Translate this page into:
Antiarthritic activity of Majoon Suranjan (a polyherbal Unani formulation) in rat
Reprint requests: Dr Surender Singh, Department of Pharmacology, All India Institute of Medical Sciences Ansari Nagar, New Delhi 110 029, India e-mail: surenderaiims@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Majoon Suranjan (MS) is a polyherbal formulation used in Unani system of medicine for the treatment of rheumatoid arthritis (RA). The present study evaluates the antiarthritic efficacy of this formulation in three different experimental models.
Methods:
The anti-inflammatory activity of MS (in doses of 450, 900 and 1800 mg/kg body wt) was evaluated using the turpentine oil induced paw oedema model and the antiarthritic efficacy was evaluated using the formaldehyde and complete Freund's adjuvant (CFA) induced arthritis models. Aspirin (100 mg/kg body wt) was used as the standard drug in all the models. In order to assess the safety of the test drug, oral acute and 28 day toxicity studies were also carried out.
Results:
MS produced a dose dependent protective effect in all the experimental models. Its antiarthritic efficacy was comparable to aspirin in formaldehyde induced arthritis and was superior to aspirin in turpentine oil induced paw oedema and CFA induced arthritis. MS also inhibited the delayed increase in joint diameter as seen in control and aspirin treated animals in CFA induced arthritis. Oral LD50 of MS was found to be >5000 mg/kg in rats. Chronic administration did not produce any significant physiological changes in the tested animals.
Interpretation & conclusions:
Results of the present study suggest that the antiarthritic activity of MS was due to the interplay between its anti-inflammatory and disease modifying activities, thus supporting its use in traditional medicine for the treatment of RA.
Keywords
Adjuvant arthritis
analgesia
formaldehyde
Majoon Suranjan
paw edema
turpentine oil
Rheumatoid arthritis (RA) is a progressive, disabling, chronic multisystem disease of unknown cause characterized by pain, swelling and stiffness of synovial joints. An inflammatory reaction, increased cellularity of synovial tissue and joint damage are the pathological hallmarks of RA1. Though conventional treatment options for this condition have improved in terms of effectiveness, the use of non-steroidal anti-inflammatory drugs (NSAIDs) like etoricoxib, disease modifying anti-rheumatic drugs (DMARDs) like methotrexate, sulphasalazine, leflunomide, hydroxychloroquine, and corticosteroids like prednisolone, methylprednisolone have all been associated with adverse effects. Because of this reason, patients suffering from chronic musculoskeletal disorders are likely to seek alternative methods for symptomatic relief and are amongst the highest users of complementary and alternative medicine2. This revival of herbal and other complementary therapies in the management of chronic diseases (RA and other inflammatory disorders) is well documented3. However, despite an increase in use, evidence for effectiveness and safety of these complementary therapies is limited.
Unani system identifies and attributes diseases like RA to a weak immune and digestive system. It suggests a number of polyherbal formulations as being effective in the treatment of this condition. Majoon Suranjan (MS) is one such polyherbal formulation composed of Lawsonia inermis, Foeniculum vulgare, Capparis spinosa, Terminalia chebula, Ipomoea turpethum, Apium graveolens, Zingiber officinalis, Convulvulus scammony, Colchicum luteum, Cassia angustifolia, Piper nigrum, Coriandrum sativum, Rosa damascus, Origanum vulgare, Pyrethrum indicum, Plumbago zelanicum, Verbascum thapus, Ricinus communis oil4. Even though this formulation has been used in the Unani system of medicine for hundreds of years, its efficacy in rheumatoid arthritis has not been validated using modern scientific parameters. Therefore, the present study was carried out to evaluate the antiarthritic potential of the polyherbal formulation Majoon Suranjan using experimental models of arthritis.
Material & Methods
Animals: The study was carried out in the Department of Pharmacology after approval of the protocol by the Institutional Animal Ethics Committee, All India Institute of Medical Sciences (AIIMS), New Delhi. Inbred adult male Wistar rats (150-200 g) from the Central Animal Facility, AIIMS, were used in the study. Animals were housed under standard laboratory conditions at 25 ± 2°C in groups of three with access to food and water ad libitum. They were acclimatized to the laboratory conditions for a period of 5 days before the study. After completion of the study, all the animals were euthanized by an overdose of anaesthetic ether and the carcasses were disposed in accordance with institute regulations.
