Translate this page into:
Tackling the malaria problem in the South-East Asia Region: Need for a change in policy?
Reprint requests: Prof. N.K. Ganguly, President, Jawaharlal Institute of Postgraduate Medical Education & Research (JIPMER) & Distinguished Biotechnology Research Professor, National Institute of Immunology, Aruna Asaf Ali Marg, J.N.U. Complex, New Delhi 110 067, India e-mail: nkganguly@nii.ac.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Malaria is largely neglected in the South-East Asia Region (SEAR), although it has the highest number of people susceptible to the disease. Malaria in the SEAR exhibits special epidemiological characteristics such as “forest malaria” and malaria due to migration across international borders. The Greater Mekong Subregion (GMS) has been a focal-point for the emergence of drug resistant malaria. With the recent emergence of artemisinin resistance, coupled with the limited availability of insecticides, malaria control efforts in the SEAR face a steep challenge. Indirect man-made factors such as climate change, as well as direct man-made factors such as the circulation of counterfeit drugs have added to the problem. Increased monitoring, surveillance, pharmacovigilance as well as cross-border collaboration are required to address these problems. Regional networking and data-sharing will keep all stakeholders updated about the status of various malaria control programmes in the SEAR. Cutting-edge technologies such as GIS/GPS (geographical information system/global positioning system) systems and mobile phones can provide information in “real-time”. A holistic and sustained approach to malaria control by integrated vector management (IVM) is suggested, in which all the stakeholder countries work collaboratively as a consortium. This approach will address the malaria problem in a collective manner so that malaria control can be sustained over time.
Keywords
Climate change
insecticide resistance
malaria
policy
SEAR
vector control
Introduction
The South-East Asia Region (SEAR) has been home to the malaria scourge for countless centuries. This disease has been “part-and-parcel” of social upheavals, economic transitions as well as shifts in the demographic pattern of the region. Historically, malaria has had a severe negative impact on the economic development of nations. The disease pattern in the SEAR can be correlated with the socio-economic changes that the Region has undergone over the years. In particular, the rapid urbanization has had an immense impact on the vector population in the Region. Besides population size, other factors such as population density, population mobility or migration also have a significant impact on vector dynamics. Although the malaria problem has historically been associated with Africa, the SEAR is slowly but surely becoming an epicenter of multi-drug resistant falciparum malaria, which, if not checked in time, is likely to spiral out of control.
The last four decades have taught us that comprehensive malaria control/eradication programmes are required to face the new emerging challenges. During this forty years malaria was almost eradicated in Sri Lanka, but resurged. It was under control in India, but here also, it resurged1. In Cambodia, war and violence had a lot to do with the emergence of drug resistant malaria2. Similar was the case for Myanmar3. Since a malaria vaccine is currently not available, thus all malaria control programmes will have to rely heavily on vector control and other strategies to reduce the malaria disease burden. These strategies have to be multi-pronged and all-inclusive, in the sense that these have to take into account the multiple facets that contribute to the malaria problem as a whole. Importantly, the activities need to be sustained, well-coordinated and managed, with a robust monitoring system in place to avoid duplication and fragmentation so that wastage of scarce (and hence valuable) resources does not occur.
Malaria burden in the SEAR
The SEAR is home to about 2.2 billion people potentially at risk of contracting malaria. This equates to approximately 67 per cent of the world population at risk of malaria, largely stemming from the fact that six of the most populous countries in the world are located in this Region, including India, China, Indonesia, Bangladesh, Vietnam and the Philippines4. In countries such as Bangladesh, India, Indonesia, Myanmar and Vietnam, about 91 per cent of the population lives in areas of high transmission for both Plasmodium vivax and P. falciparum5. In 2009, the SEAR had reported 2.4 million parasitologically confirmed malaria cases and 3320 deaths, reflecting a 7 per cent decrease in cases since 20003. India, Myanmar and Indonesia accounted for approximately 94 per cent of the reported malaria cases in the Region in 2008, with India bearing the brunt of the burden at 65 per cent6. A recent study estimated that the number of malaria deaths in India could well be as much as 6-fold higher than the current World Health Organization (WHO) estimates7.
Distribution of malaria parasites in the SEAR
Though all four known malaria parasites (MPs) are found in the SEAR, the most prevalent are P. vivax and P. falciparum, each accounting for nearly 50 per cent of the total malaria cases. In Vietnam, P. vivax and P. falciparum occur at almost the same rate except for the woody regions where the latter is more prevalent (75%)8. A PCR-based approach in south-eastern Laos indicated that the most common parasite was P. falciparum, while mixed infections accounted for 23 per cent of the samples assayed9. In Myanmar and Timor-Leste, transmission is by both parasites, while in North Korea, it is almost exclusively due to P. vivax6. Parasite mapping along the Thai border indicated that there has been a shift from P. falciparum to P. vivax along the western border with Myanmar; northern border with Laos; and eastern border with Cambodia, while an opposite trend has been observed along the southern border with Malaysia10. In India, although there have been a higher number of P. vivax cases, the trend is shifting towards P. falciparum cases, with approximately 57 per cent of cases in 2009 being caused by the latter6. In recent years, P. knowlesi, a zoonotic malaria parasite usually associated with natural infections of long-tailed and pig-tailed monkeys is being recognized as the fifth human Plasmodium species since it has been found to be an important cause of human malaria in Sarawak and Sabah (Malaysian Borneo) and in Pahang (Peninsular Malaysia), where human infections as well as fatality have been reported11.
