Translate this page into:
In vitro & in vivo estrogenic activity of glycoside fractions of Solanum nigrum fruit
Reprint requests: Dr S. Manjula, Division of Plant Molecular Biology, Rajiv Gandhi Centre for Biotechnology Thiruvananthapuram 695 014, India e-mail: smanjula@rgcb.res.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The mature fruits of Solanum nigrum contains steroidal glycosides. These are often used as vegetable and there are evidences on tribal use of these fruits as an oral contraceptive. The present study was carried out to evaluate the estrogenic potential of S. nigrum fruits by in vitro and in vivo assays.
Methods:
Defatted methanol extract of dried S. nigrum fruits was column fractionated and the glycoside positive fractions pooled. Definite concentrations of the fraction were used for in vitro and in vivo assays. The effect on cell viability was analyzed in MCF-7 cell lines by MTT assay followed by in vitro evaluation of estrogenicity by hydroxy apatite (HAP) binding assay. The results were further evaluated in vivo by performing uterotrophic assay in ovariectomized mouse models.
Results:
At low concentration (40 μg/ml), SNGF induced a dose-dependent increase in MCF-7 cell proliferation, while higher extract concentrations (80-320 μg/ml) caused progressive cell growth inhibition. The competitive binding assay using 3H-E2 suggests that this effect is mediated by estrogen receptor. Mouse uterotrophic assay revealed a classical uterotrophic response in ovariectomized mice in response to S. nigrum glycoside fraction (SNGF). SNGF at a dose of 100 mg/kg of body wt induced the maximum height of luminal epithelial cells which indicated an increase of 30.8 per cent over control (P<0.01) with a correlated increase in uterine wet wt (150% increase over control). Higher doses (250 and 500 mg/kg body wt) of SNGF did not induce any uterotrophic effect.
Interpretation & conclusions:
Our preliminary data demonstrate the hormone like activity of Solanum glycosides both in vitro and in vivo in mouse, which needs to be further explored to evaluate the possible mechanism and clinical implications.
Keywords
Estrogen receptor
glycosides
Solanum nigrum
solasodine
uterotrophic assay
Solanum nigrum (Black night shade) is used as fruit and leafy vegetable in Southeast Asia, the Americas and several places in Africa. The plant reportedly provides appreciable amounts of minerals including calcium, iron and phosphorous, vitamins A and C, as well as proteins and amino acid methionine, scarce in other commonly marketed vegetables1. The plant extract is widely used in traditional systems of medicine for its diuretic, anti- pyretic and anti-inflammatory properties2 and has a spasmolytic action on the uterus3. In certain tribes, fruits of S. nigrum are used as an oral contraceptive, and the plant is one of the main constituents of plant based remedy prescribed for dysfunctional uterine bleeding3. The medicinal effects of the plant are attributed to the presence of solasodine, a steroidal glycoalkaloid, which is a potential alternative to diosgenin for commercial synthesis of various steroidal drugs4. Solasodine in the plant is bound to a series of sugar residues attached to the oxygen atom at C-3; most common forms are the triglycosides and solamargine5. The biological effect of mixture of Solanum glycosides is restricted to studies on certain basal cell carcinomas67. There is one report of the presence of estrogen receptor (ER)-like proteins and endogenous ligands for ER in S. glaucophyllum8. The present study was primarily aimed at assessing the estrogenic potential of the fruits of this edible and medicinal Solanum species- S. nigrum, in vitro in MCF-7 cell lines and in vivo in animal model.
Material & Methods
Plant material: Authentic certified seeds of S. nigrum (Acc No. IC 298650) procured from National Bureau of Plant Genetic Resources (NBPGR), Kerala Agricultural University Campus, Thrissur, Kerala, were planted and maintained in the green house of Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, Kerala, under uniform conditions of temperature and humidity.
