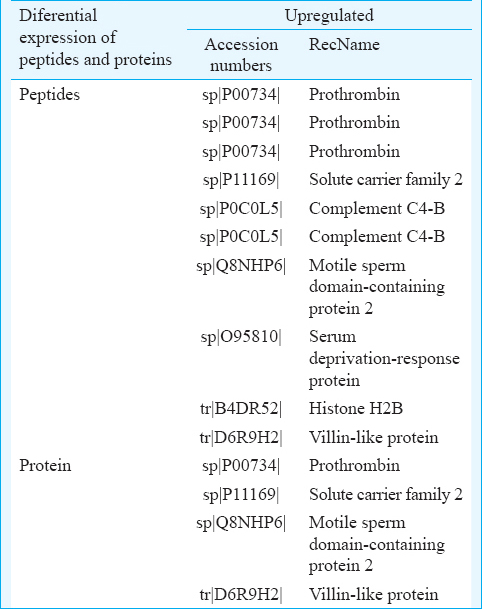
There were 38 proteins and 95 peptides with significant (adjusted P<0.001) differences in the abundance of peptides between healthy controls and RHD patients, including multiple peptides derived from histone H2B, villin-like protein, complement C4-B and motile sperm domain containing protein-2. The levels of 10 peptides were upregulated, and 85 peptides were downregulated in patients compared to controls. In addition, in patients, the levels of four proteins were upregulated and 34 were downregulated compared to controls.
Interpretation & conclusions:
This study shows that detection of significant changes in serum peptides reflects the difference between RHD patients and healthy controls. This label-free method may be helpful for clinicians to treat RHD patients during the perioperative period.
Keywords
Biological markers
peptides
peptidomics
proteins
proteomics
rheumatic heart disease
Acute rheumatic fever (RF) and chronic rheumatic heart disease (RHD) remain major causes of cardiovascular disability in school children and young adults from developing countries1. Cardiac involvement in the first attack and/or in recurrent episodes is determinant because it results in cumulative injury and permanent heart valve damage2. Rheumatic valve disease is the most severe sequela of RF which occurs in approximately 30 per cent of patients with RF3. RHD presents with varying degrees of pancarditis and is associated with valve dysfunction. Heart failure and pulmonary arterial hypertension are the most common complications of RF and RHD4. It was estimated that globally, there are 15.6-19.6 million RHD patients. Mortality of RHD in Asian countries was calculated to be 3.3 per cent/yr5. It may be helpful to diagnose and define the degree of lesions in RHD, which could be followed up with helpful advice for health or heart surgery. Doppler echocardiography is important for assessing heart valves6. Echocardiography-based surveys conducted in some developing countries showed that the prevalence of RHD was 3-10 times greater than previous assessments which were based only on clinical examination7. Therefore, a method is required to provide some assistance in diagnosing RHD and defining the grade of progression of disease.
The human body contains a broad spectrum of proteins in intracellular and extracellular compartments. The differential expression of proteins and peptides in disease has been the focus of many studies8, which aimed to understand pathogenesis of disease, and to establish therapeutic targets and relevant diagnostics markers. The biomarkers investigated for RHD include proteins involved in Streptococcus pyogenes infection9 that can be used to assist with early detection of RHD10. Peptides are short polymers of amino acids linked by amino bonds (also called peptide bonds). Polypeptides are a type of peptide with more than ten amino acids, considered to be a non-protein intermediate. Besides molecular weight, polypeptides are also different from proteins in function. Tens of thousands of polypeptides have been found in organisms and can be synthesized in all cells. Almost all cells are regulated by polypeptides which play a role in hormones, nerves, cell growth and reproduction11. Many such peptides or their parent proteins have been reported to be associated with the pathogenesis of a particular disease and/or to be useful disease markers in cases of cancer12, diabetes mellitus13, neural disease14 and collagen disease8. We have earlier reported that complement C3f des-arginine peptide, detected predominantly in the serum of patients with systemic sclerosis, enhanced proliferation of vascular endothelial cells8. Mass spectrometry (MS) is now universally used in the study of various types of body fluids, including blood15, urine16 and cerebrospinal fluid17. Gölbasý et al18 found the plasma brain natriuretic peptide (BNP) levels significantly higher in RHD than in healthy controls. There may be other peptides, which will show differences in levels between RHD patients and healthy controls.
