Translate this page into:
A non-inferiority randomized controlled clinical trial comparing Unani formulation & psoralen plus ultraviolet A sol in chronic plaque psoriasis
For correspondence: Dr Neena Khanna, Department of Dermatology & Venereology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110 029, India e-mail: neena_aiims@yahoo.co.in
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Though Unani medications have been used for centuries to treat psoriasis, there is paucity of published studies which have systematically evaluated their efficacy and safety. This study was conducted to establish non-inferiority of Unani medications (oral UNIM-401 and topical UNIM-403) vs psoralen plus ultraviolet A (PUVA) sol in treatment of moderate-severe chronic plaque psoriasis (CPP) in achieving psoriasis area severity index (PASI) 75 at 12 wk and to estimate proportion of patients who relapsed in follow up period of 12 weeks, after having achieved PASI 50.
Methods:
In this randomized, controlled trial patients with CPP were block randomized to receive either Unani treatment (147 patients) or PUVA sol (140 patients) for 12 weeks. Percentage reduction in PASI was determined in each patient at 12 wk to calculate number of patients who achieved PASI 75 as also to estimate median of percentage reduction in PASI in each group. All patients who achieved PASI 50 at 12 weeks were followed up for another 12 wk to determine proportion of patients who relapsed.
Results:
Of the 287 patients randomized, 84 of 147 in Unani group and 67 of 140 in PUVA sol group completed 12 weeks of treatment. On intention-to-treat (ITT) analysis, the response in patients on Unani medication was not inferior to those receiving PUVA sol, in attaining PASI 75 (16.3% in Unani group vs 15.7% in the PUVA sol group). Median of percentage reduction of PASI at 12 wk from baseline in Unani group (68.2%; −60, 100) and PUVA sol group (63%; −15.7, 100) was comparable. Proportion of patients who relapsed at 24 wk was comparable in both groups. However, frequency of clinical side effects was significantly higher (P =0.001) in PUVA sol group (16.4%) compared to Unani group (2%).
Interpretation & conclusions:
The findings of the present study indicated that oral UNIM-401 and topical UNIM-403 were effective and well tolerated therapeutic options in patients with moderate-severe CPP.
Keywords
Psoriasis
plus ultraviolet A sol
safety
side effects
Unani medication
Unani medicine, which is based on treating the disease depending on the patient's temperament1, is an ancient Greko-Arabic system of medicine which was introduced in India during the Mughal era. Unani preparations have been used to successfully treat a host of chronic dermatoses including psoriasis and eczemas. The preparations available for treating psoriasis include those containing Psoralea corylifolia, Mexican sarsaparilla, Santalum album, Plumbum oxidum, Fumaria parviflora, Swertia chirata, Azadirachta indica and Cinnamomum camphora2. These have been used either singly but more often in combination. Now, several of these are available as ‘ready-to-use combinations' which have been prepared using standard operating principles, thus circumventing the need for laborious extemporaneous preparation. One such frequently used oral preparation in the treatment of psoriasis is UNIM-401 which is a coded Unani formulation containing a combination of F. parviflora (Barg-e-shahtra), S. chirata (Charata talegh), P. corylifolia (Babchi) and Terminalia chebula (Halela siyah) in the ratio of 5:5:5:2 formulated as dried aqueous extract. Similarly, a frequently used topical formulation is UNIM-403 which contains inner part of bark of A. indica (neem) and C. camphora (camphor, kafoor) dispensed in sesame oil3. In a multicentric preliminary clinical study, their efficacy ranged from 60 to 68 per cent3. In another study, in a per-protocol analysis of 53 of 109 patients with psoriasis who completed the study, about 60 per cent of the patients had >60 per cent response4. However, in neither of the studies was there a mention of the inclusion and exclusion criteria and whether the outcome measures had been predefined.
