Translate this page into:
A dermoepidermal suspension spray (skin gun) for coverage of large post-burn & post-traumatic raw areas
*For correspondence: nikhil.panse@rediffmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
The idea of developing a skin gun was conceptualized and then incubated by the department of Plastic Surgery, B.J. Government Medical College and the Biomedical Engineering and Technology (incubation) Centre, College of Engineering, Pune, India, in 2017. Burns over a larger percentage of the body surface area burns have limited donor sites for skin grafting, and such patients have a higher mortality and morbidity rates. Procedures such as Meek micro-grafting with wider meshing are available, but not without limitations. The innovation proposed here aims at developing a dermoepidermal suspension spray also termed as a 'skin gun', where the skin graft is cut or pulverized into tiny pieces and sprayed over an extensive raw area (Video). These tiny pieces can act as seedlings for epidermal regeneration, and the area can heal itself in due course of time. Although there will be significant scarring, the purpose of the skin gun is to facilitate faster healing in patients with extensive burns and limited donor areas and decrease the mortality and morbidity.
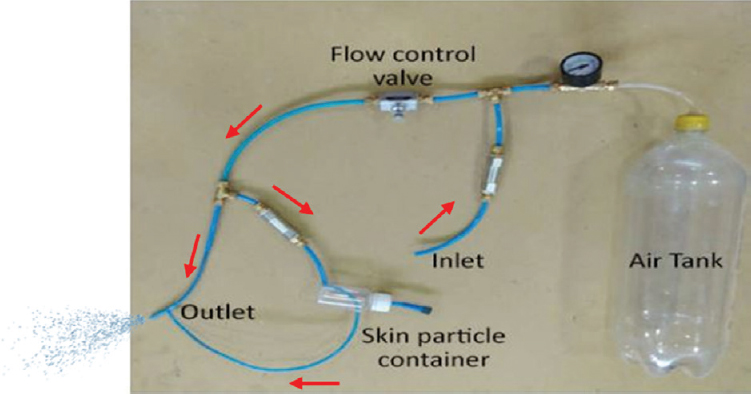
- A representative image of the rough prototype of a ‘skin gun’.
 Video available at ijmr.org.in.
Video available at ijmr.org.in.
Financial support & sponsorship: The financial support for the development of the prototype and further trials is through the Biotech Ignition Grant (BIG) Grant by BIRAC, Government of India. The patent for this innovation (201821011504) is pending approval.
Currently, we are working on developing the prototype and regularizing and standardizing the size of the tiny pieces of skin. Once the prototype is ready, animal and clinical trials will be performed, and depending on the outcomes, further course of action such as mass production or revisions in the prototype and further trials will be considered.
This project was also selected by the Society for Innovation and Entrepreneurship (IIT Mumbai) for the Academia-Industry Training Camp in Switzerland in 2019.
Conflicts of Interest: None.