Quantitative MS-based proteomics can be used to characterize relative peptide abundance across different conditions using either label-based or label-free methods19. Label-based methods involve distinct isotopic labelling of each sample and require extensive sample preparation and analysis. Several label-free methods have been shown to have a high correlation (0.99) to relative protein abundance20. One method involves identification and comparison of chromatographic precursor ion intensity from single-stage MS. Another method, called spectral count, uses the number of times that a peptide is identified with tandem MS (MS/MS). Another label-free alternative, a variation of spectral count, is the spectral index, which is the cumulative intensity of product ions in the MS/MS spectrum of an identified peptide. This allows comparison of three label-free approaches for a high dynamic range of application of neuropeptides and also provides a larger and distinct list of peptides20. Serum peptidome profiling is used to detect the exact mass of polypeptides in serum by MS and to process mass spectrum data for polypeptide profiling21. In this profiling, a peptidome identified by an accurate mass value can be further analyzed into an amino acid sequence by a tandem mass spectrometer. This can be used to identify precursor proteins and their biogenetic derivation. By contrasting differences in serum peptidome profiling of patients and controls, proteins or polypeptides specifically expressed in the disease state and biomarkers associated with diseases can be identified. These can be used to perform studies on proteins related to early diagnosis, classification and subtype and the onset mechanism of diseases.
The aim of this study was to identify peptide biomarkers for RHD using label-free MS/MS to identify biomarkers for future research and to evaluate the label-free MS/MS method.
Material & Methods
The instrument and software used in this study included the Eppendorf Freezing Centrifuge Vacuum Freeze Dryer (Thermo Savant, USA), the Eksigent nanoLC-Ultra™ 2D System (AB SCIEX, USA), ABI5800 matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF/TOF) (AB SCIEX), the TripleTOF 5600 System (AB SCIEX), Protein Pilot Software v. 4.0 (AB SCIEX), Peakview1.0 (AB SCIEX) and Markerview1.2 (AB SCIEX). Agents of nano-reversed phase liquid chromatography (RPLC) buffer A prepared with 0.1 per cent formic acid (FA) and 2 per cent acetonitrile (ACN), and nano-RPLC buffer B prepared with 0.1 per cent FA and 98 per cent ACN were also used. Serum peptide processing was performed in 0.2-ml polypropylene tubes (8 × 12-tube TempPlate II) from the USA Scientific (Ocala, FL, USA).
The study was undertaken at the Cardiovascular Surgery department of Xinqiao Hospital, The Third Military Medical University and Cardiovascular Surgery department of Chengdu Military General Hospital, PR China. The study participants were selected from December 2012 to December 2013; they consisted of 160 healthy controls with no known RHD and 160 consecutive eligible patients diagnosed with RHD. The two groups were matched in terms of gender (Fig. 1). Other characteristics were not significantly different in terms of age. The inclusion criterion for the RHD group was RHD diagnosis, with mitral valve damage but not aortic valve damage. The inclusion criterion for the healthy controls was healthy physical examinees who had attended the health examination centre of the hospital without RHD. Controls with diabetes, hypertension, renal failure, metabolic bone disease, liver failure, severe systemic illness, rheumatoid arthritis or any organic heart disease were excluded, and RHD patients with aortic valve damage were also excluded. Patients with features of overt heart failure were also not included according to Framingham diagnostic criteria22.