Distribution of malaria vectors in the SEAR
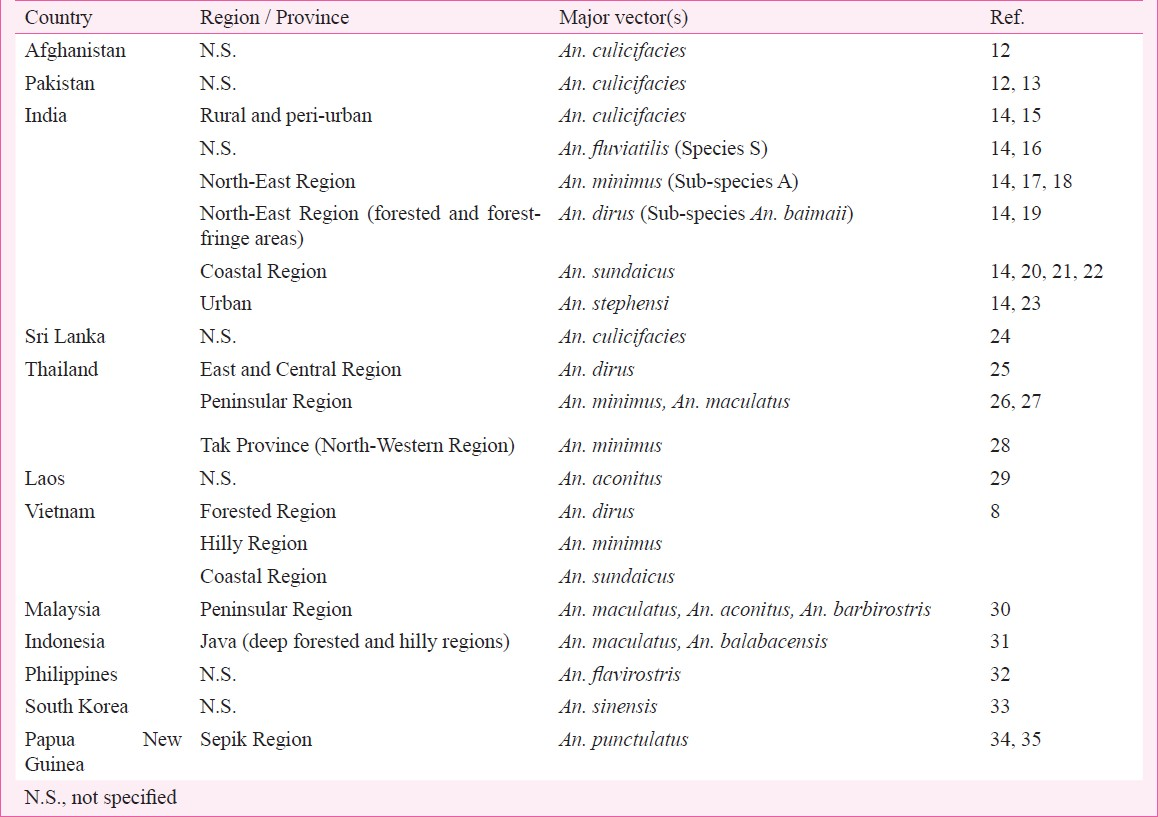
Knowledge about the distribution of malaria vectors in a vast, contrasting and divergent geographic region such as the SEAR is of paramount importance for formulating vector control strategies. All human malaria is transmitted by anophelines (genus Anopheles), albeit not all anophelines are vectors of malaria. Moreover, malaria transmission dynamics is complicated by the existence of species complexes of cryptic or sibling species in all the five major Anopheles complexes viz. (i) Culicifacies complex, (ii) Fluviatilis complex, (iii) Minimus complex, (iv) Dirus complex, and (v) Sundaicus complex. The country-wise distribution of various malaria vectors in selected countries of the SEAR is presented in the Table812–35.
Special features of malaria epidemiology in the SEAR
Malaria epidemiology in the SEAR presents features that are distinct from other regions of the globe. The SEAR is home to the largest uncharacterized population at risk from malaria. The population is highly diverse, not only from a socio-cultural and socio-economic standpoint, but also from a genetic standpoint. The role of epistasis and it's implications towards susceptibility and resistance to malaria is an area which is often underappreciated36. However, epistasis could hold the answer as well as explain the underlying phenomena associated with malaria, including rapid emergence of drug resistance, as well as why an ideal malarial vaccine is still unavailable.
Malaria in the SEAR can be classified into several ecological types, such as urban malaria (a major problem in India), coastal malaria (in Indonesia, southern Thailand and the Andaman & Nicobar Islands), and forest malaria (in Thailand, Myanmar, north-eastern States of India), which are based on the natural geographical distribution of the vectors. Other types include “man-made malaria” that essentially occurs as a result of human activities, leading to disturbance in the vector population. Importantly, the afflicted people are usually from minority ethnic groups and marginalized tribal communities who are usually the most ill-equipped to tackle the problem, and hence, constitute the so-called “hard-to-reach” populations who bear the brunt of the burden.
Special issues and challenges in malaria control in the SEAR
(i) Drug resistance: Historically, the SEAR has been a hotbed for the emergence of drug-resistant malaria. Of particular importance is the Greater Mekong Subregion (GMS) (Fig.). Resistance to anti-malarial drugs has been reported along the international borders of Thailand. First appearing in the late 1950s along the Thai-Cambodia border37, chloroquine resistance has continued to be a problem due to various reasons, including inappropriate use, territorial disputes, as well as daily mass movement of migrant gem mining workers from Thailand to the north-western part of Cambodia37. Thailand's borders with Myanmar, Cambodia and Malaysia have all produced drug-resistant MP strains. Myanmar can be considered as an information “blackhole” for malaria and other diseases of public health importance. A recent randomized controlled trial (RCT) carried out along the Thai-Myanmar border indicated that the efficacy of chloroquine treatment of P. vivax was declining, and that dihydroartemisinin-piperaquine was an effective alternative38. The fact that most of the study participants were of Burmese origin, indicates that vivax malaria could be a major problem in Myanmar. Vivax malaria could also be a major, yet under-reported problem in other parts of the SEAR. With primaquine being the only radical cure, it suffers from the problem that it cannot be used in most of the SEAR due to G6PD deficiency in majority of the population39.
