Translate this page into:
Renal expression of hypoxia inducible factor-1α in patients with chronic kidney disease: a clinicopathologic study from nephrectomized kidneys
Reprint requests: Dr Horng-Rong Chang, Division of Nephrology, Department of Internal Medicine, Chung Shan Medical University Hospital, No.110, Section 1, Jianguo N. Road, Taichung 402, Taiwan e-mail: chr@csmu.edu.tw
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Hypoxia inducible factor-1α (HIF-1α) has been shown to play a role in the pathogenesis of renal interstitial fibrosis. However, the relationship of HIF-1α expression intensity in human renal tissue with the degree of renal function or renal fibrosis has not been investigated. We therefore, undertook this study to assess the relationship between HIF-1α expression and degree of renal impairment and renal fibrosis using renal tissue from nephrectomized kidneys from patients with chronic kidney disease.
Methods:
This retrospective study was performed with 70 patients undergoing unilateral or bilateral nephrectomy because of renal cell carcinoma, urothelial cell carcinoma, or renal abscess. Immunohistochemical analysis of HIF-1α expression in non-tumourous or non-abscess renal parenchyma was performed. The patients were divided into two groups: group 1 (n=37) with low intensity HIF-1α expression and group 2 (n=33) with high intensity HIF-1α expression.
Results:
The intensity of renal HIF-1α expression was significantly associated with serum creatinine level (P=0.005), estimated glomerular filtration rate (P=0.02), fibrosis score of the interstitium (P=0.004) and glomerular sclerosis (P=0.013). A high intensity of HIF-1α expression tended to be associated with lower serum creatinine, higher estimated glomerular filtration rate, low interstitial fibrosis score and low glomerular sclerosis. In addition, multivariate analysis by step-wise logistic regression demonstrated that interstitial fibrosis was the only independent factor associated with the intensity of renal HIF-1α expression (OR 4.107, CI 1.535-11.313, P=0.005).
Interpretation & conclusions:
This study demonstrated a correlation between intensity of HIF-1α expression and degree of renal interstitial fibrosis. The association demonstrated an elevated HIF-1α expression in less severe kidney disease. The intensity of HIF-1α renal expression plays a role in the pathogenesis of chronic kidney disease.
Keywords
Chronic kidney disease
GFR
hypoxia inducible factor-1α
nephrectomy
renal fibrosis
renal function
Renal function impairment is associated with the degree of renal fibrosis which is closely related to renal tubulointerstitial damage, and the final common pathway of renal failure is mainly in the tubulointerstitium1–3. Chronic hypoxia of the kidney has been proposed to induce tubulointerstitial injury45. Under hypoxic conditions, hypoxia inducible factor (HIF) plays a major role in the genetic expression of hypoxic adaption in a coordinated manner by upregulating a number of genes involved in angiogenesis, erythropoiesis, and energy metabolism6. It also induces the hypoxia-response element for stress, and improves tissue oxygenation and cell survival in a low oxygen environment.
0 HIF is a heterodimeric transcription factor belonging to the basic helix-loop-helix PER-ARNT-SIM family of proteins7. It consists of functional α-subunits (HIF-1α, HIF-2α, and HIF-3α) and a constitutive β-subunit. HIF-1α transcriptionally upregulates a group of genes including transferrin, vascular endothelial growth factor (VEGF), glucose transporters, glycolytic pathway enzymes, insulin-like growth factor-2, endothelin-1, and inducible nitric oxide synthetase89. HIF-1α is predominantly expressed in tubular epithelial cells and it acts as the major regulator of hypoxic adaptation, whereas HIF-2α is mainly expressed in renal interstitial fibroblast-like cells and endothelial cells10. HIF-2α plays a predominant role in the regulation of erythropoietin (EPO) expression11.
Activation of HIF has been proven in various kidney disease models. In these models of acute kidney injury, HIF accumulation usually occurs near the severely damaged tubules and the ability to induce HIF is inversely related to the severity of cell damage12. VEGF, which is downstream of HIF-1α, promotes the formation of novel capillaries, reduces renal fibrosis and stabilizes renal function13. Stabilization of HIF-1α by chronic administration of cobaltous chloride ameliorated tubulointerstitial injury in a progressive Thy1 nephritis and remnant kidney model14. In addition, the expression of HIF-1α has been suggested to promote the progression of renal disease through profibrotic effects and inflammatory processes15.