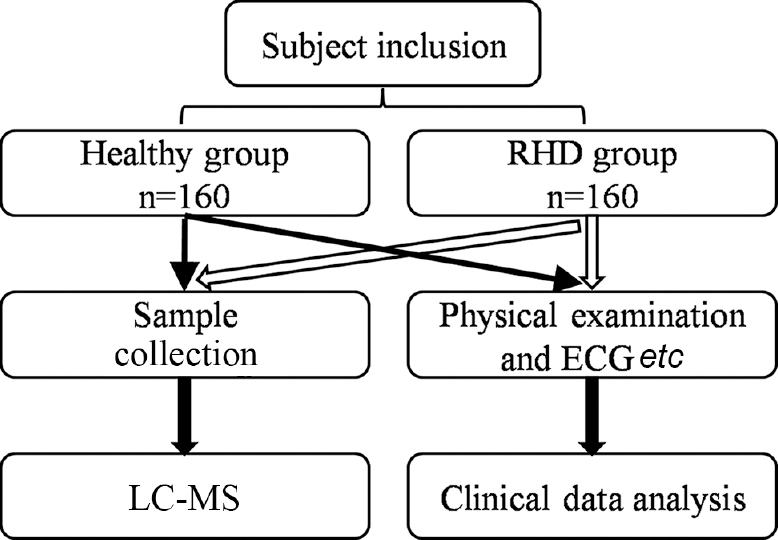
- Flow chart representing the study design and selection of participants into the healthy group and the rheumatic heart disease (RHD) groups. LC-MS, liquid chromatography- mass spectrometry.
The study protocol was approved by the ethics committee of The Third Military Medical University for biomedical research. Written informed consent was obtained from all participants.
Blood samples (3 ml) from 160 healthy controls and 160 patients diagnosed with RHD (before any corrective operation) were collected under standard clinical protocol23. Blood samples were collected in BD Vacutainer SST™ II Advance tubes (3.5 ml) (Ref number: 367957; BD Plymouth, UK) and were allowed to clot at room temperature for 1 h. The samples were centrifuged at 1500-2000×g for 15 min at room temperature. The serum was transferred to four 1-ml cryovials (Thermo Scientific, USA), with 0.5 ml serum in each, and stored at -80°C until further use. All patients’ clinical examinations were performed by attending physicians. Systolic and diastolic blood pressures were measured using standard cuff equipment in the hospital, along with pulse rate.
Echocardiography and diagnosis of rheumatic heart disease (RHD): Two-dimensional transthoracic echocardiography with targeted M-mode and Doppler were performed. The echocardiographic diagnostic criteria of RHD in this study were based on the 2012 World Heart Federation criteria24. All measurements were made by observers who were blinded to the study grouping, and average of the three readings were recorded.
Protein extraction and quantization: The sample solution was centrifuged at 15,000×g for 15 min at room temperature. The supernatant was collected and centrifuged again. The supernatant was put through a 10 kDa ultrafiltration tube at 15,000×g for 20 min to enrich the peptide. The sample was cleaned up using a ZipTip (Sigma-Aldrich, USA) and the sample was examined by ABI5800 MALDI-TOF/TOF analysis. The sample was dried in a vacuum freeze dryer and stored at -80°C for further analysis.
Reversed phase liquid chromatography (RPLC)-mass spectrometry (MS)/mass spectrometry (MS) analysis: Samples were re-suspended with nano-RPLC buffer A. The online nano-RPLC was used in the Eksigent nanoLC-Ultra™ 2D System. Samples were loaded on a C18 nanoLC trap column (AB SCIEX, USA, 100 μm × 3 cm, C18, 3 μm, 150 Å) and washed by nano-RPLC buffer A (0.1% FA, 2% ACN) at 2 μl/min for 10 min. An elution gradient of 5-35 per cent ACN (0.1% FA) in a 70-min gradient was used on an analytical ChromXP C18 column (75 μm × 15 cm, C18, 3 μm, 120 Å) with a spray tip. Data acquisition was performed with a Triple TOF 5600 System (AB SCIEX) fitted with a Nanospray III source (AB SCIEX) and a pulled quartz tip as the emitter (New Objectives, USA). Data were acquired using an ion spray voltage of 2.5 kV, curtain gas of 30 PSI, nebulizer gas of 5 PSI and an interface heater temperature of 150°C. For information-dependent acquisition, survey scans were acquired at 250 msec, and as many as 35 product ion scans were collected if they exceeded a threshold of 150 counts per second, with a 2+ to 5+ charge-state. The total cycle time was fixed to 2.5 sec. A rolling collision energy setting was applied to all precursor ions for collision-induced dissociation. Dynamic exclusion was set to one-half of the peak width (18 sec). The precursor was refreshed off the exclusion list.