Phytochemical extraction: Mature, unripe fruits from two months old plants were collected, washed and oven dried at 60°C. Uniformly dried fruits were powdered and 100 g of dried powder was used for soxhlet analysis. Extraction was carried out sequentially with 250 ml hexane and 250 ml chloroform at 50°C for 18 h to remove the less polar lipid components and finally with 250 ml methanol at a temperature of 50°C for 18 h to obtain the glycoside fraction. The defatted methanol fraction was further purified in silica gel glass column (Borosil) using the solvent mixture of 66 methanol: 33 chloroform: 1 glacial acetic acid as the mobile phase. The fractions were separately analyzed for the presence of glycosides by thin layer chromatography (TLC) performed on pre-coated TLC plates (Silica gel 60 TLC Plates, Merck, Germany). The solasodine glycoside, α - solanine was used as standard and the spots were visualized under UV light. Fractions from seven to fifteen which showed spots corresponding to the standard were pooled and dissolved in methanol followed by centrifugation at 7500 g for 5 min and the supernatant was collected. This was air-dried to remove traces of methanol and finally dissolved in distilled water at required concentrations to serve as the Solanum nigrum glycoside fraction (SNGF) used for further studies.
Cell viability assay: MCF-7 cell lines (Acc No. HTB-22) procured from ATCC (Rockville, MD, USA) were revived in Dulbecco's minimum essential medium (DMEM) phenol red free medium (Sigma, USA) containing 20 per cent foetal bovine serum (FBS) from Invitrogen (USA) and cultured in DMEM containing 10 per cent FBS. Cells were maintained in an incubator at 37°C under 5 per cent CO2. After attaining confluence, the cells were trypsinized in 0.25 per cent (w/v) trypsin to detach from the flask and the suspended cells were centrifuged at 3500 rpm for 5 min in a centrifuge. Pellets were re-suspended in 7 ml DMEM medium and 100 μl each was poured into each well of a 96 well ELISA plate (BD Falcon, USA). Cells were incubated for a period of 48 h. The stock containing the glycoside fraction was prepared in 100 mg/ml of DMSO [methyl sulfinyl methane, Sisco Research Laboratory (India)]. Five dilutions of the extract were prepared in serum free medium which include 1:500 (320 μg of SNGF), 1:1000 (160 μg of SNGF), 1:2000 (80 μg of SNGF), 1:4000 (40 μg of SNGF) and 1: 8000 (20 μg of SNGF). After allowing the cell lines to grow for a period of 48 h, the medium was removed and 100 μl each of diluted SNGF was added in each cell. After 48 h, the medium was replaced by MTT reagent [3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide] dissolved in DMEM containing 2 per cent FBS and incubated at 37°C for 2 h; 100 μl of lysis buffer (pH 7.4) containing 5 mM Tris and 1 mM EDTA was added to each well and cultures were incubated for 4 h. The absorbance of the coloured solution was measured at 570 nm in a microplate reader (Bio-Rad, Model 680, USA).
Evaluation of estrogenic activity in vitro by hydroxy apatite (HAP) binding assay: HAP binding assay for estrogen receptor binding9 was carried out for assessing the estrogenic potential of SNGF on MCF-7 cell line. Commercially available HAP (Sigma, USA) was used in this assay. Aliquots containing 15 μg of protein (MCF- 7 cytosol) were incubated overnight with varying concentrations of methanol extract and 20 nM final concentration of [3H]- estradiol (3H-E2) ± 100 fold molar excess of diethylstilbestrol (DES) (Sigma, USA) at 4°C in a final volume of 200 μl. After overnight incubation, 250 μl of a 60 per cent HAP suspension in TEM buffer (pH 7.6) was added and the mixture was kept in ice for 15 min. After washing twice with ice cold TEM buffer, the HAP pellet, collected following centrifugation, was extracted with 1 ml ethanol. The radioactivity in the ethanol extract was measured. Non-specific binding was determined in the presence of a 100-fold excess DES and was subtracted from total binding.