Previous studies on the role of HIF in kidney disease have mainly been animal studies1617. Studies on human renal expression of HIF-1α have been reported in immunoglobulin A (IgA) nephropathy, polycystic kidney disease and renal allograft biopsies18–20. Because it is difficult to obtain human renal tissue from patients with chronic kidney disease (CKD), we used nephrectomized kidneys in this study with the aim of examining the relationship between the intensity of HIF-1α expression and the degree of renal impairment and renal fibrosis.
Material & Methods
Study design and patients: This retrospective study was conducted in the Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan. From January 2006 to January 2009, 86 patients received unilateral or bilateral nephrectomy with the causes of renal cell carcinoma (RCC), urothelial cell carcinoma (UCC) or renal abscess. None of these patients had received radiation therapy or chemotherapy before surgery. The complete medical records were reviewed and the estimated glomerular filtration rate (eGFR) was calculated by the abbreviated Modification of Diet in Renal Disease (aMDRD) formula as follows: 186 × (sCr)-1.145× (age)-0.203× (0.742 if female)21. Patients were excluded if their medical records were incomplete or their levels of serum creatinine varied more than 25 per cent from baseline values (3 months before operation). Sixteen patients were excluded and a total of seventy patients were included in this study. Renal tissue specimens were then collected. CKD was defined according to the K/DOQI guidelines with the following criteria: (i) the presence of GFR < 60 ml/min/1.73 m2 for >3 months, with or without kidney damage; and (ii) the presence of structural or functional kidney damage for >3 months, with or without decreased GFR, and manifested by abnormalities in blood, urine, or imaging tests21. This study protocol was approved by the Institutional Review Board of Chung Shan Medical University Hospital.
Tissue processing: Pathologic material was processed for conventional histologic procedures. Representative sections were taken in the renal parenchyma at least 2 cm away from the tumour areas (in the cases with renal tumours). The formalin-fixed, paraffin-embedded tissues were cut into 4 mm hematoxylin- and eosin stained sections and reviewed to evaluate the glomerular, renal tubular and interstitial conditions. The scoring of fibrosis was based on Banff scoring22 for chronic lesions. The grading of chronic interstitial fibrosis and glomerular changes with quantitation was based on the percentage of cortical parenchymal involved. Interstitial fibrosis in up to 5 per cent of the cortical area was scored 0, 6-25 per cent scored 1, 26-50 per cent scored 2, and more than 50 per cent scored 3. We defined scores of 0 and 1 as low interstitial fibrosis, and scores of 2 and 3 as high interstitial fibrosis. The grading of sclerosis was as follows. No glomerulopathy, double contours in less than 10 per cent of peripheral capillary loops in the most severely affected glomerulus scored 0, double contours affecting up to 25 per cent of peripheral capillary loops in the most affected non-sclerotic glomeruli scored 1, 25-50 per cent scored 2, and more than 50 per cent scored 3. Scores of 0 and were defined 1 as low glomerular sclerosis, and scores of 2 and 3 as high glomerular sclerosis.
Analyses of HIF-1α expression by immunohistochemistry: Formalin-fixed and paraffin-embedded specimens were sectioned at a thickness of 3 Am. All sections were then deparaffinized in xylene, rehydrated through serial dilutions of alcohol, and washed in PBS (pH 7.2), the buffer which was used for all subsequent washes. For HIF-1α detection, sections were heated in a microwave oven twice for 5 min in citrate buffer (pH 6.0), and then incubated with a monoclonal anti-goat HIF-1α antibody (DAKO, Hamburg, Germany) diluted 1:2000 in citrate butter DO7; at a dilution of 1:250) for 60 min at 25°C. The conventional streptavidin peroxidase method (DAKO, LSAB Kit K675) was used to develop the signals, and the cells were counterstained with hematoxylin. Negative controls were obtained by leaving out the primary antibody. The antibody dilution buffer was used to replace antibodies to serve as a negative control.
The renal tissue was evaluated independently by three senior pathologists. The intensity of HIF-1α expression was analyzed by immunohistochemistry. Immunoreactivity for HIF-1α was scored based on the percentage of cells involved in the high power field (x400). A positive cell number less than 1 per cent was defined as (-), 1-10 per cent (1+), 11-50 per cent (2+), and more than 50 per cent (3+). We defined (-) and (1+) as a low expression, and (2+) and (3+) as a high expression.