Protein/peptide identification and quantification: Data were processed by the Paragon algorithm with Protein Pilot Software v. 4.0 (AB SCIEX) based on a human database25. Protein identification was performed with the search option: emphasis on biological modifications. The database search parameters were as follows: the instrument was a TripleTOF 5600, cysteine was modified with iodoacetamide, and biological modifications were selected as the identity focus, with no digestion. An automatic decoy database search strategy was used to estimate the false discovery rate using the Proteomics System Performance Evaluation Pipeline Software (PSPEP) integrated in the Protein Pilot Software (AB SCIEX). The false discovery rate was calculated as the false positive matches divided by the total matches. The raw peak areas, as reported by PeakView, were used for peptide quantification. In addition, MarkerView was used for differently expressed peptide/protein analysis.
Statistical analysis: The spectral count data on each peptide identified in both samples were analyzed using a Poisson model. In addition, MarkerView was used for differently-expressed peptide/protein analysing. These analyses were conducted using SAS (Cary, NC, USA). The Student's t test was applied to investigate the differences in serum peptide levels between healthy controls (n=160) and those with RHD (n=160).
Results
A total of 160 controls and 160 patients with well-defined clinical features of RHD were included in the study (Table I). To increase the probability of identifying useful biomarkers of RHD, an analytical strategy was applied that used the LC-MS elution profiles of individual peptide ions that had been detected previously in liquid chromatography with tandem MS (LC-MS/MS) experiments. PSPEP software was used for quantification of this analysis. This was a targeted quantification strategy because only those ions were quantified (by LC-MS) that had been detected previously (although not necessarily identified) in serum by data-dependent LC-MS/MS. To generate a list of quantifiable serum peptides, undigested serum peptides were pooled from the same patient group and analyzed by LC-MS/MS. These analyses were performed in triplicate, and in each replicate LC-MS/MS experiment, a list of identified peptides was generated. Approximately, the same numbers of MS/MS spectra were obtained per sample group.
As shown in Figs. 2 and 3, there were 38 proteins and 95 peptides with a significant (adjusted P<0.001) difference in abundance between healthy controls and patients, including multiple peptides which derived from histone H2B, villin-like protein, complement C4-B and motile sperm domain with protein-2. The levels of 10 peptides and four proteins were upregulated in RHD patients (Table II). The levels of 85 peptides and 34 proteins were downregulated. This analysis identified 95 candidate peptide biomarkers and 38 candidate protein biomarkers (P<0.01 and a fold change of >1.5 or <0.67). Because the criteria of the t test were variable for the purpose of candidate selection, the threshold (P<0.01 and a fold change of >1.5 or <0.67) was used to define the highest priority group.
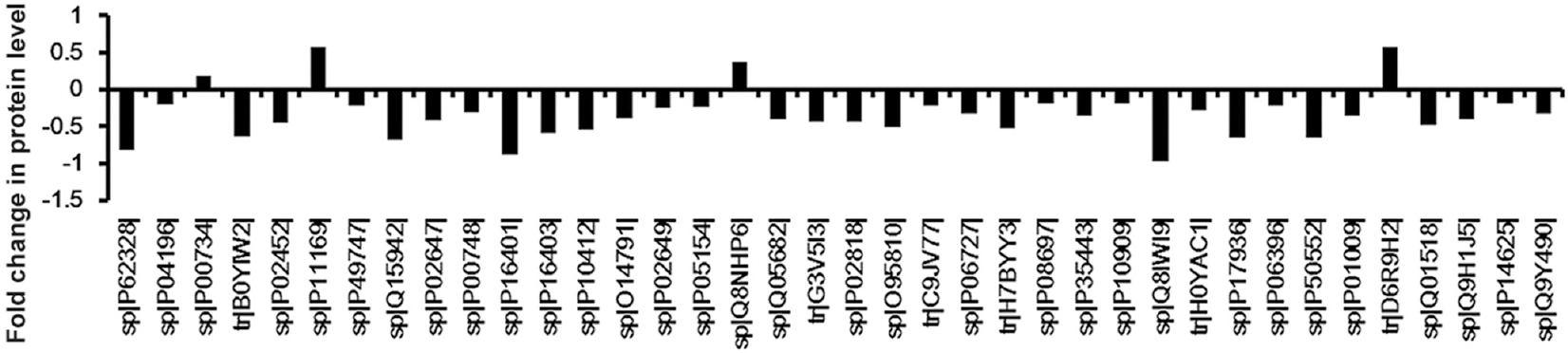
- Graphical representation of the different protein levels in serum between the rheumatic heart disease patients and healthy controls. There were 38 proteins with a significant (adjusted P<0.001) difference between healthy controls and rheumatic heart disease patients. The serum levels of four proteins were upregulated and 34 were downregulated. The number on the y axis represents the fold change in protein level. The proteins are listed according to their SWISS-PROT accession number.
