Translate this page into:
Assessing the adhesion of putative indigenous probiotic lactobacilli to human colonic epithelial cells
Reprint requests Dr Sunita Grover, Molecular Biology Unit, Department of Dairy Microbiology, National Dairy Research Institute (ICAR), Karnal 132 001, Haryana, India sungro@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Adherence of bacteria to epithelial cells and mucosal surfaces is a key criterion for selection of probiotic. We assessed the adhesion property of selected indigenous probiotic Lactobacillus strains based on their hydrophobicity and ability to adhere to human epithelial cells.
Methods:
Five human faecal Lactobacillus isolates, one from buffalo milk and one from cheese were assessed for hydrophobicity following the microbial adhesion to hydrocarbons (MATH) method and colonization potentials based on their adherence to Caco2 and HT-29 colonic adenocarcinomal human intestinal epithelial cell lines. Lactobacillus strains that adhered to Caco2 and HT-29 cell lines were quantified by plating after trypsinization and simultaneously the adhered bacteria were also examined microscopically after staining with Geimsa stain and counted in different fields.
Results:
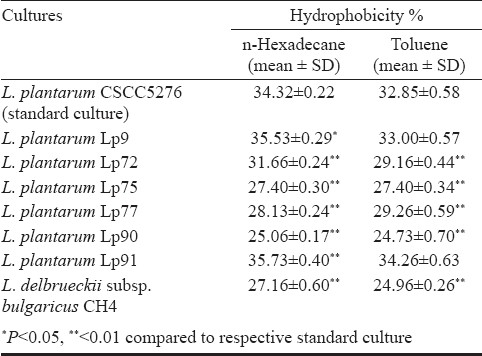
Among the tested faecal isolates, L. plantarum Lp91 showed maximum percentage hydrophobicity (35.73±0.40 for n-hexadecane and 34.26±0.63 for toluene) closely followed by L. plantarum Lp9 (35.53±0.29 for n-hexadecane and 33.00±0.57 for toluene). Based on direct adhesion to epithelial cells, L. plantarum Lp91 was the most adhesive strain to HT-29 and Caco2 cell lines with per cent adhesion values of 12.8 ± 1.56 and 10.2 ± 1.09, respectively. L. delbrukeii CH4, was the least adhesive with corresponding figures of 2.5 ± 0.37 and 2.6 ± 0.20 per cent on HT-29 and Caco2 cell lines. Adhesion of the six isolated Lactobacillus strain to HT-29 cell and Caco2 lines as recorded under microscope varied between 131.0 ± 13.9 (Lp75) to 342.7 ± 50.52 (Lp91) and 44.7 ± 9.29 (CH4) to 315.7± 35.4 (Lp91), respectively.
Interpretation & conclusions:
Two Indigenous probiotic Lactobacillus strains (Lp9, Lp91) demonstrated their ability to adhere to epithelial cell and exhibited strong hydrophobicity under in vitro conditions, and thus could have better prospects to colonize the gut with extended transit
Keywords
Adhesion
Caco2
HT-29
hydrophobicity
Lactobacillus
probiotic
A complex microbiota of more than 1,000 different bacterial species with a density of about 1014 bacterial cells inhabits the oral cavity, gastrointestinal tract (GIT), upper respiratory tract, vagina and skin, and the major part of this microflora resides in human gut1. However, there can be aberrations in the gut flora due to dietary interventions and oral drug based treatment which can be restored by application of probiotics conferring various health benefits2. Hence, for optimal expression of their general and specific physiological functions, their colonization with extended transit time is extremely crucial. In the context of their effective colonization, the ability to adhere to epithelial cells and mucosal surfaces has been suggested to be an important property of many bacterial strains used as probiotics3. Therefore, it is considered as a potential probiotic marker along with other desirable attributes for screening of novel probiotic lactobacilli that can adhere to human intestinal cells45.