Statistical analysis: Comparisons between continuous variables in non-normal distribution were analyzed by the Mann-Whitney U test, and the t test was applied for continuous variables of normal distribution. Comparisons between categorical variables were analyzed by the chi-square test. Data were expressed as mean ± SD or percentage as needed. Multivariate analysis by logistic regression was applied to detect the independent variables predicting the intensity of renal HIF-1α expression.
Results
Of the 70 patients, 35 (50%) were men. The patients had a mean age of 60.6 ± 13.8 yr, BMI of 24.4 ± 4.9 kg/m2, serum creatinine of 3.0 ± 3.5 mg/dl, eGFR of 47.3 ± 30.3 ml/min, and haemoglobin of 10.3 ± 3.1 g/dl (Table I). Twelve (17.1%) and 29 (41.4%) patients had diabetes mellitus (DM) and hypertension (HTN), respectively.
Pathological assessment: The interstitial fibrosis score (IFS) was 0 in 19 (27.1%) patients, 1 in 15 (21.5%) patients, 2 in 7 (10%) patients, and 3 in 29 (41.4%) patients. Thirty four patients (48.6%) had low IFS and 36 patients (51.4%) had high IFS. The glomerular sclerosis score (GSS) was 0 in 28 (40%) patients, 1 in 12 (17.1%) patients, 2 in 13 (18.6%) patients, and 3 in 17 (24.3%) patients. Forty patients (57.1%) had low glomerular sclerosis and 30 patients (42.9%) had high glomerular sclerosis (Table I).
Immunohistochemistry for HIF-1α: The HIF-1α expression was observed in the cytoplasm of tubule epithelium of the cortical area. No expression of HIF-1α was demonstrated in the fibrotic area. A high intensity of HIF-1α expression was predominantly observed in the cytoplasm of tubule epithelial cells in the kidneys with high eGFR and low fibrotic scores. A low intensity of HIF-1α expression was observed in the kidneys with low eGFR and high fibrotic scores (Figure). In all specimens, the expression of HIF-1α was 0 in 29 (41.5%) patients, 1+ in 8 (11.4%) patients, 2+ in 8 (11.4%) patients, and 3+ in 25 (35.7%) patients. The expression of HIF-1α was low in 37 patients (52.9%) and high in 33 patients (47.1%) (Table I).
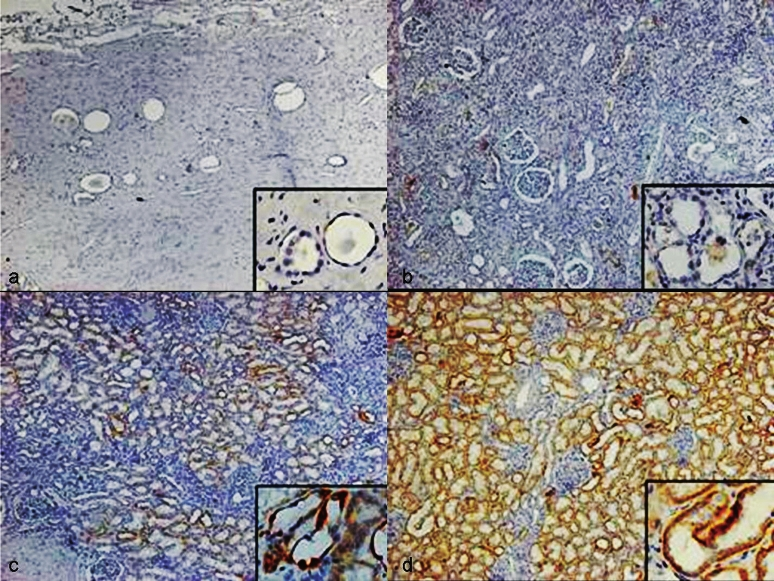
- HIF-1α expression in the epithelium of cortical tubules. (a). Interstitial fibrosis score 3, glomerulus fibrosis score 3, eGFR 7.1 ml/min: HIF-1α expression 0. (b). Interstitial fibrosis score 3, glomerulus fibrosis score 1, eGFR 38.2 ml/min: HIF-1α expression 1. (c). Interstitial fibrosis score 1, glomerulus fibrosis score 0, eGFR 50.1 ml/min: HIF-1α expression 2. (d). Interstitial fibrosis score 0, glomerulus fibrosis score 0, eGFR 101.4 ml/min: HIF-1α expression 3. The magnification for Fig. is 40× and the insets in Fig. a, b, c and d mean higher magnification of corctical tubules with HIF-1α expression (400×).