- The Greater Mekong Subregion.
It should be noted that Thailand, which is comparatively prosperous and has a good health system, shares its borders with countries characterized by social unrest, political instability and poverty – all ideal conditions for breeding drug-resistant MP strains. Hence, the recent evidence suggesting emergence of artemisinin resistance along the Thai-Cambodia border40 comes as no surprise. However, the efficacy of artesunate-mefloquine still remains high along the western borders of Thailand41. Recent studies indicate that artemisinin-resistance is still confined to the eastern border of Thailand with Cambodia, and has not spread across Thailand, since there was high susceptibility to artemisinin in eastern Bangladesh42.
(ii) Counterfeit drugs: A major compounding factor that adds to the problem of drug-resistant malaria in the GMS is the issue of fake or counterfeit anti-malarial drugs. In the GMS, countries like Cambodia, Laos, Myanmar and Vietnam have a high number of fake anti-malarial drugs, which sometimes accounts for up to 50 per cent of all antimalarials in circulation. At least 12 different types of counterfeit antimalarials are in circulation in the GMS43. Interestingly, these fake drugs contain enough of the active ingredient to test positive when tested by drug controllers, but not enough to kill the MPs, thereby encouraging resistance44.
(iii) Insecticide resistance: Insecticide resistance is a very real problem in the SEAR and is an important factor behind failure of malaria control programmes. The resistance pattern of Anopheles and Culex mosquitoes to DDT (dichloro-diphenyl-trichloroethane) has been reported from Thailand45. Moreover, both colony-reared and wild populations of An. minimus exhibited insecticide-avoidance behaviour (contact irritancy and non-contact repellency) to DDT, deltamethrin and lambda-cyhalothrin46. This behavioural avoidance response to insecticides, which is the first sign of insecticide resistance, was not only reported in An. minimus but also in other malarial vectors such as An. dirus, An. maculatus, and An. sawadwongporni47. The MALVECASIA Project (2001-2004) attempted to monitor insecticide resistance in malaria vectors in the GMS countries, including Cambodia, Laos, Thailand, and Vietnam48. The study revealed that while An. dirus was susceptible to pyrethroids in forested areas of Vietnam, it was resistant in central Vietnam. Anopheles minimus was found to be both susceptible as well as resistant in Vietnam, whereas in Cambodia, Laos and Thailand it was susceptible. Only two populations of An. minimus were resistant to DDT. In the Mekong delta, An. epiroticus was highly resistant to all pyrethroids tested. This species was mostly susceptible to DDT, except near Ho Chi Minh City, where there was indication of DDT resistance. Anopheles vagus was found to be resistant to DDT as well as several pyrethroids in both Vietnam and Cambodia.
In India, since five of the major vectors exist as species complexes of several sibling species, this has had a considerable impact on their susceptibility to commonly used insecticides in public health programmes49. Anopheles stephensi is triple resistant against the organochlorines DDT and HCH (hexachlorocyclohexane), as well as the organophosphate malathion, An. culicifacies has been shown to exhibit quadruple resistance, including all the above as well as the synthetic pyrethroid (SP) deltamethrin5051, which is a cause for concern from a public health standpoint, as pyrethroids are currently the preferred choice for insecticide-treated nets (ITNs) as well as for indoor residual spraying (IRS) in many situations. Anopheles culicifacies has been documented to be resistant to DDT in 286 districts and to DDT and malathion in 182 districts in India52, although the figures could actually be much higher. It should be noted that HCH was banned in India in 1997 due to resistance as well as environmental concerns, while DDT is still being used selectively for malaria and kala-azar vector control. One of the major reasons for resurgence of malaria in India in the mid-1970s was insecticide resistance in malaria vectors53, which indicates that there is an urgent need for controlling insecticide resistance.
Other factors impacting on malaria in the SEAR
(i) Climate change: Climate change, although a global phenomenon has important implications for malaria in the SEAR. The major climatic factors that have an impact on malaria transmission due to spatio-temporal changes in malaria vectors are temperature, relative humidity (RH) and precipitation54. Increasing atmospheric temperature causes decrease in the duration of the gonotrophic cycle as well as the extrinsic incubation period, leading to increased biting frequency, and therefore, increased rate of malaria transmission55.
The Fourth Assessment Report (2007) of the Inter-governmental Panel on Climate Change (IPCC)56 indicates that the global average surface temperature would increase unabated, if remedial measures are not instituted, and that this was likely to have an impact on vector borne diseases due to changes in vector dynamics. Moreover, it is also predicted that the mega-deltas of the large river systems in the SEAR are likely to face flooding from the sea due to the rising sea level. Increased salinity in the delta areas of the large rivers in the SEAR could have an impact on mosquito populations such as An. sundaicus that preferentially breed in saline water.
The Government of India initiated the National Communication (NATCOM) Project under the Ministry of Environment and Forests, to assess and evaluate the impact of climate change on malaria57. The comparative climatic data analyses over the past decade indicated that climate change could spread malaria to heights above 1800 m and 10 per cent more States may exhibit climatic conditions conducive for malaria vector breeding throughout the year. The transmission windows (TW) developed by NATCOM indicate that by 2050, malaria is likely to be the most endemic in central (Madhya Pradesh) and eastern (Jharkhand, Chhattisgarh, Odisha, West Bengal and Assam) States of India58.