Cell adhesion is a complex process involving contact between the bacterial cell membrane and interacting surfaces. Difficulties experienced in studying bacterial adhesion in vivo, especially in humans, have stimulated interest in the development of in vitro models for preliminary screening of potentially adherent strains. The physical and chemical characteristics of the cell surface could be assessed critically based on bacterial cell surface hydrophobicity (depends on surface components of bacteria)6 and electrical mobility/charge (rate of migration under electric field due to bacterial surface charges)7. Both the hydrophobicity and the electric charge are the consequences of the chemical composition of the bacterial surfaces. As microbial adhesion is a complicated interplay of long-range van der Waals and electrostatic forces and various other short-range interactions, strains adhering well to the hydrocarbons are considered to be hydrophobic and strains adhering poorly are considered hydrophilic.
HT-29 and Caco2 cells, the two colonic adenocarcinomas are human intestinal epithelium derived, expressing structural and functional features of normal human enterocytes have been extensively used as in vitro models in the study of human enterocytic function8–10. Chauviere et al8 have reported previously that not all strains of Lactobacillus developed adhesiveness to enterocytes such as Caco2 cells, thereby, indicating that this property is highly strain specific. The present investigation was undertaken with the objective to elucidate the adherence potential of indigenous probiotic Lactobacillus strains isolated from faecal samples from human gut and other sources under in vitro conditions based on their cell surface hydrophobicity and ability to adhere Caco2 and HT-29 cells.
Material & Methods
The study was conducted in the Department of Dairy Microbiology, National Diary Research Institute, Karnal, Haryana, India.
Bacterial strains and growth conditions: Lactobacillus plantarum 9, 72, 75, 77, 90, 91 and L. delbrueckii subsp. bulgaricus CH4 were the laboratory isolates recovered from human gut, buffalo milk and cheese were investigated for their adhesion potential on adeno-carcinomal Caco2 and HT-29 cell lines. The study also included L. plantarum CSCC5276 (also designated as NCDO82 or VTTE-71034)11 (received from Dr N.P. Shah from Victoria University, Australia) which was used as a reference culture. All lactobacilli were grown in MRS broth (deMan, Rogosa and Sharp broth; HiMedia, Mumbai, India) at 37°C for 18–24 h and maintained as glycerol stocks until further use. All the bacterial cultures used in this study were activated by sub-culturing twice in fresh MRS broth prior to cell surface hydrophobicity and adhesion test.
Cell surface hydrophobicity: Cell surface hydrophobicity of isolates and standard culture was determined by microbial adhesion to hydrocarbons (MATH) method described by Geertsema-Doornbusch et al12 using hexadecane and toluene as solvents. The isolates and standard cultures were grown in MRS broth for 16-18 h at 37°C. Cultures were harvested by centrifugation (2000 × g, 15 min, 4°C), washed twice in PUM buffer (K2HPO4: 22.2 g/l; KH2PO4: 7.26 g/l; urea: 1.8 g/l; MgSO4: 0.2g/l; pH 7.1±0.2) and finally suspended in the same buffer. The initial absorbance (A0) at 600 nm of the suspension was adjusted to 0.70±0.02 units. Five ml of cell suspension in PUM buffer was dispensed in clean and dry round bottom test tubes followed by addition of one ml of hexadecane or toluene. The contents were vortexed for 2 min. The tubes were left undisturbed for 1 h at 37°C to allow the phase separation. The lower aqueous phase was carefully removed with a sterile pasteur pipette and absorbance (A1) was recorded at 600 nm. Cell surface hydrophobicity in terms of per cent (H %) was calculated using the following formula:
H % = (1 - A1/A0) × 100
Propagation and maintenance of cell lines: The human adenocarcinoma cell lines namely HT-29 (mucus secreting) and Caco2 (non-mucus secreting) for adhesion assay were procured from Dr Tapas Mukhopadhay, Punjab University; Chandigarh, India, and National Center of Cell Sciences, Pune, India respectively. Both cell lines were cultured in Dulbecco's modified Eagle's minimal essential medium (DMEM; Sigma, USA) supplemented with 10 per cent (v/v) heat-inactivated (30 min, 56°C) foetal bovine serum (Sigma, USA), 25 mM HEPES (Sigma, USA), 100 U/ml penicillin (Sigma, USA), and 100 μg/ml streptomycin (Sigma, USA) in 25 cm2 culture flask at 37°C in an atmosphere of 5 per cent CO2/95 per cent air. The cultures were fed with fresh medium every alternate day. When reached about 80 per cent confluency, cells were harvested by incubating adhered cells with 3 ml of 0.25 per cent Trypsin-EDTA solution (Sigma, USA) at 37°C. The cells were occasionally observed between 5 and 15 min of trypsin addition under inverted microscope. When nearly 60 per cent cells detached from the surface, 7 ml of complete DMEM was added. The cell suspension was repeatedly but gently aspirated to make single cell suspension. The contents were centrifuged (1000 X g, 5 min at room temperature) and the pellet was resuspended in complete DMEM medium. The final cell counts in suspension were measured with the help of haemocytometer (MBG, Germany).