In contrast, in allopathic system of medicine, there are several validated options available for treating moderate-to-severe chronic plaque psoriasis (CPP). Phototherapy and photochemotherapy using 8-methoxypsoralen followed by exposure to UVA [psoralen plus ultraviolet A (PUVA)] are frequently used therapeutic options5. Although for many decades, systemic PUVA has been used as an effective therapy in extensive plaque psoriasis, yet its use is limited by side effects, predominantly upper gastrointestinal6. Bath PUVA was devised to circumvent the side effects of systemic PUVA, and the response to bath PUVA in CPP has been comparable to that of systemic PUVA7. In resource-limited settings, especially in geographic areas where sunlight is abundant throughout the year, PUVA sol (where the sun is used as a source of UVA) is a frequently used alternative in CPP, having been found to be twice as cost-effective as PUVA8.
There is a paucity of clinical evidence on the efficacy and safety of UNIM-401 orally and UNIM-403 topically, thus this study was conducted as a randomized controlled clinical study to establish their non-inferiority to PUVA sol in the treatment of moderate-to-severe CPP.
Material & Methods
This non-inferiority randomized, active-controlled, clinical trial was initiated only after the safety of UNIM-401 systemically and UNIM-403 topically was established in animal toxicological studies in the department of Pharmacology, All India Institute of Medical Sciences (AIIMS), New Delhi (unpublished data), and after getting approval from Institute's Ethics Committee (IEC/NP-223/2010) and registration in Clinical Trial Registry of India (2010/091/001253). Patients clinically diagnosed as CPP attending the outpatient department of Dermatology, AIIMS, New Delhi, India, between January 2011 and October 2014, were screened for the study. Of the 560 patients screened during this period, 287 patients older than 18 yr of age with moderate-to-severe CPP involving body surface area (BSA) of ≥10 per cent or a psoriasis area severity index (PASI) of ≥10 were included after obtaining informed written consent. Patients younger than 18 yr of age, those with erythrodermic/pustular psoriasis, pregnant and lactating women, patients with previous intolerance to photochemotherapy, those with photosensitivity or photoaggravated psoriasis, those with renal, hepatic, or severe cardiac comorbidities and participants with personal or family history of premalignant or malignant skin tumours were excluded from the study. Patients who were unsure of complying with the treatment protocol, those who did not have a suitable place for sun exposure and those who had received systemic therapy for psoriasis in previous eight weeks were also excluded.
The study consisted of two phases, an initial 12 wk phase of treatment to evaluate the response to therapy and a follow up phase of 12 wk to determine the relapse rate. A detailed history, physical examination (including calculation of BSA involved with psoriasis and PASI) and baseline investigations were done for all patients. The patients were allocated to two treatment groups with block randomization (block size of 16) using numbered sealed envelopes, procured from the department of Biostatistics, AIIMS.
Unani treatment group (study group): Patients were given UNIM-401 orally in the dose of two capsules of 500 mg twice a day. In addition, patients were advised to apply UNIM-403 oil on the lesions once a day, followed two hours later by exposure to sunlight, starting from five minutes, gradually increasing by five minutes every exposure, up to a maximum of 30 min.
Psoralen plus ultraviolet A (PUVA) sol group (active control group): Patients were given 8-methoxsalen in a dose of 0.6 mg/kg on alternate days as a single dose after breakfast, followed two hours later by application of petrolatum and sun exposure, starting from five minutes, gradually increasing by five minutes every exposure, up to a maximum of 30 min or development of trace of erythema, whichever was reached earlier. All patients were also advised to apply petrolatum at night.
In the treatment phase, response to treatment was assessed at weeks 4, 8 and 12 using PASI (taking mean of PASI estimated by two clinicians, blinded to the therapeutic regimen). Endpoint of treatment was taken as either complete clearance of psoriasis or completion of 12 wk of treatment, whichever was earlier. Patients were considered to be responding to treatment if there was ≥25 per cent reduction in their PASI at eight weeks. Patients were withdrawn from the study (and put on alternate therapy) if there was <25 per cent improvement in PASI, or increase in PASI at eight weeks of therapy (when they were designated as treatment failure) or if they developed unacceptable clinical or investigational adverse effects at any time of the study. Patients were also withdrawn if they missed eight consecutive days of Unani medication or four consecutive exposures of PUVA sol (when they were designated as protocol deviation).