(ii) Human behaviour: Human behaviour, an often under-recognized factor in malaria transmission, has been reported from the GMS59, Bangladesh60, India61, Thailand62 as well as other countries in the SEAR. The major risk factor is the movement of people to-and-from the forested areas63, primarily related to socio-economic as well as socio-cultural reasons6465. For example, in Cambodia, inhabitants from forest-fringe areas frequently make overnight visits to the forest to collect wood or to hunt. Temporary migrants like loggers, sandalwood collectors, soldiers and gem miners also visit the forest at night without personal protection, thereby being exposed to mosquito bites66. Again, Indian tribals often visit the forests at night-time to collect “Mahua” (Madhuca indica) flowers for making country liquor and get bitten by vector mosquitoes. Falciparum malaria has been reported from Panna district near Jabalpur in Madhya Pradesh, India, which was spread in this manner67.
Other factors related to human behaviour that impact malaria transmission include not using ITNs at night. This has been reported from China68, Indonesia69 and other countries in the SEAR. This type of behaviour primarily stems from lack of knowledge about malaria, as has been shown in Laos, where the level of malaria prevention was correlated with the level of education70. In Thailand, the use of ITNs for preventing malaria in children was higher amongst mothers who had a better knowledge of the disease71.
Strategies for malaria control in the SEAR: Implications for changes in policy
Malaria control strategies in the SEAR will ideally involve a multi-pronged approach addressing not only vector control but also early detection and case management. Management of malaria cases in the SEAR is complicated by the liver-stage parasite P. vivax that is difficult to cure without a radical approach such as treatment with primaquine, which is contraindicated in G6PD deficiency that is present in majority of the population in the SEAR. Moreover, artemisinin-based combination therapy (ACT) also needs to be used with care due to possible emergence of widespread resistance against the main active ingredient in the ACT formulation. Given the limited resources for most of the countries in the SEAR, it is important to choose the right intervention tools for the right situations, which is likely to vary from country to country. This requires an in-depth knowledge of the local epidemiology, various geo-meteorological parameters such as vegetation, temperature, RH and precipitation, coupled with a thorough understanding of socio-cultural and socio-economic aspects that have a direct bearing on the success of any and all malaria control programmes.
(i) Tackling drug resistance: Need for a multi-pronged approach: Emergence of drug resistance is a major problem in the SEAR. Of the many approaches that are required to tackle the problem, early case detection or diagnosis followed by prompt treatment (EDPT) will be an integral component of the overall strategy. Treatment needs to be carefully balanced with prevention, since it is the latter approach that will eventually contribute to the reduction of the malaria burden. The EDPT approach has been adopted in India by the National Vector Borne Disease Control Programme (NVBDCP)72, but the current rapid diagnostic tests (RDTs) have low sensitivity for vivax malaria, as a result, there is still a continued need for microscopy73, which requires trained staff as well as electricity, both scarce commodities in field settings. Thus, there is a need for RDTs with high sensitivity and specificity, which are cheap and suitable for national programmes, both for diagnostic purposes as well as for surveillance. The EDPT approach is advocated by the Global Fund, since the syndromic treatment approach has led to the emergence of drug resistance. Almost all the WHO-SEARO Member countries have incorporated this into their National Drug Policy. The emergence of artemisinin resistance in P. falciparum in the Thai-Cambodia border is of concern, which re-emphasizes the need for policy changes in order to encourage more inter-country collaboration for monitoring of drug resistance along international borders, as well as the establishment of a viable network of institutes in the SEAR that are involved in monitoring of anti-malarial drug resistance, so that sharing of the latest information becomes smooth and streamlined. In this context, it should be indicated that mobile phones have been successfully utilized to contain multi-drug resistant malaria on the Thai-Cambodian border74.
To tackle the issue of illegal manufacturing and cross-border trafficking of fake or counterfeit drugs, stringent legislations and their strict enforcement will be required that should ideally be applicable across international borders. For this, inter-country co-operation is a pre-requisite, with mobilization of funds towards strengthening manpower and infrastructure for a higher level of pharmacovigilance across the SEAR. Moreover, biochemical tests such as the GPHF-Minilab® Dye Test Kit developed by The Global Pharma Health Fund, for checking the quality of antimalarials such as artesunate should be widely introduced. If the situation escalates out of control and spills over the geographical confines of the GMS, it could spell disaster for malaria control worldwide.
(ii) Insecticides: Need to exercise caution and use judiciously: A number of insecticides exhibiting residual effect are used for IRS as adulticides that are recommended by the WHO Pesticide Evaluation Scheme (WHOPES)75. However, with very few effective insecticides at our disposal, there is a need to exercise caution, and to use these judiciously. Since insecticide resistance varies from region to region, the decision on which insecticide to use should ideally be taken at a regional level. However, an inter-country network should be in place, much like that for the monitoring of drug resistance. The network should ensure that all stakeholders have access to the latest information as to which area is using which insecticide, and for what duration and other such essential information. It needs to be explored whether a network for monitoring insecticide resistance such as MALVECASIA48, could be expanded throughout the SEAR.
It should also be kept in mind that the ground realities associated with IRS activities include inadequate supply-chain, interruption in spray schedules due to high cost, as well as low quality spraying and failure to deposit required insecticide doses on walls, and also due to exophilic nature of many vector species. Due to these shortcomings, there are sometimes strong refusals for IRS by the community. In such situations, it would be relevant to explore the introduction of other vector control options.