Adhesion assay: Adhesion assay was carried out after 60-90 passages for HT-29 and 40-70 for Caco2 cell lines. Adhesion of the Lactobacillus cultures was measured as per the method described by Jacobsen et al13. The cell suspension with 1 × 105 cells prepared in 4 ml complete DMEM medium was transferred to each well of six-well tissue culture plates. The medium was changed every alternate day. When cells reached 80 per cent confluency, the medium was replenished each day consecutively for 20 days for both the cell lines. The spent medium was completely removed 24 h before adhesion assay and cells were fed with DMEM medium lacking antibiotics. The cells were then washed twice with 3 ml phosphate-buffered saline (PBS, pH 7.4). An aliquot of two ml of DMEM (without serum and antibiotics) was added to each well and incubated at 37°C for 30 min. Different Lactobacillus cultures (at 1 × 109 cfu) suspended in 1 ml DMEM medium (without serum and antibiotics) were added to different wells. The plates were incubated at 37°C in 5 per cent CO2-95 per cent air for 2 h. The monolayers were washed five times with sterile PBS (pH 7.4). The adhesion score was measured by enumerating adhered bacteria per 20 different microscopic fields. Per cent adhesion was determined by plating method.
Adhesion score: Methanol was added to each well of six-well plate at the rate of 3 ml followed by incubation for 10 min at room temperature. Methanol was completely removed and fixed cells were stained with Giemsa stain (0.72% w/v; BDH, London) for 20 min at room temperature. The wells were washed with ethanol to remove excess stain. The plates were air dried and examined under oil immersion microscope (Leica, Germany). The number of bacteria was counted in 20 random microscopic fields and were grouped into non adhesive (≤40 bacteria), adhesive (41-100 bacteria) and strongly adhesive (>100 bacteria)13.
Per cent adhesion: Cells from monolayers were detached by trypsinization. One ml 0.25 per cent trypsin-EDTA solution (Sigma, USA) was added to each well of six-well plate and plate was incubated for 15 min at room temperature. The detached cells were repeatedly but gently aspirated to make homogenous suspension. The cell suspension was then serially diluted with saline solution and plated on MRS agar. The plates were incubated for 24-48 h at 37°C and colonies were counted (B1 cfu/ml). Bacterial cells initially added to each well of six-well plates were also counted (B0 cfu/ml). The adhesion percentage was then calculated as:
% adhesion= (B1 / B0) * 100
Statistical analysis: Statistical package SYSTAT (version 6.0.1 1996, SPSS INC., USA) software was used to analyze the data. ANOVA- Post-hoc test (Bonferroni) was used to compare the difference among the test strains.
Results
All the five Lactobacillus strains isolated from human faecal samples namely L. plantarum Lp72, L. plantarum Lp75, L. plantarum Lp77, L. plantarum Lp90, L. plantarum Lp91 along with one milk isolate L. plantarum Lp9 and one cheese isolate L. delbrueckii subsp. bulgaricus CH4 were investigated for their adhesion potential based on in vitro cell surface hydrophobicity and adherence on Caco2 and HT-29 cell lines. L. plantarum Lp9 and Lp91 exhibited significantly (P<0.05, P<0.01, respectively) higher hydrophobicity compared to L. plantarum CSCC 5276 in n-hexadecane. However, when toluene was used in the assay, the hydrophobicity of these two isolates was found to be almost similar to the standard culture. Other isolates viz. L. plantarum Lp72, L. plantarum Lp75, L. plantarum Lp77, L. plantarum Lp90 and L. delbrueckii subsp. bulgaricus CH4 had significantly (P<0.01) lower per cent hydrophobicity with n-hexadecane as compared to the standard culture (Table.). Hence, on comparative analysis, Lp91 was the most efficient culture expressing significantly higher per cent hydrophobicity in n-hexadecane versus the standard culture.