Drugs and chemicals: The polyherbal formulation Majoon Suranjan was procured from Majeedia Hospital Pharmacy, Jamia Hamdard (Hamdard University), New Delhi. Aspirin (acetylsalicylic acid) was used as commercially available powder (Sigma-Aldrich, USA). Both drugs were suspended in 1 per cent gum acacia (vehicle) and administered by oral gavage. Aspirin was administered in a dose of 100 mg/kg body wt and MS was administered in a dose of 450, 900 or 1800 mg/kg body wt. The doses that were used in the study were calculated from clinically used anti-arthritic doses of MS in man4. Formaldehyde and turpentine oil were purchased from Sigma-Aldrich, USA and complete Freund's adjuvant (CFA) was purchased from Difco Laboratories, USA.
Turpentine oil induced paw oedema: Five groups of male Wistar rats (n=6) were used in this study. Animals were fasted overnight with free access to water before the experiment. On the day of the experiment, baseline paw volume was recorded by using a plethysmometer (Ugo Basile 7140, Italy). Thereafter group I received the vehicle (2 ml/kg body wt), group II received aspirin (100 mg/kg body wt) and groups III, IV and V received MS in doses of 450, 900 and 1800 mg/kg body wt, respectively. Thirty minutes after administration of the vehicle/drug, oedema was induced by administration of 0.05 ml of turpentine oil into the subplantar surface of the left hind paw of the animal56. Increase in volume of the injected was measured at 1, 3 and 6 h post turpentine oil administration.
Formaldehyde induced arthritis: Five groups of male Wistar albino rats (n=6) were used in this study. Baseline recording of the joint diameter was made by using a micrometer screw gauge. Grouping of animals and drug treatments was same as for turpentine oil induced paw oedema. Drugs/vehicle was administered for a duration of 10 days. Thirty minutes after administration of vehicle/drugs, arthritis was induced by subplantar administration of 0.1 ml formaldehyde (2% v/v) into the left hind paw of all the animals on days 1 and 378. Increase in joint diameter of the injected paw was measured on days 8, 9 and 10,30 min after administration of the respective vehicle/drug treatment.
Adjuvant induced arthritis: Five groups of male Wistar albino rats (n=6) were used in this study. Baseline recording of the joint diameter was made by using a micrometer screw gauge. Grouping of animals and drug treatments was same as for turpentine oil induced paw oedema. Thirty minutes after administration of the vehicle/drug, arthritis was induced by subplantar administration of 0.1 ml of CFA (0.05% w/v Mycobacterium butyricum in mineral oil) into the left hind paw of all the rats910. This was designated as day 0. After immunization with CFA, all the groups were maintained on vehicle/drug treatment for 20 more days. Joint diameter of the injected paw was again measured on days 7, 14 and 21, 30 min after vehicle/drug administration.
Toxicity studies of the plant extract: Evaluation of oral acute toxicity of MS was carried out according to the Organisation for Economic Co-operation and Development (OECD) guidelines for testing of chemicals (425)11. A limit test (5000 mg/kg body weight) was performed using five male Wistar rats (150-200 g) from our breeding stock. All the animals were observed for behavioural changes and mortality till 14 days after administration of the dose.
Evaluation of oral 28 day toxicity of MS was carried out according to the OECD guidelines for testing of chemicals (407)12. Twelve male Wistar rats (150-180 g) from our breeding stock were divided into two groups (n=6). Group I received the vehicle (2 ml/kg body weight, 1% gum acacia) and served as normal control and group II received MS in a dose of 1800 mg/kg body weight (maximum dose tested in antiarthritic studies). Drug/vehicle was administered daily for a duration of 28 days.
Statistical method: Difference between groups was compared by using One-way ANOVA followed by Dunnett's Multiple Comparison. P<0.05 was considered significant.
Results
Effect of MS on turpentine oil induced paw joint oedema in rats: Subplantar administration of turpentine oil produced paw oedema that was maintained throughout the entire observation period (Fig. 1). Even though aspirin and MS treatment reduced the paw oedema as compared to control animals, the difference was significant only at 6 h after turpentine oil administrations. MS produced a dose dependent reduction in paw oedema throughout the observation period. Even though maximum inhibition of paw oedema was produced by MS at a dose of 1800 mg/kg, there was only a marginal difference in the efficacies of the higher two doses (900 and 1800 mg/kg).