Synthetic pyrethroids (SP) are used for impregnating bednets such as ITNs and long-lasting insecticidal nets (LLINs), since only this class of insecticide has minimal toxicity on humans, and hence approved by the WHOPES75. Evidence exists for community preference of ITNs over IRS76, as well as acceptability and willingness to purchase and use LLINs77. Therefore, upscaling, establishment of distribution systems through social marketing, creation of awareness to available interventions and behavioural change to use the interventions appropriately and regularly will expedite the uptake of LLINs. Efforts are already in place in the SEAR to scale-up the use of LLINs78. In India, which has a robust rural health system under the National Rural Health Mission (NRHM) that includes ASHAs (Accredited Social Health Activists) who have immensely contributed to the rural Maternal and Child Health Programmes, there is a distinct possibility of utilizing their expertise in the upscaling process for the LLINs. The comparatively higher cost of the LLINs might be an impediment to initial up-scaling programmes, but is likely to be a good investment for the future, considering the fact that these last much longer than ITNs. Hence, there is a need to sensitize all stakeholders, including the respective governments from all malaria controlling countries in the SEAR, non-governmental organizations (NGOs), private sector partners, and most importantly, civil society about the importance of LLINs as one of the most important preventive measures against malaria.
The problem of insecticide resistance arising particularly from IRS activities is real, and can become exacerbated by the use of the same class of insecticide, both in the health as well as the agriculture sector. Moreover, since the number of anti-malarial insecticides is very limited, development of insecticide resistance can seriously pose a threat to malaria control programmes. Therefore, strategies to prolong the life of insecticides, such as rotation between different chemical classes should be encouraged, but under strict supervision. Other strategies include avoiding the use of insecticides that simultaneously select for resistance to other chemically related insecticides, leading to the development of multiple resistance. Also, the same insecticide should not be used as an adulticide as well as a larvicide. In order to strengthen these activities, monitoring mechanisms should be in place to monitor early signs of insecticide resistance as per established WHO guidelines79. Emergence of resistance against pyrethroids could spell disaster for malaria control programmes that are aiming at upscaling the distribution of LLINs. Since successful trials have been carried out comparing SPs with alternative insecticides such as organophosphates and carbamates8081 for impregnating mosquito nets, there is a window of opportunity for rotating the insecticides, as and when required.
(iii) Creating awareness: Crucial for successful malaria control programmes: Information, education and communication (IEC) are key components for the success of malaria control programmes. A thorough understanding of the epidemiology and pathogenesis of malaria in the community will likely bring about changes in attitude, which will eventually bring about behavioural changes towards intervention measures, as well as a possible increase in “treatment-seeking behaviour”. Urban malaria can be completely prevented with community involvement82. Therefore, there is a need for making community members feel that they are also important stakeholders in the malaria control programme. It should be remembered that IEC activities need to be a continual process, requiring sustained efforts involving training of trainers, field personnel and health educators attached to the various outreach programmes within the malaria control programme.
IEC activities need to be based upon sound data generated by operational research (OR). OR is an important aspect of socio-behavioural studies impacting on malaria transmission, such as human migration and “forest malaria”. OR studies have already indicated that awareness can be created and behavioural changes brought about, resulting in an increased acceptability of LLINs83. OR could also look at the feasibility of introduction of LLINs along with IRS activities in areas where multiple vector species with different behavioural characteristics exist, to study their synergistic effect. OR will also be required to evaluate the logistic feasibility of using edible larvivorous fish, such as grass carps (Ctenopharyngodon idella) for mosquito larvae control in rice fields. Hence, OR will be an important component of malaria control programmes in the long-run.
(iv) Integrated vector management: Need for a holistic approach: Integrated vector management (IVM) is essentially a decision-making process targeted at reducing vector populations to interrupt the transmission of vector-borne diseases84. IVM consists of (i) selection of methods based on knowledge of local vector biology, disease transmission and morbidity; (ii) using a range of interventions, often in combination and synergistically; (iii) intra- and inter-sectoral collaboration; (iv) engagement with local communities and other stakeholders; (v) a public health regulatory and legislative framework; (vi) rational use of insecticides; and (vii) good management practices. An IVM approach takes into account the available health infrastructure and resources and integrates all available and effective measures, whether chemical, biological, or environmental.
The primary factor that needs to be considered for vector management is a thorough knowledge and understanding of the vector population as well as vector bionomics. Importantly, geographical information system (GIS) can be used for collection as well as analysis of spatial data with a great deal of flexibility. GIS, coupled with remote sensing (RS) technologies is capable of describing local and landscape level features influencing disease vector distribution such as that for malaria. GIS has the capability of determining the exact co-ordinates of the vector population hot-spots on the basis of meteorological data and land use features85. Moreover, GIS technology can be adapted for micro-level mapping of vector distribution within small areas such as villages86. GIS can also be used for monitoring and evaluation of malaria control activities such as IRS activities87, as well as mapping of indicators for Roll Back Malaria (RBM), such as distribution of ITNs/ LLINs, malaria prophylaxis for pregnant women, and data on prompt and effective case management88. The GIS-based technology can be coupled with RS technology to give real-time information on surges in disease transmission, particularly at times of emergency, such as epidemics. This technology will help to effectively channelize limited and scarce resources to areas where malaria is more prevalent.
An integral component of IVM is stopping the growth of the vector population at the larval stage itself. Several bio-environmental approaches for controlling malaria are available that are not only effective, but also have the advantage of not being plagued by resistance issues like the chemical approaches. These include the use of larvivorous fish, including mosquito fish (Gambusia affinis) and guppy fish (Poecilia reticulata), which are capable of surviving in presence of the native fish Aplocheilus, and also in a wide range of temperatures and salinity. The potential of these fish in larva control has been successfully demonstrated in field conditions in India89. However, long-term use of larvivorous fish would require constant monitoring since these fish, which originate from the Caribbean, could have adverse effects on the local fish population.
Another bio-environmental approach involves the use of bio-insecticides, including toxins derived from bacteria such as Bacillus thuringiensis var. israelensis and B. sphaericus, which act as larvicides. Though B. sphaericus has been reported to have developed resistance90, B. thuringiensis has been found to be useful as a larvicide in urban areas, but has a short half-life, and frequent re-treatment is required, which is not cost-effective.