The quantitative binding of the Lactobacillus test cultures was also investigated on HT-29 and Caco-2 cell lines by two independent methods i.e. direct microscopic examination after Giemsa staining and enumeration by plating on MRS (Figs 1, 2). All the test cultures adhered to HT-29 cell lines albeit at different levels. On comparative evaluation, L. plantarum Lp91 (342.7 ± 50.52), L. plantarum Lp9 (321.3 ± 20.50) and L. plantarum Lp77 (260.7 ± 16.07) were the most adhesive strains based on their respective adhesion score while there was no significant difference with that of control strain L. plantarum CSCC5276 (278.7 ± 16.50) (Fig. 3). Remaining four isolates Lp72 (188.7 ± 14.30), Lp75 (131.0 ± 13.89), Lp90 (144.0 ± 7.55) and CH4 (34.0 ± 7.55) differed significantly from positive control strain (P<0.01). L. delbrueckii subsp. bulgaricus CH4 (34.0 ± 7.55) was the least adhesive strain. More or less, a similar trend in adhesion property of the test cultures was recorded with Caco-2 cell line (Fig. 2) although relatively at a lower rate as indicated by their adhesion scores. In this case also, L. plantarum Lp91 was the most adhesive strain (315.7 ± 35.47) followed by L. plantarum Lp9 (256.7 ± 13.58), L. plantarum Lp77 (192.7 ± 16.50), L. plantarum Lp90 (172.0 ± 17.06), L. plantarum Lp72 (162.3 ± 19.22), and L. plantarum Lp75 (137.7 ± 8.33) respectively. In comparison to these, adhesion score of CSCC5276 was 264.7 ± 29.02. L. delbrueckii subsp. bulgaricus CH4 (44.7 ± 9.29) again showed least adhesion property among the isolates. There was no significant difference in the adhesion score of L. plantarum Lp9 and L. plantarum Lp91 with that of L. plantarum CSCC5276 (positive control). However, the adhesion score of L. plantarum Lp77 was significantly lower (P<0.05). The remaining four strains demonstrated much lower level of adhesion score (P<0.01). All the lactobacilli isolates were categorized as strongly adhesive (>100 bacteria / 20 microscopic fields) except CH4 which was considered as non adhesive strain (≤ 40 bacteria / 20 microscopic fields) in both the cell lines.
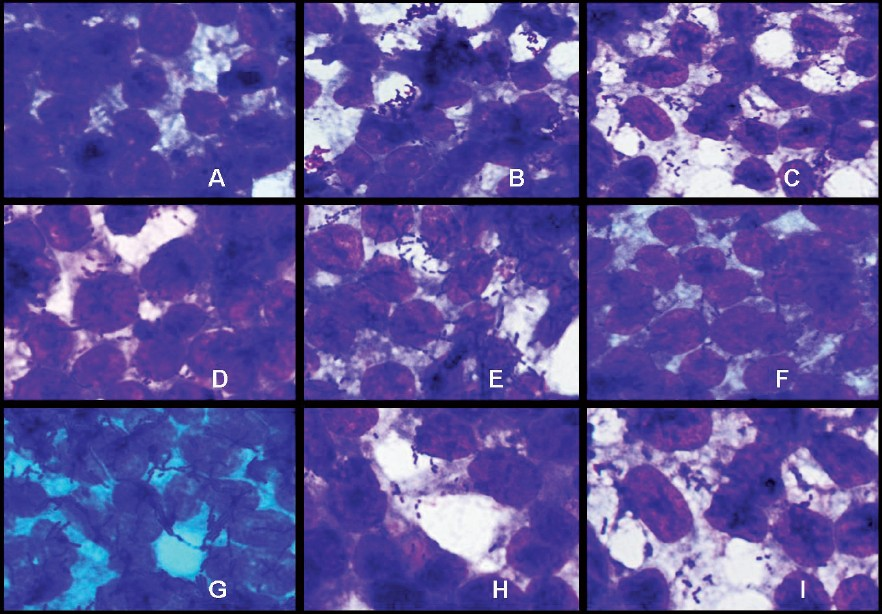
- Adhesion of Lactobacillus strains on HT-29 cell cultures observed under oil immersion microscope (100X) after staining with Geimsa strain. A-Blank HT-29 cell line, B-L. plantarumCSCC 5276, C- L. plantarumLp91, D-L. plantarumLp9, E-L. plantarumLp72, F-L. plantarumLp75, G- L. plantarumLp77, H- L. plantarumLp90, I- L. delbrueckiisubsp. bulgaricusCH4.