In the follow up phase (weeks 12-24), all the patients who had achieved ≥50 per cent reduction in PASI at week 12 (as compared to baseline) were advised to use petrolatum twice a day and were assessed using PASI score at weeks 16, 20 and 24 to determine the number of patients who relapsed. Relapse in these patients was defined as an increase of ≥50 per cent in the PASI achieved at week 12.
Statistical analysis: The sample size calculation was based on an earlier study8, in which 46 per cent of patients of CPP treated with PUVA sol achieved PASI 75. Hence, in the present study, to conclude non-inferiority of Unani medication (PASI >75 = 44%) vis-a-vis PUVA sol in achieving PASI 75 at 12 wk, taking a difference of <20 per cent in response rate between the treatment groups, with assumed power and alpha of 90 and 5 per cent, respectively, a sample size of 131 patients per group was calculated. Taking into consideration a 10 per cent loss to follow up, 144 patients were randomized per group.
Statistical analysis was carried out using Stata 12.0 (College Station, Texas, USA). Data were presented as number (%), mean±standard deviation (SD) or as median (range) wherever appropriate. Baseline categorical and continuous variables were compared between the groups using Chi-square/Fisher's exact test and Student's t test for independent samples/Wilcoxon rank-sum test, respectively. The clinical side effects were compared between the two groups using Fisher's exact test.
Intention-to-treat (ITT) and per-protocol (PP) analysis were performed to determine the non-inferiority of Unani medication vis-a-vis PUVA sol. The primary outcome was a reduction of ≥75 per cent in PASI from baseline to 12 week. The lower limit of 95 per cent confidence interval (CI) of ≥20 per cent indicated that the Unani intervention was non-inferior to the standard therapy. The results were also reported as relative risk (95% CI).
Results
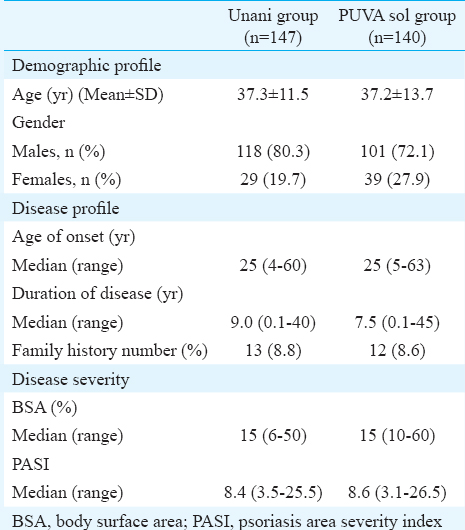
A total of 560 patients with CPP were screened between January 2011 and October 2014, and of these, 287 were randomized with 147 patients receiving Unani treatment and 140 receiving PUVA sol (Fig. 1). The demographic and disease profile of the patients was comparable in the two groups (Table I).
