Translate this page into:
γδ T cells response to Mycobacterium tuberculosis in pulmonary tuberculosis patients using preponderant complementary determinant region 3 sequence
Reprint requests: Dr Zhendong Zhao, State Key Laboratory for Molecular Virology and Genetic Engineering, Institute of Pathogen Biology, Chinese Academy of Medical Sciences & Peking Union Medical College, 6 Rong Jing Dong Jie, Beijing 100176, PR China e-mail: timjszzd@163.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The unique immunological functions of γδ T lymphocytes to contribute immunity against Mycobacterium tuberculosis attracted interest of researchers. However, little is known about the specificity of γδ Τ cell in tuberculosis patients and the lack of exact tuberculosis antigen recognized by γδ T cells limited its application. The analysis of complementary determinant region (CDR)3 sequence characteristic in γδ T cells of tuberculosis patients would contribute to understand the distribution specificity of γδ T cell. In present study, we investigated the diversity of the γ9/δ2 T cell immunorepertoire and analysed the specificity of the expressed CDR3 in pulmonary tuberculosis patients.
Methods:
The total RNA in peripheral blood mononuclear cell of 50 pulmonary tuberculosis patients and 10 healthy controls was extracted. The polymerase chain reaction was used to specifically amplify the CDR3 region of γ9 and δ2 chain. The PCR products were ligated into the pGEM-T easy vector. The plasmid DNA was sequenced using the ABI3700 and the T7 primer.
Results:
Our findings showed that predominant CDR3 sequence of δ2 chain in pulmonary tuberculosis patients was CACDTLVSTDKLIFGKG. The sequence specifically exists in almost all pulmonary tuberculosis patients. The conserved hydrophobic acid residue in 97 positions is present in the γδ T cell reactive to M. tuberculosis. The length of δ2 CDR3 in pulmonary tuberculosis patients has no relation with the disease progress.
Interpretation & conclusions:
Our results suggest that γδ T cells appear to use CDR3 sequence to recognise M. tuberculosis antigen. γδ T cells reactive to M. tuberculosis were diverse and polyclonal.
Keywords
CDR3 region
gammadelta T cells
predominant sequence
pulmonary tuberculosis
Tuberculosis (TB) has affected humanity since the beginning of the recorded time and is associated with poverty, malnutrition, overcrowding, and immuno-suppression1. In recent years, the vaccine of M. tuberculosis based on γδ T cells attracted attention of researchers, however, little is known about the structural basis of antigenic recognition by γδ T cell, and the lack of exact tuberculosis antigen recognized by γδ T cells limited its application.
γδ T cells are a distinct subset of CD3+ T cell featuring T cell receptors (TCRs) that are encoded by Vγ and Vδ gene segments. In peripheral blood of healthy individuals, γδ T cells represent 2-10 per cent of total T cells, and of these, the majority express Vγ9Vδ2 TCRs. Studies in humans and animal models suggested that γδ T cells play an important role in immune response to M. tuberculosis2. In human, γδ T cell are present in increased proportions in peripheral blood of a fraction of tuberculosis patients3. γδ T cells could inhibit the proliferation of M. tuberculosis through the secretion of IFN-γ, but also participate in the anit-tuberculosis immune response elicited by other immune cells such as NK cells4, dendritic cells5 and CD8+ αβ T cells6. Thus, the interaction net composed by many immune cells might play more important role in the infection of M. tuberculosis. Further studies are needed to define a precise subset of V γ9/δ2 T cells in immunity to M. tuberculosis infections as well as to find tuberculosis antigen recognized by the antigen-specific γδ T cells.
The antigen binding site of the γδ TCR is primarily formed from three complementary determinant regions (CDRs) contributed by each Vγ or Vδ domain. Both CDR1 and CDR2 regions are encoded by germline V genes, while the CDR3 region is formed by somatic rearrangement of V (D) and J fragments. Sequence diversity in antigen receptors is not evenly distributed among all six CDRs. The diversity is highly concentrated in one or two CDR3s7. To determine the characteristics of amino acid sequences of CDR3δ, Xu et al8 cloned and sequenced Vδ2 cDNA from tumour infiltrated lymphocytes in rectal cancer and ovarian epithelial cancer. They used synthesized CDR3δ peptide as a probe to screen putative protein ligands in tumour protein extracts by affinity chromatography analysis and successfully identified a new antigen human mutS homolog 2 (hMSH2) that is recognized by human γδ TCR8. The analysis of CDR3 sequence characteristic in γδ T cells would contribute to understand the distribution specificity of γδ T cell in pulmonary tuberculosis patients. Predominant CDR3 sequence could also be used to construct the transfectant cell lines expressing M. tuberculosis specific γδ TCR, which could be applied to evaluate the importance of γδ CDR3 sequence1011 and identify new ligands for γδ TCR12.