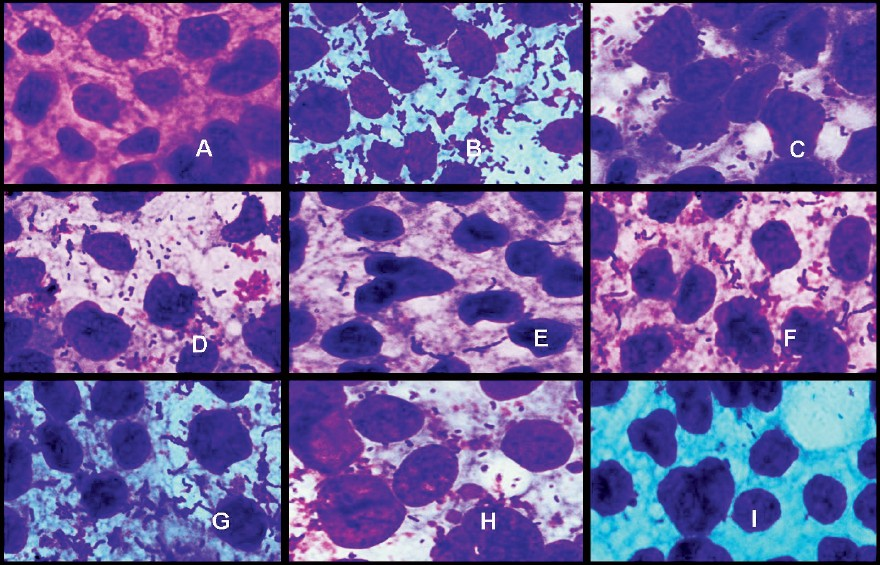
- Adhesion of Lactobacillus strains on Caco2 cell cultures observed under oil immersion microscope (100X) after staining with Geimsa strain. A-Blank Caco2 cell line, B- L. plantarumCSCC 5276, C- L. plantarumLp91, D- L. plantarumLp9, E- L. plantarumLp72, F- L. plantarumLp75, G- L. plantarumLp77, H- L. plantarumLp90, I- L. delbrueckiisubsp. bulgaricusCH4.
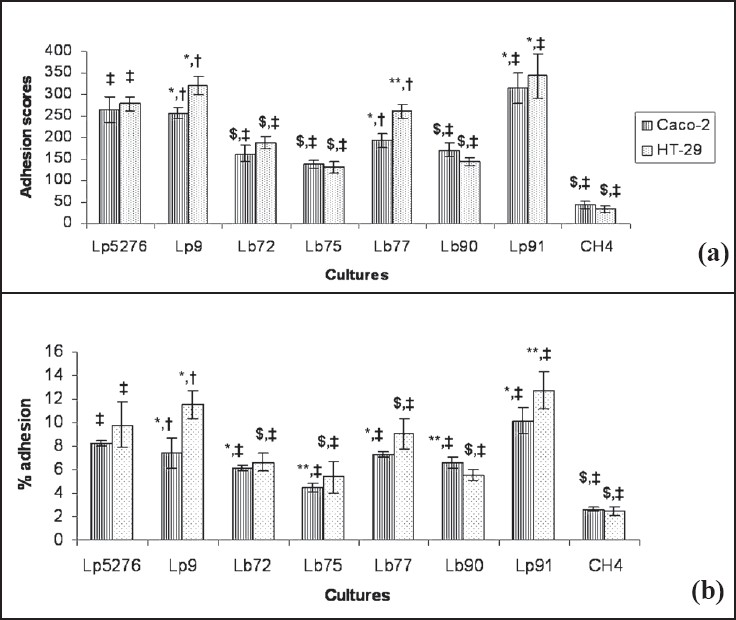
- Adhesion of Lactobacillus strains to Caco2 and HT-29 cell lines. Lp5276 positive control. *There is no significant difference with positive control with in the cell line. **Significant difference from positive control (ANOVA pair-wise test, P<0.05). $Significant difference from positive control (ANOVA pair-wise test, P<0.01). #There is no significant difference when isolates were compared with two different cell lines. +Significant difference when isolates were compared with two different cell lines (ANOVA pairwise test, P<0.05).