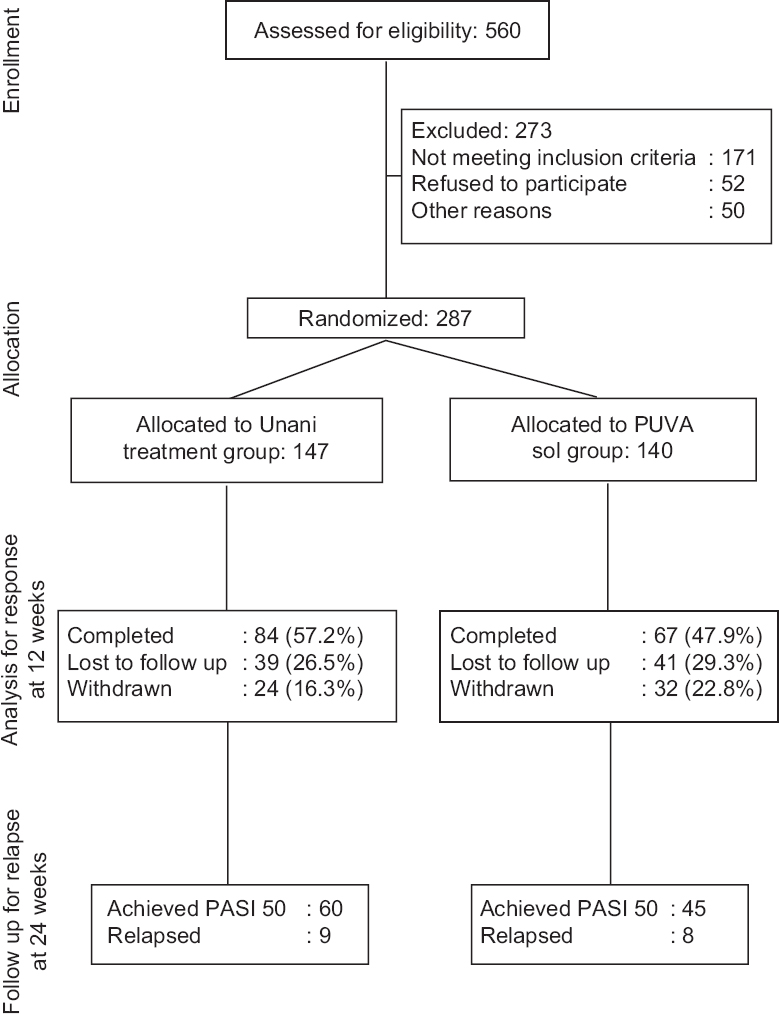
- Consort flow chart of recruitment and allocation of patients to Unani treatment and psoralen plus ultraviolet A sol groups.
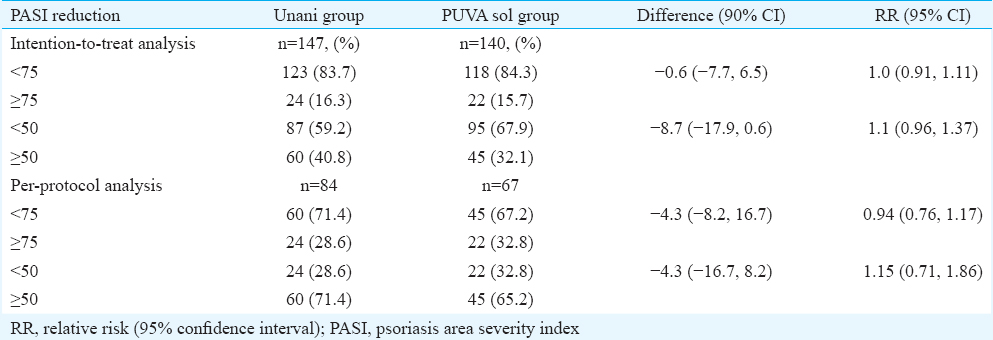
ITT analysis was done to determine the non-inferiority of Unani therapy to PUVA sol, based on the number of patients who attained PASI 75 and PASI 50 at 12 wk in the two groups (Table II), with the primary outcome measure being attainment of PASI 75 or more (Figs. 2A, B and 3A, B). Twenty four (16.3%) patients in Unani treatment group and 22 (15.7%) in the PUVA sol group attained PASI 75 at 12 wk, giving a difference of –0.6 (–7.7, 6.5) with the lower confidence interval being –7.7 per cent, which was greater than the predefined non-inferiority margin (Δ of –20%), implying that the response in attaining PASI 75 at 12 wk in Unani treatment group was non-inferior to PUVA sol group (Table II). Similarly, the Unani medications were non-inferior to PUVA sol in achieving PASI 50 at 12 wk (Table II). In PP analysis, the Unani medications were non-inferior to PUVA sol in achieving PASI 75 and PASI 50 at 12 wk (Table II).