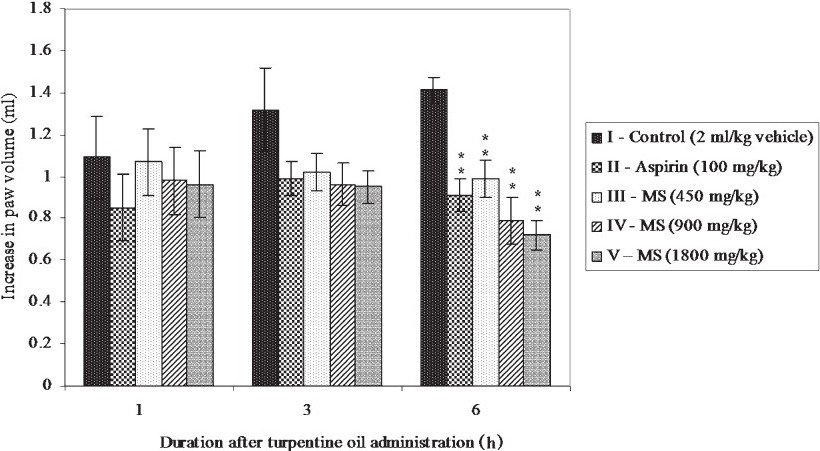
- Effect of MS on turpentine oil induced paw oedema in rats. All values are mean ± SE from 6 animals in each group. Statistical analysis by One-way ANOVA followed by Dunnett's Multiple Comparison. **P<0.01 compared to control.
Effect of MS on formaldehyde induced arthritis in rats: Administration of 2 per cent formaldehyde on days 1 and 3 produced ankle joint swelling in the injected limb all the animals. This joint swelling was sustained throughout the observation period of 10 days (Fig. 2). The increase in joint diameter was less in the aspirin and MS treated groups as compared to the control, and this difference was significant (P<0.01) on all observational days. Even though MS produced a dose dependent inhibition of joint swelling, maximum inhibition was produced by aspirin. Only a marginal difference was observed in the efficacies of the higher two doses of MS (900 and 1800 mg/kg).
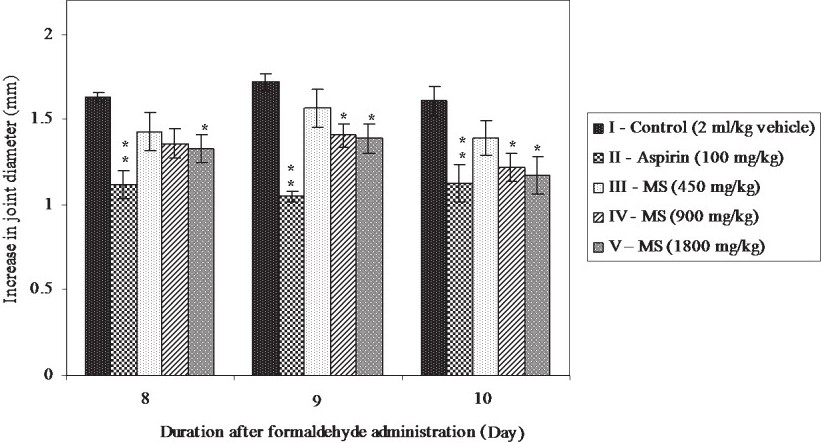
- Effect of MS on formaldehyde induced arthritis in rats. All values are mean ± SE from 6 animals in each group. Statistical analysis by One-way ANOVA followed by Dunnett's Multiple Comparison. *P<0.05, **P<0.01 compared to control.
Effect of MS on CFA induced arthritis in rats: Immunization with CFA produced an increase in the ankle joint diameter of the injected limb in all the animals (Fig. 3). The standard drug aspirin produced a significant decrease in the joint diameter as compared to control on all observation days. Even though MS produced a dose dependent reduction in joint swelling as compared to control, the difference was significant only on day 21 in the higher two dose treated groups. Maximum joint swelling was observed in all the groups on day 7. However, in the control and aspirin treated groups, after the initial decrease in joint diameter, from day 7 to 14 there was a slight increase in the joint diameter up to day 21. This trend was not seen in any of the MS treated groups. Maximum reduction of joint swelling was produced by MS on day 21 in the highest dose treated group (1800 mg/kg).