The observations with regard to adhesion scores obtained using HT-29 can be further corroborated by the per cent adhesion values i.e. 12.8 ± 1.56, 11.5 ± 1.21 and 9.0 ± 1.30 in respect of Lp91, Lp9 and Lp77 vis a vis the standard culture CSCC5276 (9.8 ± 1.95). L. plantarum Lp72, Lp75 and Lp90 also exhibited moderate adhesion property with per cent adhesion values of 6.6 ±.0.81, 5.4 ± 1.37 and 5.6 ± 0.48 with HT-29 cells. L. delbrueckii subsp. bulgaricus CH4, cheese isolate showed least adhesion property (2.5± 0.37 %) as compared to other isolates from human faecal samples. Lp75 and Lp90 showed significant difference in per cent adhesion (P<0.05) from the positive control. Per cent adhesion values like adhesion score obtained with Lactobacillus isolates on Caco2 cells were again relatively lower as compared to HT-29 cell lines Fig. 3. Based on per cent adhesion, L. plantarum Lp91, L. plantarum Lp9 and L. plantarum Lp77 were the most adhesive strains (10.2 ± 1.09, 7.4 ± 1.34 and 7.3 ± 0.28%, respectively) as compared with other isolates viz. 6.1 ± 0.24, 4.4 ± 0.38 and 6.6 ± 0.47 per cent for L. plantarum Lp72, L. plantarum Lp75 and L. plantarum Lp90, respectively. No significant difference was recorded between L. plantarum Lp9 and L. plantarum CSCC5276 (positive control). L. plantarum Lp91 (P<0.05) and L. plantarum Lp72, L. plantarum Lp75, L. plantarum Lp77, L. plantarum Lp90 and L. delbrueckii subsp. bulgaricus CH4 (2.6 ± 0.20%) showed significant difference (P<0.01) from that of control.
Irrespective of the methods and cell lines used for adhesion assay, there was no significant difference in per cent adhesion between all the isolates except L. plantarum Lp9 which showed significant difference (P<0.05) with both the methods in different cell lines. Binding of L. plantarum Lp9 and L. plantarum Lp77 was significantly higher (P<0.05) on HT-29 as compared to Caco2 cells (Fig. 3). The binding of cheese isolate L. delbrueckii subsp. bulgaricus CH4 was lowest amongst all the isolates and significantly lower (P<0.01) to that of L. plantarum CSCC5276 with both the methods on both cell lines.
Discussion
One of the important properties of probiotic bacteria including lactobacilli is their ability to adhere to the target sites for their colonization in the gut for expressing optimal functionality. Caco2 and HT-29 cell line in vitro models for probiotic adherence studies have been extensively used to screen putative probiotic cultures8914–16. The organisms must adhere to mucosal epithelial cells lining the gut to be designated as probiotic67 which also depends on the number of bacteria added17. The level of adhesion of bacterial strains positively correlates with the number of bacteria added upon certain point when the saturation of potential binding sites on cell lines probably occurs18. Screening of isolates based on per cent adhesion to HT-29 and Caco2 cells is preferred as it simulates to in vivo situations.