Evaluation in vivo on animal models: Swiss albino mice from Sree Chitra Thirunal Institute of Medical Science and Technology (SCTIMST), Thiruvananthapuram, Kerala, were housed within temperature controlled (22 ± 2°C) conditions in the animal house facility of Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram. Mice were maintained on a 12 h light: 12 h darkness photoperiod and food and water were available ad libitum. Female mice (3 months old, 20-25 g) were ovariectomized and rested for ten days before receiving treatment. The treatment groups were administered orally for seven days with 200 μl each of SNGF alone at concentrations of 100, 250 and 500 mg/kg body wt/ day, distilled water alone (control group), or with 17β-estradiol (E2) alone (Sigma, USA) at a dose of 1 mg/kg body wt, which served as the positive control group. After seven days, mice were weighed and sacrificed by cervical dislocation and their uteri were dissected out, blotted and the wet wt recorded. All the protocols for animal experiments were approved by the Ethics Committee for Animal Experiment of Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram.
Histological analysis: The uteri were immediately fixed in 10 per cent formaldehyde. One horn of each uterus was dissected transversely and microtome sections of the tissues were stained by haematoxylin and eosin method10. The sections were used for morphometric determination of changes in luminal epithelium.
Morphometric analysis: The height of the luminal epithelium was determined using optical micrometer attached to the fluorescent microscope (Nikon Instruments Inc, NY, USA). Four measurements were made within four areas of each uterus.
Statistical analysis: All experiments were performed in 5 replicates and results are presented as mean ± SEM. Data were analyzed by one-way ANOVA. The differences between treatments were tested by Fisher's least significant difference test.
Results & Discussion
SNGF treatment at 40 μg/ml resulted in proliferation of MCF-7 cells, whereas it inhibited MCF-7 cell proliferation at concentrations ranging from 80 to 320 μg/ml (Fig. 1), which is 30 to 60 per cent lower than MCF-7 control. SNGF significantly inhibited the binding of [3H]-E2 to cytosolic estrogen receptor in a dose dependent manner. The IC50 value of SNGF is 40 ± 5 μg/ml (Fig. 2). The results of competitive binding assay using [3H]-E2 suggest that stimulation of cell proliferation at low concentration is likely to be mediated via the estrogen receptor. This observation is in consonance with similar findings with soy isoflavone phytoestrogen, genistein1112. In a recent report, extracts of Chinese licorice (Glycyrrhiza uralensis) exhibited ER-dependent MCF-7cell proliferation at low concentrations and an anti-proliferative activity at higher concentrations which was found to be ER- independent13. Our observations in S. nigrum are in agreement with these reports but further studies are needed to conclude whether the anti-proliferative activity of SNGF at higher concentrations is ER-independent.
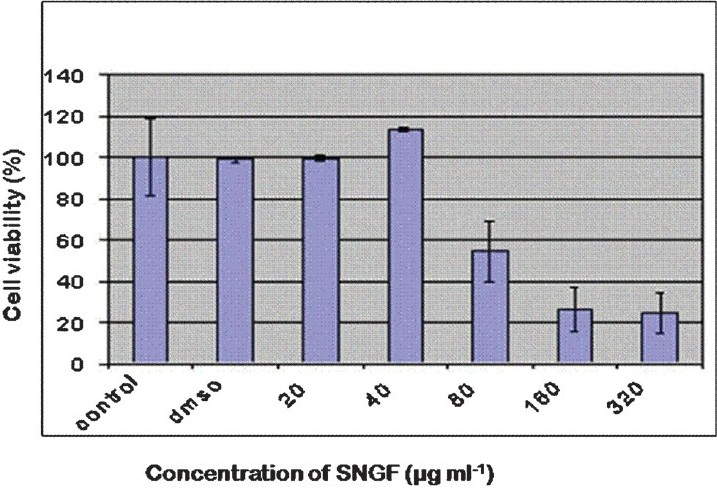
- MTT assay shows proliferative effect of SNGF on MCF-7 cells at a concentration of 40 μg/ml concentration and an inhibition of cell proliferation at higher concentrations (80-320 μg/ml). The MCF-7 cells were treated with SNGF at concentrations ranging from 20-320 μg/ml. Values are means ± SE of 5 replicates.