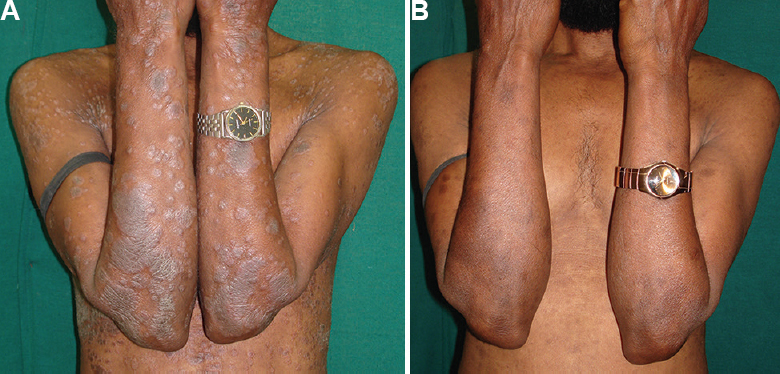
- (A) Patient with psoriasis area severity index (PASI) of 7.5 randomized to Unani group at baseline, (B) Response to Unani treatment at 12 wk.
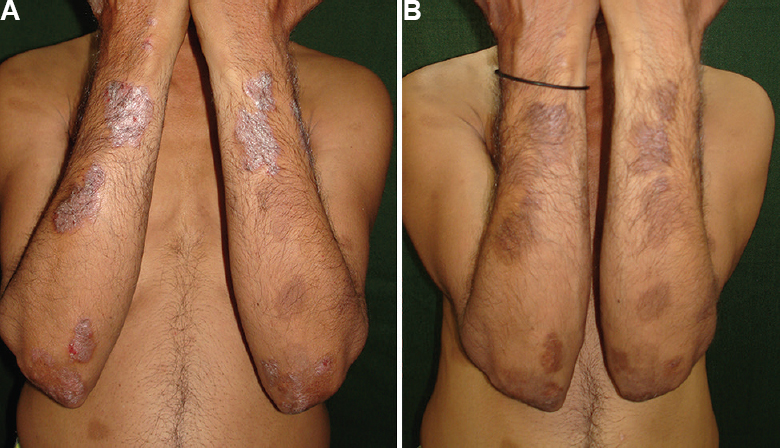
- (A) Patient with psoriasis area severity index (PASI) of 10.3 randomized to psoralen plus ultraviolet A sol group at baseline, (B) Response to psoralen plus ultraviolet A sol treatment at 12 wk.
The median of percentage reduction of PASI at 12 wk (Fig. 4) from baseline in the Unani treatment group (68.2%; –60, 100) and PUVA sol group (63%; –15.7, 100) was not significantly different. Similarly, the median of percentage reduction of PASI at four and eight weeks in the Unani treatment group was not significantly different to that in the PUVA sol group.
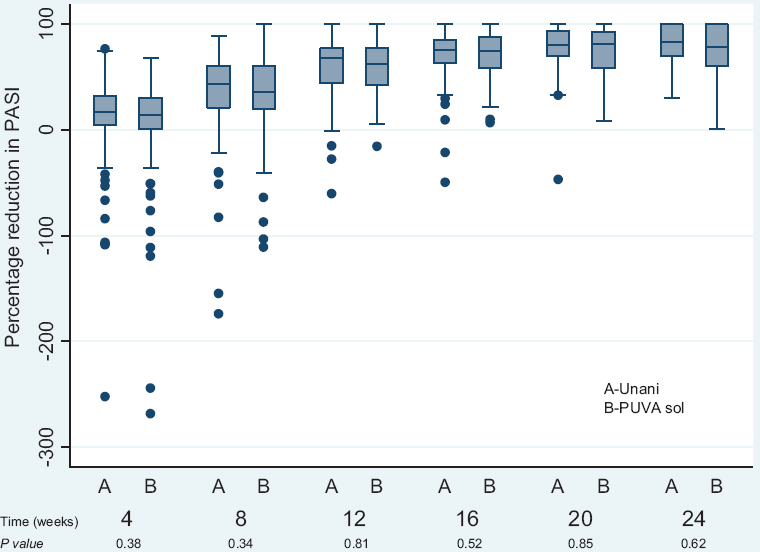
- Box plot showing percentage reduction from baseline in psoriasis area severity index (PASI) at 4, 8, 12, 16, 20 and 24 wk in Unani treatment group versus psoralen plus ultraviolet A (PUVA) sol group.