In the present study, we attempted to investigate the diversity of the γ9/δ2 T cell immunorepertoire by sequence analyses of the expressed CDR3 in pulmonary tuberculosis patients.
Material & Methods
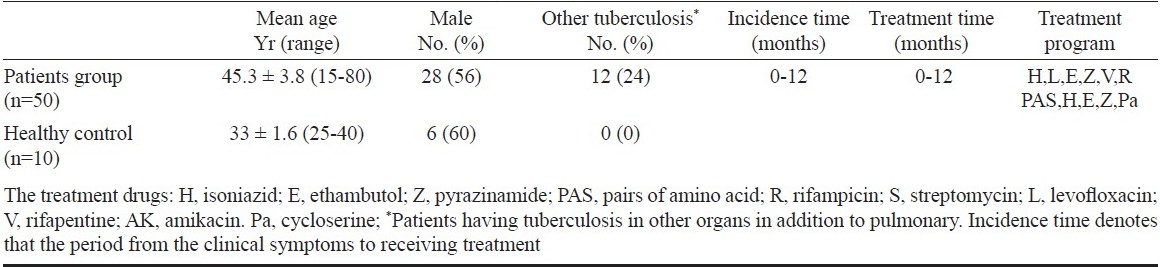
Patients and study design: The study was performed on randomly selected 50 pulmonary tuberculosis patients (mean age, 45.3 ± 3.8, 28 men and 22 women) who had been admitted to the Beijing Tuberculosis and Thoracic Tumor Research Institute during 12 months (April 2008 - April 2009). Pulmonary tuberculosis was diagnosed by the clinical parameters: presence of cough/expectoration, chest X-ray showing infiltrated and /or cavities, a minimum of one positive sputum smear and culture result for acid-fast bacilli. The exclusion criteria were human immunodeficiency virus positivity, diabetes mellitus, pregnancy and immunological or autoimmune diseases (Table I). Ten healthy volunteers (mean age, 33 ± 1.6, 4 women and 6 men) were included as control group. Healthy subjects did not have any changes on X-ray and tuberculosis history or other underlying disease. Exclusion criteria for the healthy control group were smoking, medication, pregnancy and any abnormalities in renal and liver function tests. Permission for the study was obtained from the Clinical Ethics Committee of Institute of Pathogen Biology, Beijing, and all subjects were informed and gave their oral consent to participate.
The extraction of RNA and reverse transcription polymerase chain reaction: Total RNA was harvested from peripheral blood mononuclear cells (PBMC) of the pulmonary tuberculosis patients and healthy subjects following the Qiagen RNeasy protocol (http://www.qiagen.com), including the optional DNase treatment. One microgram of total RNA was then converted into cDNA using reverse transcription system kit (Qiagen, China). First strand synthesis was primed by anchored oligo(dT) primers to generate a cDNA library representative of the entire cellular mRNA pool. Primer sequences complementary to upstream V regions and downstream C regions were used to amplify CDR3 regions. The primer sequence is from the report of Xu et al8. The primer was TCRγ9CDR3-up 5’-AATGTAGAGAAACAGGAC-3, TCRγ9CDR3-down ‘5’-ATCTGTAATGATAAGCTTT-3’, TCRδ2CDR3-up 5’-GCACCATCAGAGAGAGATGAAGGG-3’, TCRδ2CDR3-down 5’-AAACGGATGGTTTGGTATGAGGC-3’. The PCR products were separated on 1 per cent agarose/tris-acetate-EDTA (TAE) gels.