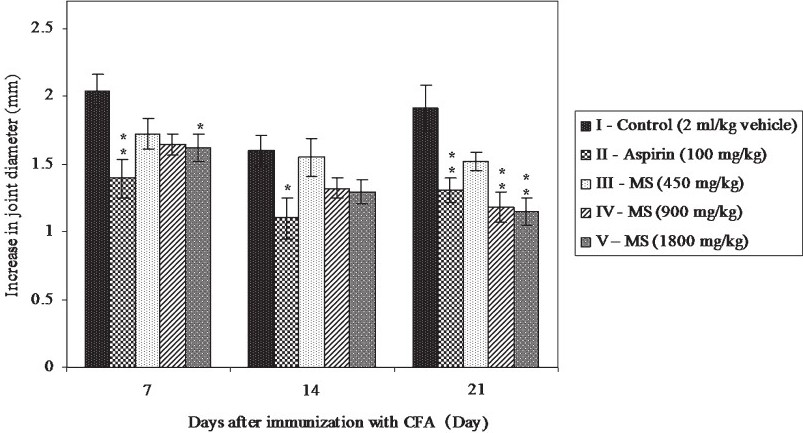
- Effect of MS on Complete Freund's Adjuvant (CFA) induced arthritis in rats. All values are mean ± SE from 6 animals in each group. Statistical analysis by One-way ANOVA followed by Dunnett's Multiple Comparison. *P<0.05, **P<0.01 compared to control.
Toxicity profile of the test plant: Administration of MS in a dose of 5000 mg/kg body weight did not produce any behavioural abnormalities in the animals. As all tested animals survived, the oral LD50 of MS in rats was found to be >5000 mg/kg body weight.
Chronic administration of MS in a dose of 1800 mg/kg body weight for 28 days did not produce any significant physiological changes in the tested animals as compared to normal control (data not shown). There was a marginal increase in body weight, bleeding time, RBC count and SGOT (serum glutamate oxaloacetate transaminase) levels as compared to normal control, but this increase was not significant. WBC count, %Hb and percentage organ weight of liver showed a marginal decrease from normal control, but this difference was also not statistically significant. All other parameters remained unaltered.
Discussion
Majoon Suranjan (MS) is a polyherbal formulation that is used in the Unani system of medicine for treatment of RA and other joint disorders4. It is composed of the extracts of 18 individual medicinal plants which are formulated in a sugar base. Some of the individual constituents of this polyherbal formulation have been evaluated for their anti-inflammatory activity. Lawsonia inermis has been shown to be efficacious in cotton pellet granuloma, granuloma pouch and formalin induced paw oedema models of inflammation in rats13. Chebulagic acid from the immature fruit of Terminalia chebula has been shown to suppress the onset and progression of collagen induced arthritis in mice14. Colchicum luteum has been shown to afford symptomatic relief in patients with rheumatoid arthritis in a 90 day trial1516, Coriandrum sativum has been shown to be efficacious in reducing carrageenan induced paw oedema17, Pyrethrum indicum has been shown to induce synoviocytes apoptosis and suppress proliferation of synoviocytes in adjuvant-induced arthritis rats18, Zingiber officinalis has been shown to decrease pain and swelling in arthritis patients19 and Foeniculum vulgare has been found to be effective in reducing carrageenan induced paw oedema20. In present study turpentine oil induced paw oedema was used to evaluate the anti-inflammatory activity and the formaldehyde and CFA induced joint arthritis models were used to evaluate the antiarthritic efficacy of the formulation.
Turpentine oil induced paw oedema is characterized by a triphasic release of inflammatory mediators. The initial phase is mediated by histamine and serotonin, intermediate phase by kinin like substance and the late phase by cycloxygenase and lipoxygenase products56. In the present study, inhibition of turpentine oil induced paw oedema was observed in the test drug treated groups throughout the observation period. This suggests that MS influenced all the phases of turpentine oil induced inflammation in the rat paw. However, maximum inhibition of paw oedema was seen during the late phase of inflammation, thus suggesting a prominent cycloxygenase/lipoxygenase inhibitory activity.
In formaldehyde induced inflammatory arthritis MS was able to significantly reduce joint swelling in the treated group. Even though the reduction in joint swelling in the MS treated groups was lower than that observed in the aspirin treated group, on day 10 at the higher doses tested (900 and 1800 mg/kg) efficacy of MS was comparable to aspirin.