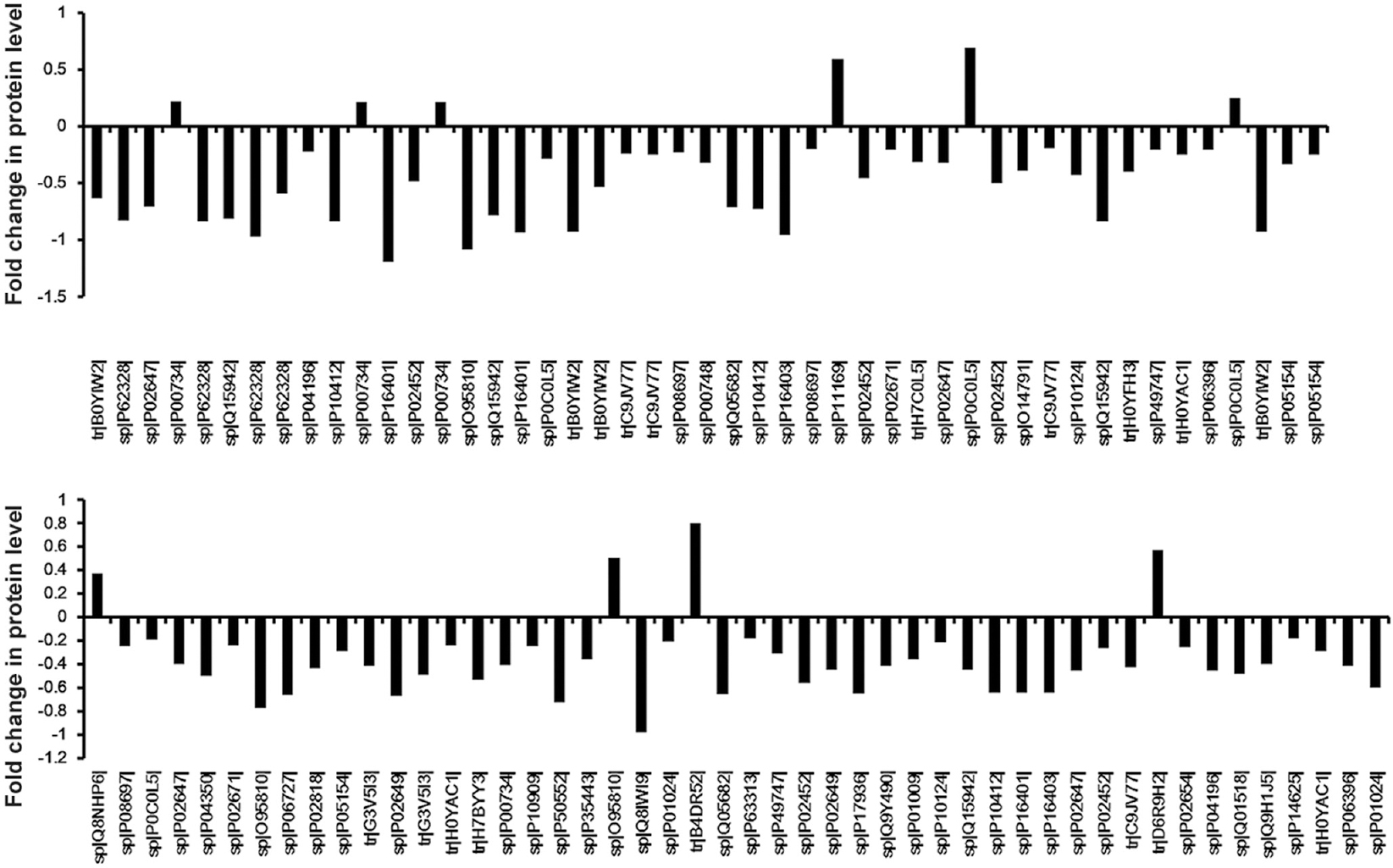
- Graphical representation of the different peptide levels in serum between the rheumatic heart disease patients and healthy controls. There were 95 peptides with a significant (adjusted P<0.001) difference in abundance of peptide between healthy controls and rheumatic heart disease patients. The serum levels of 10 peptides were upregulated and 85 peptides were downregulated. The number on the y axis represents the fold change in peptide level. The peptides are listed according to their SWISS-PROT accession number.
