Translate this page into:
A cost-effective anaerobic culture method & its comparison with a standard method
Reprint requests: Dr Kashi Nath Prasad, Department of Microbiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow 226 014, Uttar Pradesh, India e-mail: kashinprasad@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Twenty six anaerobes were recovered from 150 deep-seated abscess samples cultured by the proposed two-step combustion-modified candle-jar system and Anoxomat. The degree of growth and colony size were similar in both systems, except for Clostridium difficile. The modified candle-jar system was found to be a sensitive and cost-effective alternative that might be used in resource-limited settings.
Keywords
Anaerobes
Anoxomat
iron wool
modified candle-jar technique
Anaerobic organisms are aetiologic agents of serious infections in human and have been documented to be the major causative agents of otitis media, oro-dental infections, puerperal sepsis, lung abscesses and intra-abdominal sepsis12. Culture and isolation of these organisms are not easy due to their fastidious nature. Their recovery in the microbiology laboratory requires properly collected and transported samples for isolation. The fact that anaerobic infections are normally polymicrobial makes their recovery in cultures difficult as clinical samples are processed routinely for aerobic bacteria.
The introduction of the McIntosh-Fildes jar was an important breakthrough in the field of anaerobic bacteriology3. Subsequently, other methods were introduced with different principles or modification of old ones. These were GasPak, anaerobic glove box, Coy anaerobic chambers, Anoxomat, pre-reduced anaerobically sterilized media and sodium azide selective medium. These complex and expensive systems are needed to isolate anaerobic flora and are suitable for well-equipped laboratories.
In developing countries anaerobic microbial bacteriology has been neglected and most laboratories do not use any technique to isolate and identify anaerobic organisms. We, therefore, attempted to evaluate the modified candle-jar system, a new cost-effective method developed by Maiti et al4, and to compare its results for isolation of anaerobes from clinical samples using Anoxomat system.
The study was conducted from March 2014 to March 2015 in the Microbiology department of the Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, a tertiary care centre, in northern India. A total of 150 consecutive deep-seated pus samples received from various departments such as gastrosurgery, neurosurgery and intervention radiology were included in the study. The clinical samples and known anaerobic bacterial control strains were inoculated on Wilkins-Chalgren agar plates (HiMedia Laboratories, Mumbai) supplemented with five per cent sheep blood in duplicate. After inoculation, one plate was incubated anaerobically by standard Anoxomat (Mart Microbiology B.V., The Netherlands) system and another by the two-step combustion-modified candle-jar system as described below. Control strains of Bacteroides fragilis ATCC 25285, Clostridium perfringens ATCC 13124 and laboratory isolated strains of Fusobacterium nucleatum, Peptostreptococcus anaerobius, Veillonella species, Clostridium difficile and Propionibacterium spp. were used as controls.
In our study, a lighted candle was kept instead of bicarbonate solution as a CO2 generator and one per cent methylene blue strip was used instead of one per cent methylene blue solution as an indicator of anaerobiosis. Acidified copper sulphate (Sigma, USA) was prepared by mixing 5 ml of (10% w/v) copper sulphate solution, 5 ml of 10 per cent Tween 80 (Sigma), 3 ml of (2 mol/l) sulphuric acid (prepared by adding 11 ml of concentrated acid to 89 ml water) and distilled water up to 200 ml. Five grams of grease-free (grade 0/grade 1) steel wool (commercially available from local market for scrubbing) was dipped in freshly prepared acidified copper sulphate solution until the wool appeared dark grey. Excess solution was drained and the steel wool was moulded into a loose pad to fit an open Petri plate. To prepare methylene blue strip, Whatman filter paper (number-1) was first cut into long strips of about five cm and then soaked in one per cent (w/v) methylene blue solution and dried.
The inoculated Wilkins-Chalgren blood agar plates were placed at the bottom of 1000 ml capacity jar. Then, the open plate containing acidified copper sulphate treated steel wool and a candle was kept on top of the inoculated plates. Methylene blue strips were kept inside the jar as an indicator of anaerobiosis. Candle was lighted and jar lid was closed tightly. The lighted candle in the jar initially burnt oxygen and produced CO2. Iron wool treated with acidified copper sulphate absorbed the remaining oxygen. The jar was then placed in an incubator at 37°C for 48 h.
Growth and colony size were examined at 48 h. The overall growth appearance and the average diameter of well-isolated colonies were recorded. After identical inoculations, the degree of growth was noted. Growth was labelled as ‘0’ for no growth, ‘1+’ for scanty growth, ‘2+’ for moderate growth and ‘3+’ for heavy growth.