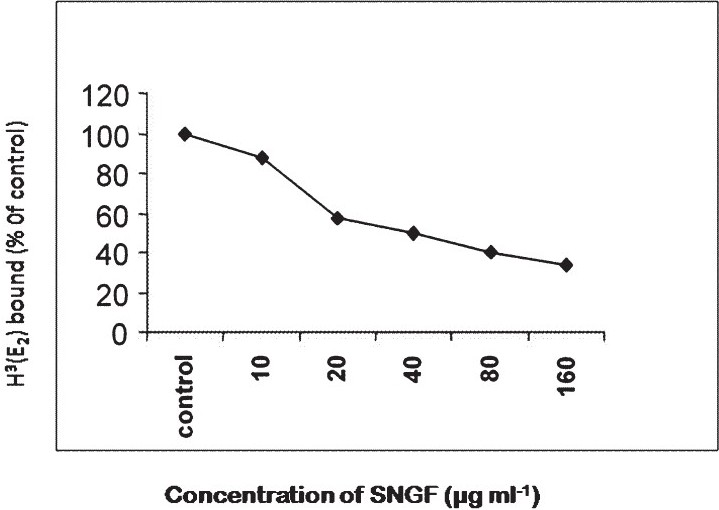
- HAP binding assay result shows that SNGF at a concentration of 40 μg/ml reduces the specific radioligand binding of the control (17 μ - E2) to the ER by 50 per cent.
The uteri from mice treated with 100 mg/kg of body wt appeared stouter and swollen, which upon sectioning, was found filled with fluid. Histological observations revealed that this lowest dose of SNGF induced the maximum height of luminal epithelial cells (Figs 3, 4b) which indicated an increase of 30.8 per cent over control (P<0.01), in addition to effecting stromal cell differentiation and glandular proliferation (Fig. 4c), which were not observed in uterus from control mice. Treatment with E2 induced an increase of luminal epithelial cell height of 84.6 per cent (Fig. 3; P<0.001) over control (Fig. 4a). Higher concentrations did not affect a significant increase in cell height relative to control. The SNGF when fed to the ovariectomized mice at a concentration of 100 mg/kg body wt also significantly increased the wet wt of the uterus relative to the control group (P<0.001). There was a 150 per cent increase in uterine wet wt in response to100 mg/kg concentration of extract and a 283 per cent increase in response to positive E2 (P<0.001). Wet wt of the uterus calculated as a percentage of the body wt showed a similar change in pattern in response to treatment. Higher concentrations (250 and 500 mg/kg of body wt/day) of SNGF did not produce a significant increase in uterine wet wt compared to control (Fig. 5).

- Effect of SNGF on ovariectomized mouse uterine epithelial cells. Water fed ovariectomized mice served as control; treatment group mice were fed with 100, 250 & 500 mg/kg body wt of SNGF. They were sacrificed after 7 days of treatment and the uteri were collected. Result indicated an increase in uterine cell height of animals treated with 100 mg/kg SNGF which is 30.8 per cent more than the control. Values represent the mean ± SE values obtained from 5 animals per treatment. P**<0.01, P**<0.001 compared to control.