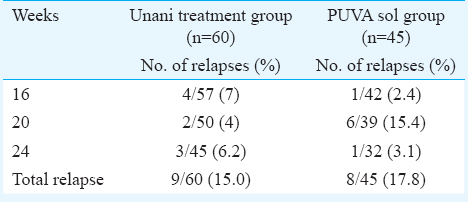
Of the patients who completed 12 wk of therapy, 60 of 84 (71.4%) in the Unani treatment group and 45 of 67 (65.2%) in PUVA sol group achieved PASI 50, and these patients were followed up to determine the proportion of patients who relapsed at 24 wk (Table III). The relapses in Unani treatment group (9; 15%) and in PUVA sol group (8; 17.8%) were comparable. Even after stopping therapy and despite relapses, there was a reduction in PASI from 12 to 24 wk both in Unani treatment group (from 68.2%; –60, 100 at 12 wk to 87.7%; 30.3, 100 at 24 wk) and in PUVA sol group (from 63%; –15.7, 100 at 12 wk to 86.3%; 1.2, 100 at 24 wk). Moreover, the difference in the median of percentage reduction at each time point between the Unani treatment groups and PUVA sol group was not significant (Fig. 4).
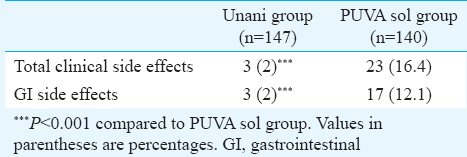
The total number of patients who developed clinical side effects was much higher in the PUVA sol group (23; 16.4%) than in the Unani treatment group (3; 2%), and this difference was significant (P=0.001). Seventeen (12.1%) patients on PUVA sol developed gastrointestinal side effects vis-a-vis three (2%) in Unani treatment group Table IV. The other adverse effects observed in patients in PUVA sol group included phototoxicity (1.4%), headache (1.4%) and palpitation (0.7%). None of the patients in Unani treatment group experienced any such side effects. There were transient and mild alterations in haematological and biochemical parameters in 19 patients (Unani treatment group: 9 and PUVA sol group: 10), but these did not necessitate withdrawal of treatment.
Discussion
Unani formulations have been used for centuries in treatment of several skin diseases including psoriasis29. However, most of these studies are based on personal experiences, anecdotal reports, and case series using subjective/qualitative outcome measures such as ‘good/excellent/significant' response. There is a paucity of systematic studies evaluating the efficacy of Unani medications in psoriasis with clearly defined objectives, inclusion and exclusion criteria and predetermined outcome parameters.
In Allopathic system of medicine, there are several options available for treating moderate-to-severe psoriasis. Of these, PUVA (as bath or as systemic PUVA)10 and PUVA sol have been used successfully in the treatment of psoriasis for more 40 yr, with PUVA sol being twice as cost-effective as PUVA8. Gahalaut et al11 in a PP analysis of 40 patients of psoriasis treated with PUVA sol observed that 20 per cent of their patients achieved PASI 75, 40 per cent achieved PASI 50, while 10 per cent had an aggravation of their disease at 12 wk. The response of psoriasis to PUVA sol is enhanced by the concomitant use of retinoids12. Kar et al13 reported complete clearance of CPP in 32 per cent patients and more than 50 per cent improvement in 44 per cent of patients treated with PUVA sol for eight weeks. Similarly, Talwalkar et al14 reported 95 per cent improvement in 40.7 per cent of patients and more than 50 per cent improvement in 22 per cent of patients treated with PUVA sol. In a study of 36 patients with CPP, of whom 20 were treated with PUVA sol, Aggarwal et al8 reported that 75 per cent of their patients attained PASI 50 at 12 wk on a PP analysis.