Correlation between renal HIF-1α expression and clinical characteristics: The patients were divided into two groups: those with low (n=37) or high (n=33) intensity HIF-1α expression. There were significant differences in the IFS, GSS, serum creatinine, and eGFR between these two groups. HIF-1α expression was not significantly different with age (P=0.96), body mass index (P=0.51), haemoglobin (p = 0.19), gender (P=0.473), smoking (P=0.906), diabetes mellitus (P=0.828) or hypertension (P=0.774). A high intensity of HIF-1α expression tended to be associated with a low fibrosis score in both the glomerulus (P=0.013) and interstitium (P=0.004) (Table II).
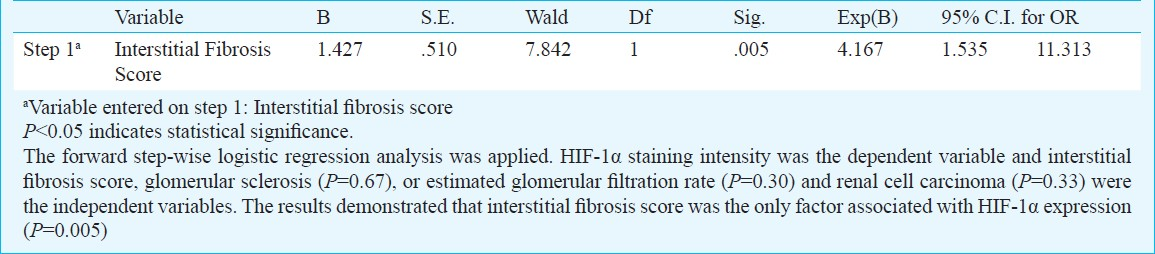
To determine which factors affected the renal expression of HIF-1α, a forward step-wise logistic regression analysis was performed with HIF-1α staining intensity as the categorical dependent variable, and eGFR, severity of glomerular sclerosis or interstitial fibrosis, and RCC as the independent variables. The four variables were entered into the model. Logistic regression analysis showed that IFS (OR 4.107, CI 1.535-11.313) (P=0.005) was the only independent predictor of HIF-1α staining intensity (Table III).
Microenvironmental hypoxia of tumours is an important mechanism of HIF induction, and HIF-1α immunostaining is observed throughout a tumour in clear-cell renal carcinoma and haemangioblastoma23. There was no statistically significant difference in the distribution of UCC, RCC or renal abscess between the groups with high or low HIF-1α expression (P=0.054) (Table II). To confirm that the tumours had no impact on the HIF-1α expression of the renal tissues adjacent to the tumours, the expressions of HIF-1α with RCC (P=0.32), UCC (P=0.51) and their tumour T stages were analyzed, and no association was revealed. To confirm that the infections had no impact on the HIF-1α expression of the renal tissues adjacent to the abscess, the expression of HIF-1α with abscess or no abscess was analyzed, and no association was revealed.
Discussion
Our results demonstrated that HIF-1α was expressed predominantly in the cytoplasm of tubular epithelium in the kidneys with better renal function and less fibrosis. The expression of HIF-1α was decreased in the kidneys with higher fibrosis and lower eGFR. A high fibrosis score of the interstitium was consistently associated with a decreased expression of HIF-1α. An elevated HIF-1α expression was found in less severe kidney disease.
CKD typically displays loss of peritubular capillaries in areas of tubulointerstitial fibrosis. Extensive tubulointerstitial injury results in decreasing capillary blood supply and hypoxia in the region2024. Hypoxia may initiate the development and progression of renal disease, but the molecular mechanism remains unclear. Yuan et al25 found that loss of HIF-1α favours progression of interstitial fibrosis.
HIF activity is primarily regulated by oxygen-dependent proteasomal degradation of the α-subunit. Under normoxic conditions, the α-subunit is hydroxylated by HIF prolyl-hydroxylases that marks HIF as a target for von Hippel-Lindau (VHL) E3 ubiquitin ligase leading to proteasomal degradation. At low oxygen tension or in the absence of von Hippel-Lindau E3 ubiquitin ligase, HIF-α escapes degradation and heterodimerizes with HIF-β. The heterodimer then binds to the transcriptional coactivator CBP/p300. Besides hypoxia, several other co-regulators including reactive oxygen species, ascorbate, succinate, fumarate or NO, and acetyltransferase ARD1 have been described recently26. In the cells with deficient or aberrant VHL protein, HIF-α escapes degradation and accumulates, binding to HIF-β27.