Adhesion of bacteria is a complex process involving contact between both the bacterial cell membrane and interacting surfaces. One of the important properties of bacteria is cell surface hydrophobicity. Bacterial adherence has been suggested to be the result of two essentially different mechanisms: specific and nonspecific binding19. Non specific binding involves electrostatic or hydrophobic interactions of lower affinity than in specific binding. Piette and Idziak19 have reported that cell-surface charge and hydrophobicity can considerably influence the strength of adhesion. The role of nonspecific hydrophobic interactions in bacterial adherence has led to the development of a wide variety of investigative methods such as microelectrophoresis, contact angle measurements20 or MATH in a two-phase system21. In spite of several studies on the cell surface hydrophobicity and charges of lactobacilli, these physico-chemical aspects remain poorly understood. In the present investigation, MATH was used to determine the cell surface characteristics and the potential ability of Lactobacillus strains to adhere to a support. The results pertaining to hydrophobicity of the test cultures used in this study showed similar trends with both n-hexadecane and toluene indicating that either of the solvents can be employed in hydrophobicity assay. Based on the results emerging from this study, L. plantarum Lp9 and L. plantarum Lp91 can be explored as potentially putative probiotic strains as these both exhibited a strong hydrophobicity which was comparable or even better than that of CSCC5276 used as a positive control. Most of the lactobacilli tested in this study exhibited strong hydrophobicity both in hexadecane as well as in toluene. Our results in this regard are in agreement with those obtained from previous studies2223.
In the present investigation, the numbers of bacteria adhering to HT-29 and Caco2 cell lines were measured examining them directly under microscope after staining and also by colony count on agar after trypsinization. On comparative evaluation based on adhesion score, isolates L. plantarum Lp91 and L. plantarum Lp9 were the most adhesive strains while there was no significant difference with that of control strain L. plantarum CSCC5276 in both the cell lines. However, the remaining four isolates (Lp72, Lp75, Lp77 and Lp90) differed significantly (P<0.01) from positive control strain. L. delbrueckii CH4 showed least adhesion property among the isolates. Adhesion scores of all the isolates except L. delbrueckii subsp. bulgaricus CH4 were more than 100 on both cell lines and therefore, L. plantarum Lp9, Lp72, Lp75, Lp77, Lp90 and Lp91 can be regarded as strongly adhesive to both cell lines as per classification suggested by Jacobson et al13. Tuomola and Salminen17 studied adhesion of 12 different Lactobacillus strains using Caco2 cell line as an in vitro model for intestinal epithelium using flow cytometer of bacteria stained with LIVE/DEAD® Bac Light™ Bacterial Viability Kit to check viability of bacteria after adhesion, radiolabelled bacteria (incubating with methyl-1,2-[3H]-thymidine) by liquid scintillation and Gram's staining of adhered bacteria under microscope and reported no significant difference in the adhesion of the strains by all the methods.
The adhesion assay especially microscopic enumeration is prone to error since Lactobacillus exits as in chains and these are not uniformly distributed in microscopic field. L. plantarum Lp75 and L. plantarum Lp77 were present in long chain and were similar to standard culture. In contrast, promising isolates L. plantarum Lp9 and L. plantarum Lp91 were present in short chains. The number of adhered bacteria to the cell lines was determined by colony count on agar after trypsinization, since it enables to enumerate all the bacteria attached to the cells, while a limited number of microscopic fields can be examined under microscope24. Percentage of adhesion to Caco2 and HT-29 cell lines was high among the strains isolated from the human feacal samples and buffalo milk than that has been isolated from cheese. This shows that adhesive Lactobacillus strains have host-residential characteristics specific to the population from which it has been isolated. Thus, our indigenous Lactobacillus strains will have more beneficial effect in the Indian population other than strains that are commercially available in the international market. This is also consistent with the earlier studies on the adhesion8925. Adhesion of Lactobacillus isolates to HT-29 and Caco2 was strain specific and varied within the same species. This was in agreement with results obtained from previous studies8.
In conclusion, the Lactobacillus strains isolated from the human faecal samples showed better hydrophobicity and ability to adhere epithelial cells under in vitro conditions as compared to the one derived from a cheese sample. These indigenous strains hold great promise and could serve as the ideal candidate probiotics and can be targeted as the subject for more intensive in vivo studies to explore their novel health promoting functions due to better colonization in the gut. Although, the in vitro assays used for assessing the adherence potential of probiotic strains may not exactly mimic the gut environment, these can be valuable in short listing the promising probiotic strains for establishing their functional efficacy in human subjects in subsequent clinical studies.