Our study was designed to evaluate the non-inferiority of Unani medications vis-a-vis oral PUVA sol in patients with CPP at 12 wk, in terms of number of patients who achieved PASI 75 in each group as also comparing the median of percentage reduction of PASI. It was observed both on ITT analysis and also on PP analysis (done to validate the non-inferiority), the Unani medications were not only non-inferior to PUVA sol in achieving PASI 75 but also PASI 50. Similarly, the median of percentage reduction in PASI at 12 wk was comparable in both the groups. However, less than 50 per cent of the initial cohort in ITT analysis in both the groups achieved PASI 50 at 12 wk, indicating that 12 wk may not be adequate duration of therapy both with PUVA sol and Unani medications.
The drop out in the present study was 27.9 per cent, and while calculating the sample size, we had factored in for a drop out of 10 per cent. However, the drop out in our study was similar to that in previous study in psoriasis8, where therapy needs to be taken for long periods of time in a chronic disease, especially if therapy is cumbersome, as is the case with PUVA/PUVA sol as also the Unani medications.
Despite the response, the problem with therapy of psoriasis is that relapses are generally the rule when treatment is stopped with all therapies including biologicals1516. Yones et al17 observed relapses in 32 per cent of their patients within 24 wk of stopping PUVA treatment, while Melski et al18 reported relapses in 35 per cent of their patients on stopping PUVA therapy. At 24 wk of follow up, we observed relapse in 15 per cent of patients in Unani treatment group and 18 per cent in the PUVA sol group, with the relapse rate in the two groups was comparable. Even during the follow up period at week 24, there was a decrease in the median of percentage change in PASI in both the groups, indicating that despite stopping treatment and some patients relapsing, in most patients, the PASI continued to decrease.
Side effects are frequent with psoralen-based therapies. Gahalaut et al11 and Aggarwal et al8 reported that 60-70 per cent of their patients on PUVA developed side effects with more than half of these being nausea and vomiting. Parrish et al19 and Singh et al6 also reported 23 and 55.6 per cent of their patients on PUVA, respectively, complained of nausea and vomiting. However, Handa et al20 in a retrospective analysis observed that only 3.5 per cent of their patients on photochemotherapy with psoralens in vitiligo had developed nausea and vomiting. We observed 16 per cent of patients on PUVA sol developed clinical side effects and most of these were gastrointestinal. It is here that Unani medication significantly scored over PUVA sol as only two per cent of patients on Unani formulations developed side effects.
The limitation of our study was that only those patients who had a facility for sun exposure at their residence could be recruited. The disadvantage of using sunlight as the source of UV radiation includes its variation with time, place, season and atmospheric conditions. In addition, its use is restricted in the case of female patients because of lack of privacy. Furthermore, PASI 75 was used as the primary endpoint and this was possibly very stringent as it placed potentially useful therapies at risk of failing to demonstrate efficacy. It was also believed that 12 wk of treatment with both PUVA sol and Unani formulations might not be an adequate duration of therapy and these treatments should be given for at least 20 week.
In conclusion, a combination of UNIM-401 orally and UNIM-403 topically may be an effective and safe treatment alternative in patients with psoriasis who have contraindications to other therapies.
Acknowledgment
Authors acknowledge the Ex-Director Generals of CCRUM, Dr K.M. Siddiqui and Dr S. Shakir Jamil for providing facilities and their cooperation for conducting the trial, and also co-operation of Drs S. Rasool, R. Jarupula and M. Nayak, who assisted in the study.
Financial support & sponsorship: Authors acknowledge the Central Council for Research in Unani Medicine (CCRUM), Ministry of AYUSH, New Delhi, for providing funding support
Conflicts of Interest: Drs Neena Khanna and M. Kalaivani declared no conflict of interest. Drs Rais-ur-Rahman, Khalid M. Siddiqui and Tamanna Nazli were employees of Central Council for Research in Unani Medicine (CCRUM), a government organization, which was the funding agency.