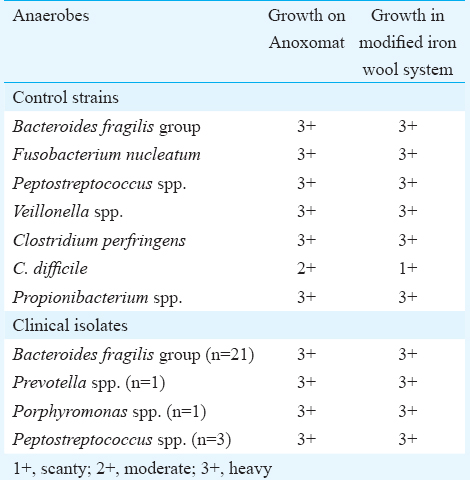
Of the 150 samples from deep-seated abscesses, 120 were abdominal abscesses, 15 were brain abscess, four each were breast and psoas abscess and two were pelvic abscesses. Five samples were from other body sites. Anaerobes were recovered from 25 samples. The organisms included B. fragilis group (21), Peptostreptococcus spp. (3), Porphyromonas spp. (1) and Prevotella spp. (1). One brain abscess culture grew both B. fragilis and Peptostreptococcus spp. All isolates showed similar growth in both systems (Table). There were no observable differences between the degree of growth (heavy, moderate, scanty) and colony size between the two systems. The methylene blue indicator strip remained colourless till the jar was opened.
Interest in anaerobic bacteriology has re-emerged due to increasing reports of infections with anaerobic bacteria including Bacteroides group, C. difficile, C. perfringens and Veillonella parvula1. As per literature, more than 300 different kinds of anaerobic apparatus were used between 1888 and 19185. Although several techniques are available for maintaining an oxygen-free environment during the processing of specimens for anaerobic culture, the anaerobic jar is most common. User-friendly methods such as Anoxomat technique6 and anaerobic glove box technique7 are used in some sophisticated laboratories. In developed countries, procedures for cultivation and identification of anaerobic bacteria are well established. However, equipment and resources are lacking in developing countries for establishing a separate anaerobic section in the diagnostic laboratory. Therefore, a cost-effective, user-friendly technique suitable for initiation of anaerobiosis at collection point was developed as modified candle-jar system8 and in this study we attempted to evaluate this for clinical setup by comparing results of the same with Anoxomat system.
For optimum culture of anaerobes, the initial goal is to reduce the oxygen tension from the environment where inoculated plates are held and maintain a reduced state in the medium. The GasPak system (BD Biosciences, Sparks, Maryland, USA) is an acceptable method for most small-scale laboratories. However, it is a single use and costly technique and takes more than an hour to attain critically low levels of oxygen. By combustion of white wax candle in candle jar system, 4-5 per cent CO2 is generated with instantaneous lowering of oxygen level inside the sealed jar leaving approximately 1-2 per cent un-burnt oxygen4.
In the modified candle-jar system, acidified copper-coated steel wool is used for removal of residual oxygen after candle combustion. As one g iron wool absorbs approximately one ml of oxygen per minute, 5 g of acidified copper sulphate treated steel wool can theoretically remove 10-20 ml residual oxygen within 2-4 min from a 1000 ml sealed jar after candle combustion. The candle jar is an alternative technique that can be used to reduce the major bulk of oxygen rapidly. The residual oxygen in the air can then be slowly reduced by devising alternative methods such as use of alkaline pyrogallol. This may not be good substitute for iron wool as suggested by Parker9 because it often produces some amount of carbon monoxide which is inhibitory to some organisms. In addition, reaction kinetics is faster in steel wool for availability of larger reactant surface. However, any single method is not good enough since rapid combustion does not completely exhaust all the oxygen while the slow method has the limitation of delayed response. Combination of both methods in the same system will be more efficient. Our results showed that all control strains and anaerobes from clinical samples grew well in both the systems and there was no difference in the degree of growth and colony sizes, except for C. difficile which was scanty in modified iron wool method compared to Anoxomat system where the growth of C. difficile was moderate (Table). The use of bi-layered buffer-charcoal medium7 can enhance growth and colony size by removing accumulated inhibitory metabolites from growing colonies. This modification needs to be evaluated, particularly for growth of C. difficile.
In bag culture method, iron wool was used as a sole agent for chemical combustion from smaller container, but it took longer time to attain a critical low oxygen level. This might be due to trapped air in inoculated plate, which came out slowly by weak convection current in small container to react with iron wool. Modified candle-jar system overcame this limitation by adding a rapid combustion step that helped to bring out trapped air quickly by creating negative pressure10.
In conclusion, the modified iron wool method was found to be an effective method of anaerobiosis and the results were comparable with the Anoxomat system. The method was cost-effective as well which could be easily introduced in resource-limited settings.
Conflicts of Interest: None.
References
- Nosocomial infections in leukemic and solid-tumor cancer patients: distribution, outcome and microbial spectrum of anaerobes. Future Microbiol. 2012;7:1423-9.
- [Google Scholar]
- Clinical microbiology: reemphasizing the role of anaerobic bacteria in human infections. J Med Microbiol Diagn. 2012;1:e109.
- [Google Scholar]
- An improved form of Mcintosh and Fildes’ anaërobic jar. Br J Exp Pathol. 1921;2:153-4.
- [Google Scholar]
- Anaerobic culture on growth efficient bi-layered culture plate in a modified candle jar using a rapid and slow combustion system. Indian J Med Microbiol. 2013;31:173-6.
- [Google Scholar]
- Evaluation of the anoxomat: a new technique for anaerobic and microaerophilic clinical bacteriology. J Clin Pathol. 1989;42:640-4.
- [Google Scholar]
- Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969;17:568-76.
- [Google Scholar]
- Anaerobic culture on growth efficient bi-layered culture plate in a modified candle jar using a rapid and slow combustion system: few comments. Indian J Med Microbiol. 2014;32:351-2.
- [Google Scholar]