Discussion
The aim of this study was to identify a list of potential peptide diagnostic markers for RHD by label-free MS/MS methods. The results showed that 38 proteins and 95 peptides had significantly different levels in RHD patients compared to healthy controls.
Although proteomics techniques have helped to improve the understanding of molecular cell biology26, the impact of proteomics in clinical practice has not yet reached initial expectations due to some technological limitations16. The combination methods, such as novel LC-MS techniques for quantitative proteomics, may prove beneficial for biomarkers identification and validation27. Online LC-ESI-MS due to the nature of the initial LC separation step can help reducing the amount of analytes, thereby reducing the possibility of ion suppression, because ion formation by electrospray ionization is proportional to analyte concentration28. Ideal strategies to derive quantitative information from LC-MS experiments are based on differential sTable isotope labelling of proteins or peptides, which are mixed and quantified relative to each other in single multidimensional LC-LC-MS experiments. However, this technique is not ideal for biomarker discovery because of problems associated with protein derivatization in the clinical setting and because of its limited throughput. In addition, isotope labelling techniques make it difficult to compare a large number of specimens. Labelling reagents can be used to compare up to eight protein samples29.
Novel analytical strategies for quantitative proteomics, without the need for isotope labelling, can quantify polypeptides with the same precision and accuracy as those based on isotope labeling30. In addition, such label-free quantitative LC-MS approaches can compare an unlimited number of samples. Therefore, these are ideal for biomarker discovery because experimental designs normally involve comparing a large number of specimens to validate the results.
In this study, protocols for peptidomic analysis of serum peptides were described. These included (i) collection of peripheral blood from patients, (ii) separation of serum from the blood samples, (iii) purification of peptides from the serum samples, (iv) detection of individual peptides, (v) pattern recognition and clustering, and (vi) sequence identification of the peptides of interest. The large number of proteins and peptides identified in this study provided important information for the identification of RHD biomarkers that would need to be further evaluated by other studies. Biological and pathological functions of the identified peptides and their usefulness as RHD biomarkers can be elucidated using synthetic peptides and their parent proteins.
Of the 38 proteins and 95 peptides identified in this study, several biomarkers have been identified, including multiple peptides derived from histone H2B and complement C4-B both of which are known to be correlated with RHD. While those that have until now not been identified as related to RHD suggest important directions for future research to elucidate the mechanism of cardiac dysfunction in RHD. In addition, this list should provide biomarkers to determine levels of heart failure and RHD progression, and to assist in the identification of useful markers in the future.
This study had some limitations. This was a single centre study, with a relatively small sample size, and these results should be considered as a preliminary screening because the correlations between the biomarker and the disease were not studied. The patients screened for the biomarkers were patients undergoing treatment for RHD. Newly diagnosed treatment-naive patients could not be included because of the limited numbers available at the time of study. Serum was selected rather than plasma as the fluid for investigation; this was to avoid complications from hypercoagulability caused by diseases such as nephrotic syndrome, chronic heart disease, chronic cor pulmonale, ischaemic cerebrovascular disease and diabetes mellitus. However, as RHD is a hypercoagulable (prothrombotic) state, coagulation proteins and peptides may hold immense importance for biomarker discovery and should be considered in future studies.
In conclusion, using a novel label-free method, 38 proteins and 95 peptides were identified that were present at different levels in the serum of RHD patients compared to healthy controls. These markers may assist in the discovery of more valuable peptides which may become biomarkers for disease severity and provide methods of treating RHD patients during the perioperative period.
Acknowledgment
Authors acknowledge the funding support received from National Science Foundation (No. 2011BAI11B18) China.
Conflicts of Interest: None.