References
- History of science, philosophy and culture in Indian civilization Vol IV, Part 2. New Delhi: Centre for Studies in Civilizations, Project of History of Indian Science, Philosophy and Culture; 2001. p. :298-325.
- [Google Scholar]
- Kantoori GH, ed. Al quanoon fil tib. Vol 4. Delhi: Ejaz Publishing House; 2010. p. :345.
- Unani treatment for Nar-e-Farsi (eczema) and Daus Sadaf (psoriasis): A success story. New Delhi: Central Council for Research in Unani Medicine; 2015.
- Treatment with 401 O and 403 L (Coded formula) both systemic and topical formulation in the treatment of psoriasis - An open label study. Planta Med. 2012;78:116.
- [Google Scholar]
- Comparison of efficacy and side-effect profile of oral PUVA vs. oral PUVA sol in the treatment of vitiligo: A 36-week prospective study. J Eur Acad Dermatol Venereol. 2013;27:1344-51.
- [Google Scholar]
- Efficacy of bath psoralen plus ultraviolet A (PUVA) vs. system PUVA in psoriasis: A prospective, open, randomized, multicentre study. Br J Dermatol. 2013;169:704-8.
- [Google Scholar]
- Comparison of clinical and cost-effectiveness of psoralen + ultraviolet A versus psoralen + sunlight in the treatment of chronic plaque psoriasis in a developing economy. Int J Dermatol. 2013;52:478-85.
- [Google Scholar]
- Traditional Knowledge Digital Library. New Delhi: CSIR-Ministry of AYUSH, Government of India; 2017.
- [Google Scholar]
- Indian Association of Dermatologists, Venereologists and Leprologists. Photochemotherapy (PUVA) in psoriasis and vitiligo. Indian J Dermatol Venereol Leprol. 2014;80:497-504.
- [Google Scholar]
- Effect of oral PUVAsol on the quality of life in Indian patients having chronic plaque psoriasis. Dermatol Res Pract. 2014;2014:291586.
- [Google Scholar]
- Clinical efficacy of psoralen + sunlight vs. combination of isotretinoin and psoralen + sunlight for the treatment of chronic plaque-type psoriasis vulgaris: A randomized hospital-based study. Photodermatol Photoimmunol Photomed. 2014;30:294-301.
- [Google Scholar]
- Evaluation of psoralen with solar ultraviolet light (puva sol) and adjunctive topical tar therapy in psoriasis. J Indian Med Assoc. 1994;92:120-1.
- [Google Scholar]
- Evaluation of 8-methoxypsoralen and solar ultraviolet light (puva sol) in psoriasis. Indian J Dermatol Venereol Leprol. 1981;47:17-20.
- [Google Scholar]
- How long does the benefit of biologics last? An update on time to relapse and potential for rebound of biologic agents for psoriasis. Psoriasis Forum. 2010;16:36-42.
- [Google Scholar]
- Efficacy and safety of efalizumab in patients with moderate-to-severe plaque psoriasis resistant to previous anti-psoriatic treatment: Results of a multicentre, open-label, phase IIIb/IV trial. Arch Drug Inf. 2010;3:9-18.
- [Google Scholar]
- Randomized double-blind trial of the treatment of chronic plaque psoriasis: Efficacy of psoralen-UV-A therapy vs. narrowband UV-B therapy. Arch Dermatol. 2006;142:836-42.
- [Google Scholar]
- Oral methoxsalen photochemotherapy for the treatment of psoriasis: A cooperative clinical trial. J Invest Dermatol. 1977;68:328-35.
- [Google Scholar]
- Photochemotherapy of vitiligo. Use of orally administered psoralens and a high-intensity long-wave ultraviolet light system. Arch Dermatol. 1976;112:1531-4.
- [Google Scholar]
- Vitiligo: A retrospective comparative analysis of treatment modalities in 500 patients. J Dermatol. 2001;28:461-6.
- [Google Scholar]