HIF-1α stimulates the expression of vasculogenic genes such as EPO and VEGF to maintain oxygen delivery and to protect cells from ischaemia. HIF-1α exerts a beneficial effect on renal tissues4. At the same time, HIF-1α also induces expression of profibrogenic genes such as tissue inhibitor of metalloproteinase 1 (TIMP1), connective tissue growth factor (CTGF), and plasminogen activator inhibitor 1. HIF-1α accelerates tissue fibrosis by upregulating the profibrogenic factors28.
HIF-1α has been reported to play a role in kidney protection. In the remnant kidney rat model of systemic and glomerular hypertension, the kidney presents with increased renin-angiotensin activity-related glomerular sclerosis and hypocellular tubulointerstitial fibrosis. The cobalt treated group, in which the HIF-1α expression can be stabilized, showed lower scores of tubulointerstitial injury meaning that HIF-1α plays a role in tubulointerstitial protection14. In a rat model of obese and hypertensive type 2 diabetes metabolic diseases, Ohtomo et al29 reported that upregulation of HIF reduced proteinuria and histological kidney injury.
Increased HIF-1α expression has been reported in biopsies of human renal tissue from chronic allograft nephropathy18 and IgA nephropathy20. In human renal allograft biopsies18, abundant HIF-1α expression is present and correlates with a cold ischaemic time of more than 15 h and/or functioning grafts with an age of more than 50 years. A low HIF-1α score correlates with primary non-function, likely reflecting a loss of oxygen consumption for tubular transport23. The renal biopsy specimens from 23 patients with IgA nephropathy were classified according to interstitial injury score: grade 0 (0%), grade 1 (1-25%), grade 2 (26-50%), and grade 3 (51-100%)20. In tubular epithelium, HIF-1α was weakly expressed in grade 0, with increased progression in grade 2, but a marked decrease in grade 3. HIF-1α expression was strong in tubular epithelium but negative in glomerular cells. A decreased intensity of HIF-1α expression in the fibrotic renal tissues but not in the normal parts was found in this study. The reason for the loss of HIF-1α expression in fibrotic kidneys may result from the loss of oxygen consumption in advanced interstitial tubulopathy, as there was an inverse correlation between HIF-1α intensity and the severity of interstitial fibrosis in this study.
HIF is activated in response to hypoxic renal injury. An increased HIF expression has been shown in biopsies from patients with diabetic nephropathy, IgA glomerulonephritis and chronic allograft nephropathy. The degree of HIF expression correlates with the extent of tubular injury. One target gene for HIF is pro-fibrotic connective tissue growth factor (CTGF)30. A stable expression of HIF-1α in tubular epithelial cells promotes interstitial fibrosis28.
It is unclear whether an increased expression of HIF leads to a stabilization of the disease process and thus is nephroprotective or contributes to interstitial fibrosis27. Our finding that HIF expression was inversely associated with fibrosis is contrary to most of the published reports, which suggest that HIF expression is a promoter of fibrosis. Several experimental and human studies have shown this, especially through epithelial mesenchymal transition (EMT). One possible explanation could be that upregulation occurs in the initial stages when EMT is actively taking place, but shuts off once the fibrosis is fully established. Whether the increased activity of HIF is beneficial or harmful is unclear, it may depend on the underling disease and the duration of HIF expression27.
Our findings showed that HIF-1α expression was inversely associated with fibrosis and eGFR. Short-term HIF-1α expression may be beneficial, but prolonged HIF-1α activation may be pro-fibrotic. An elevated HIF-1α expression is protective or elevated in early CKD when active tissue damage is ongoing. Once fibrosis is advanced in the later stages of CKD, the disease burns out resulting in a lower HIF-1α expression.
Overexpression of HIF-1α has been demonstrated in multiple types of human cancer3132. Although we selected renal tissues at least 2 cm away from the tumor areas to prevent the influence of UCC or RCC on the expression of adjacent renal tissue HIF-1α, we found that the presentation of low or high HIF-1α expressions in RCC, UCC or renal abscess was with borderline significance (P=0.054). This is partly because UCC is the most common malignancy in dialysis patients in Taiwan, and chronic tubulointerstitial nephritis is the most likely underlying disease in haemodialysis patients with UCC33.