Another recent development in the area of bio-environmental control is the use of the endosymbiotic bacterium Wolbachia, which has been demonstrated to protect mosquitoes such as Aedes aegypti91 and Ae. albopictus92 against dengue virus infection, resulting in reduced dengue transmission. Importantly, the same approach has been shown to inhibit P. falciparum in An. gambiae93, the major malaria vector in sub-Saharan Africa. If Wolbachia are able to inhibit malaria parasites in the Anopheles spp. prevalent in the SEAR, it could become a promising strategy for reducing malaria transmission in the Region.
Other essential components of vector control involve rigorous surveillance coupled with public education and implementation of the various control measures. Hence, the IVM approach should rationally apply all the knowledge gained from the interventions and strategies discussed so far, in a holistic and all-inclusive manner to achieve its aims and objectives.
The way forward: Suggestions for changes in policy
It should be remembered that currently there are neither any new classes of antimalarials nor new insecticides at our disposal. Malaria control must be achieved as well as sustained over time, before embarking on any ambitious efforts for elimination. One has only to look retrospectively at our past efforts for elimination of malaria to gain insights into planning for the future. It is suggested that the plans and policies for controlling malaria should be streamlined to tackle the problem in a sustained manner for achieving the long-term goal of elimination in the future. Some suggestions for changes in policy include: (i) The various global malaria control programmes need to focus collectively on the malaria problem in the SEAR, which has not been given the due attention that it deserves. (ii) Community participation, women's co-operatives as well as micro-financing by Gramin (rural) banks should be encouraged for sustainable financing and empowering women for increasing awareness about the disease. (iii) There is a need to capture the vector surveillance as a unified resource system, which should also incorporate the issue of insecticide resistance. (iv) The public health measures for vector control should be further strengthened in the policy, in order to be more effective and implementable. (v) Common public health interventions, particularly the bioenvironmental control measures should be incorporated seamlessly into the IVM programme. (vi) R&D should be directed at exploring the opportunity of incorporating a common biological control strategy against arboviral diseases as well as malaria using Wolbachia. (vii) All the IVM technologies as well as LLINs should be brought into the policy framework as quickly as possible. (viii) There is a need to strengthen the Research-Policy Interface by bringing together all the relevant stakeholders, including academia, industry, and government onto a single platform.
Acknowledgment
The authors thank all those who generously provided their original published work on various aspects of malaria research pertaining to the South East Asia Region, which has immensely enriched this review. The authors would also like to thank the Department of Biotechnology, Government of India for funding and continued support in various studies.
References
- Burden of malaria in India: Retrospective and prospective view. Am J Trop Med Hyg. 2007;77:69-78.
- [Google Scholar]
- Fake malaria drugs fuel rise of drug-resistant disease. Shots Health News from NPR. Available from: http://www.npr.org/blogs/health/2012/12/20/167282184/fakemalaria-drugs-fuel-rise-of-drug-resistant-disease
- [Google Scholar]
- World Malaria Report 2008. World Health Organization, Geneva. Available from: http://www.who.int/malaria/publications/atoz/9789241563697/en/index.html
- [Google Scholar]
- Roll Back Malaria: Global Malaria Action Plan for a malariafree world. Available from: http://www.rollbackmalaria.org/gmap/3-4.htm
- [Google Scholar]
- World Malaria Report 2010. World Health Organization, Geneva. Available from: http://www.who.int/malaria/world_malaria_report_2010/en/index.html
- [Google Scholar]
- Adult and child malaria mortality in India: a nationally representative mortality survey. Lancet. 2010;376:1768-74.
- [Google Scholar]
- Malaria in Vietnam: environment, prevention and treatment. Bulletin de la Société de Pathologie Exotique. 1993;86:494-9.
- [Google Scholar]
- A field study on malaria prevalence in southeastern Laos by polymerase chain reaction assay. Am J Trop Med Hyg. 2001;64:257-61.
- [Google Scholar]
- Trend of malaria incidence in highly endemic provinces along the Thai borders, 1991-2001. Southeast Asian J Trop Med Public Health. 2003;34:486-94.
- [Google Scholar]
- Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165-71.
- [Google Scholar]
- Anopheline species complexes in Southeast Asia, Technical Publication, SEARO No. 18. New Delhi: World Health Organization Regional Office for South-East Asia; 1998.
- [Google Scholar]
- Malaria vectors in the changing environment of the southern Punjab, Pakistan. Trans R Soc Trop Med Hyg. 2004;98:442-9.
- [Google Scholar]
- Anopheles fluviatilis complex: host feeding pattern of species S, T and U. J Am Mosq Control Assoc. 1996;12:147-9.
- [Google Scholar]
- Molecular characterization and species identification of the Anopheles dirus and Anopheles minimus complexes in north-east India using rDNA ITS-2. Acta Trop. 2006;100:156-61.
- [Google Scholar]
- Anopheles minimus: its bionomics and role in the transmission of malaria in Assam, India. Bull World Health Organ. 1996;74:61-6.
- [Google Scholar]
- Feeding pattern of Anopheles dirus, the major vector of forest malaria in North-east India. Southeast Asian J Trop Med Public Health. 1996;27:378-81.
- [Google Scholar]
- Highly efficient dry season transmission of malaria in Thailand. Trans R Soc Trop Med Hyg. 1990;84:22-8.
- [Google Scholar]
- Detection of Plasmodium vivax and Plasmodium falciparum circumsporozoite antigen in anopheline mosquitoes collected in southern Thailand. Am J Trop Med Hyg. 1996;54:114-21.