Complete Freund's adjuvant induced arthritis is one of the most widely used models as it has been shown to share a number of clinical and immunological features with human arthritis9. Therefore, this model is used with a relatively high degree of validity for evaluating agents with potential antiarthritic activity. In the vehicle treated animals (control), there was an increase in the joint diameter after day 14, which can be attributed to the delayed immunological flare in the disease21. This increase in joint diameter was also seen in the aspirin treated group, demonstrating the absence of disease modifying activity in the standard drug. However, this trend of delayed increase in joint diameter was not observed in the MS treated groups, suggesting the involvement of mechanisms other than inhibition of inflammatory autocoids in the antiarthritic activity of the test drug. The most probable mechanism might be the inhibition of proinflammatory cells by Colchicum luteum22 and Terminalia chebula14, which could have led to an alteration in the immunological milieu during the delayed phase of the response.
Results of the present study contribute towards validating the traditional use of this polyherbal formulation in the treatment of rheumatoid arthritis. However, no animal model completely depicts the pathophysiology and disease progression in this debilitating disease. Therefore, further investigational studies are required to elucidate the exact mechanism of antiarthritic activity of this polyherbal formulation.
The research work was supported by an ad-hoc project grant from Indian Council of Medical Research, New Delhi, India. Authors also thank Prof. Mohammed Ali, Department of Pharmacognosy and Phytochemistry, Jamia Hamdard (Hamdard University), New Delhi, for his valuable suggestions regarding phytochemical analysis of the polyherbal formulation.
Conflict of Interest: None to declare.
References
- Rheumatoid arthritis. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, eds. Harrison's principles of internal medicine (16th ed). New York: McGraw Hill; 2005. p. :1968-77.
- [Google Scholar]
- Prevalence of the use of unconventional remedies for arthritis in a metropolitan community. Arthritis Rheum. 1989;32:1604-7.
- [Google Scholar]
- Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280:1569-75.
- [Google Scholar]
- Indian Medical Science Series, No. 55. In: Said HM, ed. Hamdard pharmacopoeia of eastern medicine. Delhi: Sri Satguru Publications; 1997.
- [Google Scholar]
- Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971;104:15-29.
- [Google Scholar]
- Anti-inflammatory property of 401 (MCD-peptide), a peptide from the venom of bee Apis mellifera (L.) Br J Pharmacol. 1974;50:383-92.
- [Google Scholar]
- Effect of deoxycortone and ascorbic acid on formaldehyde-induced arthritis in normal and adrenalec-tomized rats. Lancet. 1950;1:157-9.
- [Google Scholar]
- Antiarthritic activity of Glycyrrhiza glabra Linn. Indian J Physiol Pharmacol. 1959;3:39-47.
- [Google Scholar]
- Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br J Pharmacol Chemother. 1963;21:127-36.
- [Google Scholar]
- Effect of fixed oil of Ocimum sanctum against experimentally induced arthritis and joint edema in laboratory animals. Int J Pharmacognosy. 1996;34:218-22.
- [Google Scholar]
- Anti-inflammatory activity of some active principles of Lawsonia inermis leaves. Indian J Pharmacol. 1986;18:113-4.
- [Google Scholar]
- Suppression of the onset and progression of collagen-induced arthritis by chebulagic acid screened from a Natural Product Library. Arthritis Rheum. 2005;52:345-53.
- [Google Scholar]
- Effect of Colchicum luteum baker in the management of rheumatoid arthritis. Indian J Tradit Know. 2005;4:421-3.
- [Google Scholar]
- Evaluation of anti-arthritic and anti-inflammatory activity of Sudard, a poly herbal formulation. Iran J Pharmacol Ther. 2007;6:71-5.
- [Google Scholar]
- Study of the anti-inflammatory activity of some medicinal edible plants growing in Egypt. J Islamic Acad Sci. 1997;10:113-22.
- [Google Scholar]
- Effect of total flavonoids of Chrysanthemum indicum on the apoptosis of synoviocytes in joint of adjuvant arthritis rats. Am J Chin Med. 2008;36:695-704.
- [Google Scholar]
- Ginger (Zingiber officinale) in rheumatism and musculoskeletal disorders. Med Hypotheses. 1992;39:342-8.
- [Google Scholar]
- The anti-inflammatory activity of the Foeniculum vulgare L. essential oil and investigation of its median lethal dose in rats and mice. Int J Pharmacol. 2005;1:329-31.
- [Google Scholar]
- Studies of polyarthritis and other lesions induced in rats by injection of mycobacterial adjuvant. I. General clinical and pathological characteristics and some modifying factors. Arthritis Rheum. 1959;2:440-59.
- [Google Scholar]
- Colchicine down-regulates lipopolysaccharide-induced granulocyte- macrophage colony-stimulating factor production in murine macrophages. J Immunol. 1997;159:3531-9.
- [Google Scholar]