The limitations of the study include the retrospective nature, cross-sectional characteristics and that the underlying causes of chronic kidney disease were incomplete. Though the renal parenchyma were sampled 2 cm away from the malignancy, the possibility of molecular events occurring there could not be ruled out.
In conclusion, our results demonstrated that the renal expression of HIF-1α was inversely associated with interstitial fibrosis in human renal tissues. The molecular basis needs further studies to clarify.
Acknowledgment
The authors acknowledge the staff of The Institute of Medical and Molecular Toxicology, Chung Shan Medical University, Taiwan, for continuous help and suggestions in conducting the study. This work was suported by grants CSH-2011-A-022 and CSH-2012-C-015 of Chung Shan Medical University Hospital.
References
- Experimental insights into the tubulointerstitial disease accompanying primary glomerular lesions. J Am Soc Nephrol. 1994;5:1273-87.
- [Google Scholar]
- Final common pathways of progression of renal diseases. Clin Exp Nephrol. 2002;6:182-9.
- [Google Scholar]
- Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern Med. 2004;43:9-17.
- [Google Scholar]
- Role of hypoxia in the pathogenesis of renal disease. Kidney Int. 2005;68(Suppl 99):S46-51.
- [Google Scholar]
- Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17-25.
- [Google Scholar]
- Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510-4.
- [Google Scholar]
- The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411-21.
- [Google Scholar]
- Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485-90.
- [Google Scholar]
- Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291:F271-81.
- [Google Scholar]
- Role of chronic hypoxia and hypoxia inducible factor in kidney disease. Chin Med J. 2008;121:257-64.
- [Google Scholar]
- Hypoxia-inducible transcription factors and their role in renal disease. Semin Nephrol. 2007;27:363-72.
- [Google Scholar]
- Impaired angiogenesis in the remnant kidney model: II.Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12:1448-57.
- [Google Scholar]
- Induction of protective genes by cobalt ameliorates tubulointerstitial injury in the progressive Thy1 nephritis. Kidney Int. 2005;68:2714-25.
- [Google Scholar]
- The VHL/HIF oxygen-sensing pathway and its relevance to kidney disease. Kidney Int. 2006;69:1302-7.
- [Google Scholar]
- Drug discovery for overcoming chronic kidney disease (CKD): prolyl-hydroxylase inhibitors to activate hypoxia-inducible factor (HIF) as a novel therapeutic approach in CKD. J Pharmacol Sci. 2009;109:24-31.
- [Google Scholar]
- Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810-20.
- [Google Scholar]
- Immunohistochemical detection of hypoxia-inducible factor-1alpha in human renal allograft biopsies. J Am Soc Nephrol. 2007;18:343-51.
- [Google Scholar]
- Involvement of hypoxia-inducible transcription factors in polycystic kidney disease. Am J Pathol. 2007;170:830-42.
- [Google Scholar]
- Implication of peritubular capillary loss and altered expression of vascular endothelial growth factor in IgA nephropathy. Nephron Physiol. 2006;102:9-16.
- [Google Scholar]
- National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-47.
- [Google Scholar]
- The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713-23.
- [Google Scholar]
- HIF-1alpha expression follows microvascular loss in advanced murine adriamycin nephrosis. Am J Physiol Renal Physiol. 2005;288:F198-206.
- [Google Scholar]
- Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Patholol. 2003;163:2289-301.
- [Google Scholar]
- Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol Dial Transplant. 2011;26:1132-7.
- [Google Scholar]
- Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol. 2008;295:F1023-9.
- [Google Scholar]
- Cobalt ameliorates renal injury in an obese, hypertensive type 2 diabetes rat model. Nephrol Dial Transplant. 2008;23:1166-72.
- [Google Scholar]
- Hypoxic induction of Ctgf is directly mediated by Hif-1. Am J Physiol Renal Physiol. 2004;287:F1223-32.
- [Google Scholar]
- Hypoxia-inducible factor-1 in human breast & prostate cancer. Endocr Relat Cancer. 2006;13:739-49.
- [Google Scholar]
- Hypoxia-inducible factor 1 alpha expression in renal cell carcinoma analyzed by tissue microarray. Eur Urol. 2006;50:1272-7.
- [Google Scholar]
- Renal diagnosis of chronic hemodialysis patients with urinary tract transitional cell carcinoma in Taiwan. Cancer. 2007;109:1487-92.
- [Google Scholar]