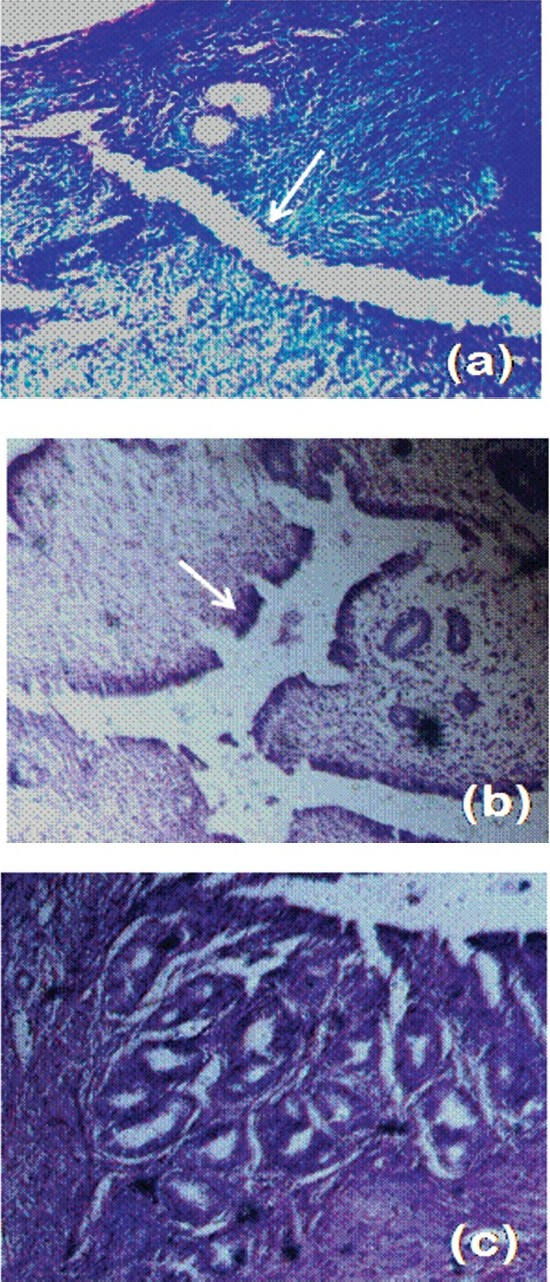
- Photomicrographs of Heamatoxylin and Eosin (H&E) stained sections of ovariectomized Swiss albino mice. (a) Water vehicle (control); (b & c) treatment with 100 mg/kg of body wt of SNGF; (b) arrow shows increase in luminal epithelial cell height compared to control; (c) increase in gland number in response to SNGF (10X magnification).
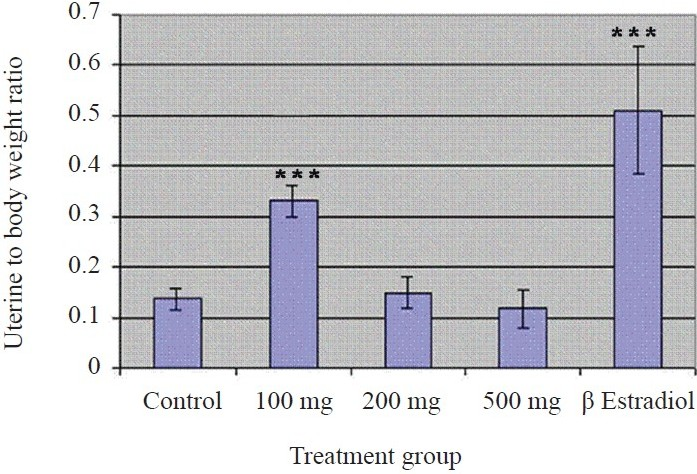
- Effect of SNGF on uterine wet wt to body wt ratio of ovariectomized Swiss albino mice. The result shows a 150 per cent increase in uterine wet wt in response to 100 mg/kg concentration of SNGF and a 283 per cent increase in response to positive E2 (P<0.001). Higher concentrations (250, 500 mg/kg body wt/day) of SNGF did not produce a significant increase in uterine wet wt compared to control. Values are means ± SE of 5 replicates. P***<0.001 compared to control.
The uterotrophic assay has been traditionally used to establish the estrogenic effects of suspected estrogens and is still used as a gold standard for in vivo assessment of estrogenic activity. The uterus responds to cyclical changes in estrogen and progesterone levels in preparation for embryo implantation and estrogen mediates the principal proliferative response of the uterus through the estrogen receptors1415. In uterus, the physiological and genomic responses to estrogen have been described as biphasic. The events include hyperemia and uterine fluid uptake or water imbibition16. The water imbibition results in rapid increase in uterine wet wt. The late phase response to estrogen includes epithelial cell proliferation and differentiation17. SNGF at low concentrations displays uterotrophic activity that is not observed at higher concentrations which seems to depress the uterotrophic response to below the control levels. Similar observations from previous studies led to the conclusion that the uterotrophic and the estrogen agonistic effect presented by various natural estrogens occur at low doses only18–20. The results confirm the calabrese's concept of hormone-like biphasic dose response model which is characterized by a low-dose stimulation which usually present a modest response (30-60% vs. control) and a high-dose inhibition21–23 as revealed in our studies on S. nigrum, indicating that a classical uterotrophic response occurs in the mouse uterus in response to SNGF.