- [Google Scholar]
- Female body size, parity and malaria infection of Anopheles maculatus in Peninsular Malaysia. J Med Entomol. 1992;29:379-83.
- [Google Scholar]
- Some entomological observations on temporal and spatial distribution of malaria vectors in three villages in northwestern Thailand using a geographic information system. Southeast Asian J Trop Med Public Health. 2003;34:505-16.
- [Google Scholar]
- Preliminary studies of Anopheles mosquitoes in eight provinces in Lao PDR. Southeast Asian J Trop Med Public Health. 2001;32:83-7.
- [Google Scholar]
- Species composition of adult Anopheles populations and their breeding habitats in Hulu Perak district, peninsular Malaysia. Southeast Asian J Trop Med Public Health. 2002;33:547-50.
- [Google Scholar]
- Epidemic malaria in the Menoreh Hills of Central Java. Am J Trop Med Hyg. 2002;66:287-92.
- [Google Scholar]
- Stream-bank shade and larval distribution of the Philippine malaria vector Anopheles flavirostris. Med Vet Entomol. 2002;16:347-55.
- [Google Scholar]
- A cost comparison of two malaria control methods in Kyunggi Province, Republic of Korea, using remote sensing and geographic information systems. Am J Trop Med Hyg. 2002;66:680-5.
- [Google Scholar]
- Polymerase chain reaction diagnosis and the changing pattern of vector ecology and malaria transmission dynamics in Papua New Guinea. Am J Trop Med Hyg. 2004;71:277-84.
- [Google Scholar]
- Negative epistasis between the malaria-protective effects of α+ - thalassemia and the sickle cell trait. Nat Genet. 2005;37:1253-7.
- [Google Scholar]
- Drug resistant malaria on the Thai-Myanmar and Thai-Cambodian borders. Southeast Asian J Trop Med Public Health. 2001;32:41-9.
- [Google Scholar]
- Dihydroartemisinin-piperaquine versus chloroquine in the treatment of Plasmodium vivax malaria in Thailand: A randomized controlled trial. Clin Infect Dis. 2011;53:977-84.
- [Google Scholar]
- Glucose-6-phosphate deficiency and antimalarial drug development. Am J Trop Med Hyg. 2007;77:779-89.
- [Google Scholar]
- Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619-20.
- [Google Scholar]
- In vivo sensitivity monitoring of mefloquine monotherapy and artesunate-mefloquine combinations for the treatment of uncomplicated falciparum malaria in Thailand in 2003. Trop Med Int Health. 2006;11:211-9.
- [Google Scholar]
- Manslaughter by fake artesunate in Asia – will Africa be next? PLoS Med. 2006;3:e197.
- [Google Scholar]
- Science and Development Network. Health Features: Malaria; Resistance spreads: malaria in South-East Asia. Available from: http://www.scidev.net/en/health/malaria/features/resistance-spreads-malaria-in-southeast-asia.html
- [Google Scholar]
- Correlation of glutathione S-transferase and DDT dehydrochlorinase activities with DDT susceptibility in Anopheles and Culex mosquitoes from northern Thailand. Southeast Asian J Trop Med Public Health. 2000;31:111-8.
- [Google Scholar]
- Insecticide-induced behavioral responses of Anopheles minimus, a malaria vector in Thailand. J Am Mosq Control Assoc. 2001;17:13-22.
- [Google Scholar]
- Excito-repellency of deltamethrin on the malaria vectors, Anopheles minimus, Anopheles dirus, Anopheles swadwongporni, and Anopheles maculatus, in Thailand. J Am Mosq Control Assoc. 2004;20:45-54.
- [Google Scholar]
- The insecticide resistance status of malaria vectors in the Mekong region. Malar J. 2008;7:102.
- [Google Scholar]
- Response of Anopheles culicifacies sibling species A and B to DDT and HCH in India: implications in malaria control. Med Vet Entomol. 1988;2:219-23.
- [Google Scholar]
- Reduced susceptibility to deltamethrin in Anopheles culicifacies S.l. in district Ramanathapuram in Tamil Nadu: Selection of pyrethroid resistant strain. Curr Sci. 2002;82:185-8.
- [Google Scholar]
- Pyrethroid resistance in An. culicifacies in Surat district, Gujarat, West India. Curr Sci. 2002;82:547-50.
- [Google Scholar]
- Combating resistance to insecticides in malaria control - gains made in India. Public Health. 2006;80:30-7.
- [Google Scholar]
- Review of malaria and its control in India. In: Sharma VP, ed. Proceedings of Indo-UK Workshop on Malaria. Delhi: Malaria Research Centre; 1984. p. :13-40.
- [Google Scholar]
- Textbook of malaria eradication (2nd ed). London: Oxford University Press; 1969.
- The epidemiology of human malaria as an explanation of its distribution, including some implications for its control. In: Wernsdorfer WH, McGregor SI, eds. Malaria, principles and practice of malariology. London: Churchill Livingstone; 1988. p. :913-98.
- [Google Scholar]
- Inter-governmental Panel on Climate Change (IPCC) Fourth Assessment Report: Climate Change. 2007. Available from: http://www.ipcc.ch/publications_and_data/ar4/syr/en/contents.html
- [Google Scholar]
- Impact of climate change on malaria in India with emphasis on selected sites. In: Proceedings of the NATCOM V&A Workshop on Water Resources, Coastal Zones and Human Health. New Delhi: Indian Institute of Technology (IIT); 2003. p. :127-31.
- [Google Scholar]
- National Workshop on Climate Change and its Impacts on Health. World Health Organization and National Environmental Engineering Research Institute, Lonavala, India. 2007 November 26-27
- [Google Scholar]
- Mekong malaria. Malaria, multi-drug resistance and economic development in the greater Mekong Subregion of Southeast Asia. Southeast Asian J Trop Med Public Health. 1999;30:1-101.