Authors acknowledge the Director, National Dairy Research Institute (NDRI), Karnal, India, for providing facilities to carry out this study, and thank Dr N.P. Shah (Australia) for providing the standard Lactobacillus culture, and Dr Tapas Mukhopadhay, Punjab University; Chandigarh, India, for providing HT-29 cell lines. The financial support from Indian Council of Agricultural Research (ICAR, India) in terms of providing Senior Research Fellowship to the first author (RKD) is acknowledged.
References
- Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev. 2008;72:728-64.
- [Google Scholar]
- FAO/WHO. In: Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Rome, Italy: Food and Agriculture Organization of the United Nations and World Health Organization Expert Consultation Report; 2001.
- [Google Scholar]
- Adherence of Lactobacillus species to human fetal intestinal cells. J Dairy Sci. 1982;65:2063-9.
- [Google Scholar]
- Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J Dairy Sci. 1987;70:1-12.
- [Google Scholar]
- Surface of Lactic Acid Bacteria: relationships between chemical composition and physicochemical properties. Appl Environ Microbiol. 2000;66:2548-54.
- [Google Scholar]
- Electrophoretic mobility distributions of single-strain microbial populations. Appl Environ Microbiol. 2001;67:491-4.
- [Google Scholar]
- Adhesion of Lactobacillus acidophilus strain LB to human enterocyte-like Caco-2 cells. J Gen Microbiol. 1992;138:1689-96.
- [Google Scholar]
- Identification of a surface protein from Lactobacillus reuteri JCM1081 that adheres to porcine gastric mucin and human enterocyte-like HT-29 Cells. Curr Microbiol. 2008;57:33-8.
- [Google Scholar]
- An in vitro study on bacterial growth interactions and intestinal epithelial cell adhesion characteristics of probiotic combinations. Curr Microbiol. 2009;60:327-35.
- [Google Scholar]
- In vitro fermentation of cereal dietary fibre carbohydrates by probiotic and intestinal bacteria. J Sci Food Agric. 2002;82:781-9.
- [Google Scholar]
- Microbial cell surface hydrophobicity: The involvement of electrostatic interactions in microbial adhesion to hydrocarbons (MATH) J Microbiol Methods. 1993;18:61-8.
- [Google Scholar]
- Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol. 1999;65:4949-56.
- [Google Scholar]
- Lactobacillus acidophilus LA1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483-9.
- [Google Scholar]
- Attachment of Lactobacillus casei strain GG to human colon carcinoma cell line Caco-2: comparison with other dairy strains. Lett Appl Microbiol. 1991;13:154-6.
- [Google Scholar]
- Human ileostomy glycoproteins as a model for small intestinal mucus to investigate adhesion of probiotics. Lett Appl Microbiol. 1999;28:159-63.
- [Google Scholar]
- Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int J Food Microbiol. 1998;41:45-51.
- [Google Scholar]
- Adhesion of two Lactobacillus gasseri probiotic strains on Caco-2 cells. Food Technol Biotechnol. 2003;41:83-8.
- [Google Scholar]
- A model study of factors involved in adhesion of Pseudomonas fluorescens. Appl Environ Microbiol. 1992;58:2783-91.
- [Google Scholar]
- Physicochemical surface characteristics of urogenital and poultry lactobacilli. J Colloid Interface Sci. 1993;156:319-24.
- [Google Scholar]
- Effect of ciprofloxacin and vancomycin on physicochemical surface properties of Staphylococcus epidermidis, Escherichia coli, Lactobacillus casei and Lactobacillus acidophilus. Microbios. 1995;82:49-67.
- [Google Scholar]
- Hemagglutination, adherence, and surface properties of vaginal Lactobacillus species. J Infect Dis. 1995;171:1237-43.
- [Google Scholar]
- Biodiversity-based identification and functional characterization of the mannose-specific adhesion of Lactobacillus plantarum. J Bacteriol. 2005;187:6128-36.
- [Google Scholar]
- Microbial interactions to intestinal mucosal models. Methods Enzymol. 2001;337:200-12.
- [Google Scholar]
- Comparison of the adherence of three Lactobacillus strains to Caco-2 and Int-407 human intestional cell lines. Lett Appl Microbiol. 1996;22:439-42.
- [Google Scholar]