In conclusion, our data showed hormone like activity of Solanum glycosides indicated by their ability to bind estrogen receptor in vitro. SNGF is less active than E2, but has potencies comparable to other known phytoestrogens. Further studies need to be done to understand the mechanism and clinical implications.
This work was financially supported by Indian Council of Medical Research, New Delhi. Authors thank Dr Santhosh Kumar S, Veterinary surgeon, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, Kerala, for helping in the animal surgical procedures and Dr Mangalam S. Nair of Natural Products Synthesis, National Institute for Interdisciplinary Science and Technology, Government of India, for help provided in carrying out the phytochemical analyses.
References
- Antinociceptive, anti-inflammatory and antipyretic effects of Solanum nigrum chloroform extract in animal models. Yakugaku Zasshi. 2006;126:1171-8.
- [Google Scholar]
- Steroid production by callus and cell suspension cultures of Solanum aviculare. J Natl Prod. 1984;47:373-6.
- [Google Scholar]
- Salinity stress enhances production of solasodine in Solanum nigrum L. Chem Pharmacol Bull. 2008;56:17-21.
- [Google Scholar]
- Solasodine glycosides as anti-cancer agents: preclinical and clinical studies. Asia Pac J Pharmacol. 1994;9:113-8.
- [Google Scholar]
- Solasodine glycoalkaloids: a novel topical therapy for basal cell carcinoma.A double- blind, randomized, placebo- controlled, parallel group, multicenter study. Int J Dermatol. 2008;47:78-82.
- [Google Scholar]
- Presence of estrogen receptor (ER)-like proteins and endogenous ligands for ER in Solanaceae. Plant Sci. 2004;166:397-404.
- [Google Scholar]
- Female sex steroids receptors and function. In: Gross F, Labhart A, Mann T, Bandes J, eds. Monographs on endocrinology. New York: Springer-Verlag; 1979. p. :28-36.
- [Google Scholar]
- Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271-5.
- [Google Scholar]
- Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58:3833-8.
- [Google Scholar]
- Estrogenic activities of extracts of Chinese licorice (Glycyrrhiza uralensis) root in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 2009;113:209-16.
- [Google Scholar]
- Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor α binding to classical estrogen response elements. J Biol Chem. 2006;281:26683-92.
- [Google Scholar]
- Estrogen and antiestrogen action in reproductive tissues and tumors. Recent Prog Horm Res. 1979;35:259-300.
- [Google Scholar]
- Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol. 2003;17:2070-83.
- [Google Scholar]
- Estrogen receptor null mice: what have we learned and where will they lead us? Endocrin Rev. 1999;20:358-417.
- [Google Scholar]
- Toxicity vs. beneficial effects of phytoestrogens. Pure Appl Chem. 2003;75:2047-53.
- [Google Scholar]
- The OECD program to validate the rat uterotrophic bioassay. Phase 2: dietary phytoestrogen analyses. Environ Health Perspect. 2003;111:1559-67.
- [Google Scholar]
- Immature rat uterotrophic assay of 18 chemicals and Hershberger assay of 30 chemicals. Toxicology. 2003;183:93-115.
- [Google Scholar]
- The hormetic dose-response model is more common than the threshold model in toxicology. Toxicol Sci. 2003;71:246-50.
- [Google Scholar]
- Morinda citrifolia has very weak estrogenic activity in vivo. Thai J Physiol Sci. 2004;17:22-9.
- [Google Scholar]
- In vivo and in vitro effects of Bidens pilosa L. (Asteraceae) leaf aqueous and ethanol extracts on primed-oestrogenized rat uterine muscle. Afr J Tradit Complement Altern Med. 2007;5:79-91.
- [Google Scholar]