- [Google Scholar]
- Magnitude of forest related malaria in the WHO Southeast Asia Region. In: Sharma VP, Kondrashin AV, eds. Forest Malaria in Southeast Asia - Proceedings of an Informal Consultative Meeting, February 18-22, 1991. New Delhi: World Health Organization; 1991. p. :29-53.
- [Google Scholar]
- Perspectives of forest malaria in India. In: Sharma VP, Kondrashin AV, eds. Forest Malaria in Southeast Asia – Proceedings of an Informal Consultative Meeting, February 18-22, 1991. New Delhi: World Health Organization; 1991. p. :81-91.
- [Google Scholar]
- Social, behavioral, housing factors and their interactive effects associated with malaria occurrence in east Thailand. Southeast Asian J Trop Med Public Health. 1986;17:386-92.
- [Google Scholar]
- Behaviors in self-prevention of malaria among mobile population in east Thailand. Southeast Asian J Trop Med Public Health. 1995;26:213-8.
- [Google Scholar]
- Poverty and malaria: a study in a Thai-Myanmar border area. Southeast Asian J Trop Med Public Health. 2001;32:608-14.
- [Google Scholar]
- Social and cultural aspects of malaria. Southeast Asian J Trop Med Public Health. 2001;32:727-32.
- [Google Scholar]
- Changing patterns of forest malaria among the mobile adult male population in Chumkiri District, Cambodia. Acta Trop. 2008;106:207-12.
- [Google Scholar]
- Migration malaria associated with forest economy in central India. Curr Sci. 2004;87:1696-9.
- [Google Scholar]
- A study on human behavior and socio-economic factors affecting malaria transmission and control in Qiongzhong, Hainan. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 1995;13:89-93.
- [Google Scholar]
- The human behavioral and socioeconomic determinants of malaria in BacanIsland, North Maluku, Indonesia. J Epidemiol. 2000;10:280-9.
- [Google Scholar]
- Knowledge and behavior relating to malaria in malaria endemic villages of Khammouane Province, Lao PDR. Southeast Asian J Trop Med Public Health. 2002;33:246-54.
- [Google Scholar]
- Maternal influence on the use of impregnated bednets in the protection of infantile malaria. Southeast Asian J Trop Med Public Health. 1998;29:702-5.
- [Google Scholar]
- World Health Organization. 2010. Guidelines for the Treatment of Malaria. (2nd ed). Geneva: WHO; Available from: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf
- [Google Scholar]
- Application of mobile-technology for disease and treatment monitoring of malaria in the “Better Border Healthcare Program”. Malar J. 2010;9:237.
- [Google Scholar]
- World Health Organization. WHO Pesticides Evaluation Scheme: “WHOPES”. Available from: http://www.who.int/whopes/en/
- [Google Scholar]
- Insecticide-treated nets, the key element for rolling back malaria in north-eastern India: policy and practice. Open Entomol J. 2008;2:14-20.
- [Google Scholar]
- Acceptability, willing to purchase and use long lasting insecticide treated mosquito nets in Orissa State, India. Acta Trop. 2009;112:149-55.
- [Google Scholar]
- World Health Organization: Regional Strategic Framework for Scaling Up the Use of Insecticide-treated Nets. World Health Organization Regional Office for South-East Asia, New Delhi. 2005. Available from: http://www.searo.who.int/LinkFiles/Reports_MAL-239_&_VBC-87.pdf
- [Google Scholar]
- World Health Organization: Report of the WHO informal consultation. Test procedures for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticides on treated surfaces. In: WHO/CDS/CPC/MAL/98.12. Geneva: WHO; 1998.
- [Google Scholar]
- Experimental hut comparisons of nets treated with carbamate or pyrethroid insecticides, washed or unwashed, against pyrethroid-resistant mosquitoes. Med Vet Entomol. 2004;18:134-40.
- [Google Scholar]
- Experimental hut evaluation of bednets treated with an organophosphate (chlorpyrifos-methyl) or a pyrethroid (lambdacyhalothrin) alone and in combination against insecticide-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes. Malar J. 2005;4:25.
- [Google Scholar]
- Community perceptions and practices about urban malaria prevention and control in Gondar town, northwest Ethiopia. Ethiop Med J. 2007;45:343-51.
- [Google Scholar]
- Knowledge, attitude and practice on malaria: a study in a tribal belt of Orissa state, India with reference to use of long lasting treated mosquito nets. Acta Trop. 2009;112:137-42.
- [Google Scholar]
- World Health Organization. Global Strategic Framework for Integrated Vector Management. 2004; WHO/CDS/CPE/PVC/2004.10. World Health Organization, Geneva. Available from: http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE_PVC_2004_10.pdf
- [Google Scholar]
- Predictive habitat modelling for forest malaria vector species An. dirus in India – a GIS-based approach. Curr Sci. 2001;80:1129-34.
- [Google Scholar]
- Enhancing malaria control using a computerized management system in southern Africa. Malar J. 2003;2:13.
- [Google Scholar]
- Geographical disparities in core population coverage indicators for roll back malaria in Malawi. Int J Equity Health. 2007;6:5.
- [Google Scholar]
- Larvivorous fish against malaria vectors. Trans R Soc Trop Med Hyg. 2009;103:210-1.
- [Google Scholar]
- Resistance to Bacillus sphaericus in Culex quinquefasciatus Say 1823. Curr Sci. 1995;69:695-8.
- [Google Scholar]
- The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010;6:e1000833.
- [Google Scholar]
- Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc Natl Acad Sci USA. 2012;109:255-60.
- [Google Scholar]
- Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011;7:e1002043.
- [Google Scholar]