References
- Global research priorities in rheumatic fever and rheumatic heart disease. Ann Pediatr Cardiol. 2011;4:4-12.
- [Google Scholar]
- Mitral valve repair and replacement for rheumatic disease. J Thorac Cardiovasc Surg. 2000;119:53-60.
- [Google Scholar]
- Cardiovascular complications in newly diagnosed rheumatic heart disease patients at Mulago Hospital, Uganda. Cardiovasc J Afr. 2013;24:80-5.
- [Google Scholar]
- The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685-94.
- [Google Scholar]
- Rheumatic fever and rheumatic heart disease: Report of a WHO expert consultation on rheumatic fever and rheumatic heart disease. World Health Organization 2004
- [Google Scholar]
- Congenital heart disease and rheumatic heart disease in Africa: Recent advances and current priorities. Heart. 2013;99:1554-61.
- [Google Scholar]
- Comprehensive investigation of disease-specific short peptides in sera from patients with systemic sclerosis: Complement C3f-des-arginine, detected predominantly in systemic sclerosis sera, enhances proliferation of vascular endothelial cells. Arthritis Rheum. 2007;56:2018-30.
- [Google Scholar]
- speB gene as a specific genetic marker for early detection of rheumatic heart disease in human. Cell Mol Biol (Noisy-le-grand). 2012;58:50-4.
- [Google Scholar]
- mga genosensor for early detection of human rheumatic heart disease. Appl Biochem Biotechnol. 2014;173:228-38.
- [Google Scholar]
- Investigating endogenous peptides and peptidases using peptidomics. Biochemistry. 2011;50:7447-61.
- [Google Scholar]
- Serum peptidome patterns that distinguish metastatic thyroid carcinoma from cancer-free controls are unbiased by gender and age. Mol Cell Proteomics. 2006;5:1840-52.
- [Google Scholar]
- Neuropeptides of the islets of Langerhans: A peptidomics study. Gen Comp Endocrinol. 2007;152:231-41.
- [Google Scholar]
- Identification of novel biomarker candidates by differential peptidomics analysis of cerebrospinal fluid in Alzheimer's disease. Comb Chem High Throughput Screen. 2005;8:801-6.
- [Google Scholar]
- Prerequisites for peptidomic analysis of blood samples: I. Evaluation of blood specimen qualities and determination of technical performance characteristics. Comb Chem High Throughput Screen. 2005;8:725-33.
- [Google Scholar]
- Clinical urinary peptidomics: Learning to walk before we can run. Clin Chem. 2007;53:375-6.
- [Google Scholar]
- Plasma brain natriuretic peptide levels in patients with rheumatic heart disease. Eur J Heart Fail. 2004;6:757-60.
- [Google Scholar]
- Less label, more free: Approaches in label-free quantitative mass spectrometry. Proteomics. 2011;11:535-53.
- [Google Scholar]
- Mass spectrometry-based label-free quantitative proteomics. J Biomed Biotechnol 2010. 2010;1:840518.
- [Google Scholar]
- The natural history of congestive heart failure: The Framingham study. N Engl J Med. 1971;285:1441-6.
- [Google Scholar]
- Correcting common errors in identifying cancer-specific serum peptide signatures. J Proteome Res. 2005;4:1060-72.
- [Google Scholar]
- World heart federation criteria for echocardiographic diagnosis of rheumatic heart disease – An evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309.
- [Google Scholar]
- The Paragon algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics. 2007;6:1638-55.
- [Google Scholar]
- Advances and challenges in liquid chromatography-mass spectrometry-based proteomics profiling for clinical applications. Mol Cell Proteomics. 2006;5:1727-44.
- [Google Scholar]
- Ultra-sensitive and quantitative characterization of proteomes. Mol Biosyst. 2006;2:221-30.
- [Google Scholar]
- Quantitative shotgun proteomics of enriched heterocysts from Nostoc sp. PCC 7120 using 8-plex isobaric peptide tags. J Proteome Res. 2008;7:1615-28.
- [Google Scholar]
- The Association of Biomolecular Resource Facilities Proteomics Research Group 2006 study: Relative protein quantitation. Mol Cell Proteomics. 2007;6:1291-8.
- [Google Scholar]