Cloning and sequencing of Vγ9 and Vδ2 chain: PCR products were purified by gel extraction using gel extraction kits (AXYGEN, China) according to the manufacturer's instructions. The purified PCR fragments were ligated into the pGEM-T easy vector (Invitrogen, USA) and the resulting plasmids were transfected by heat shock into DH5a competent Escherichia coli for propagation. Glycerol stocks were frozen to maintain the clones. Colonies were picked and grown overnight in 1-2 ml of Luria-Bertani broth containing ampicillin (50 mg/ml). Plasmids were purified using the DNeasy Miniprep kit (Qiagen, China). The plasmid DNA was sequenced using the ABI3700 and the T7 primer. Sequences were determined using the DNAman software analysis system11. CDR3 lengths were calculated as four less than the number of amino acids between the last conserved Cys residue in the V region to the conserved GXG or AXG motif in the joining segment as previously described8.
Statistical analysis: Student's t-test was used for statistical evaluation of the data. P<0.05 was considered significant.
Results
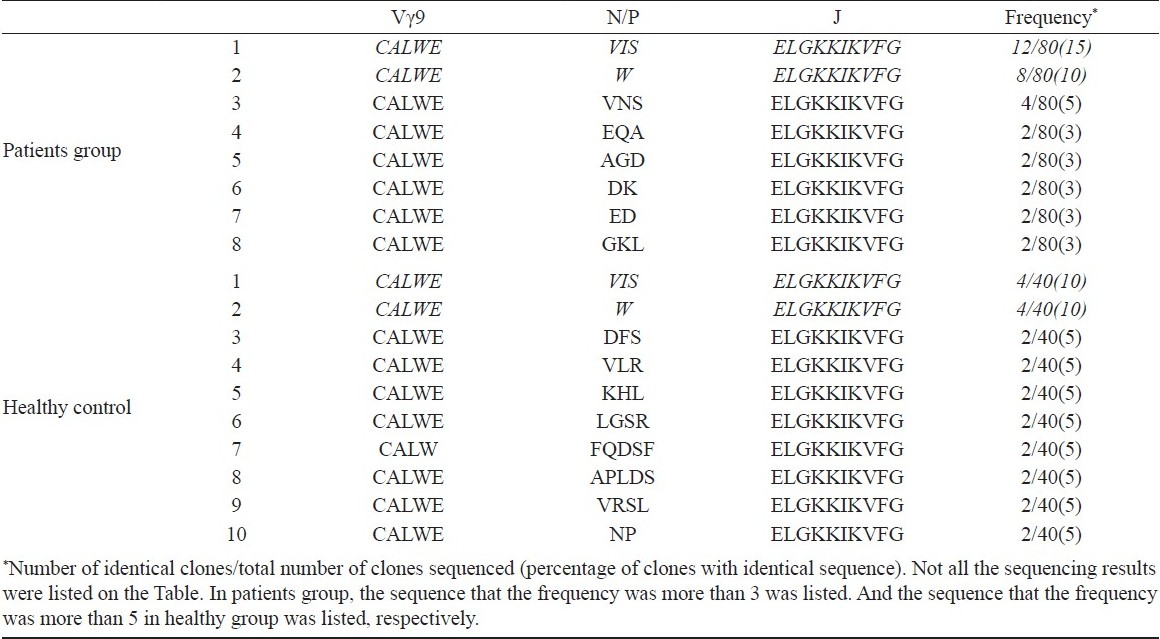
Sequencing of the CDR3 regions confirm that γ9/δ2 T cells use prominent CDR3 sequence to recognize tuberculosis antigen: Antigen specificity is largely determined by the sequences encoded by the hypervariable CDR3 regions of the TCR. To study specificities of the γ9/δ2 T cell reactive to M. tuberculosis, the diversity of the Vγ9 and Vδ2 TCR CDR3 loops expressed by this population of T cells in pulmonary tuberculosis patients was analysed. The sequencing results are shown in Tables II and III. First the difference of Vγ9 CDR3 loops between pulmonary tuberculosis patients and healthy subjects was analysed. The sequence of clone 1 appeared 12 times among 80 sequenced Vγ9 chains. So, it was regarded as a predominant motif of Vγ9 chains in tuberculosis patients (Table II). But the sequence also appeared in healthy controls (clone 1). There was no significant difference for frequency of the sequence between patients and control groups indicating that the sequence was not specific for pulmonary tuberculosis patients.
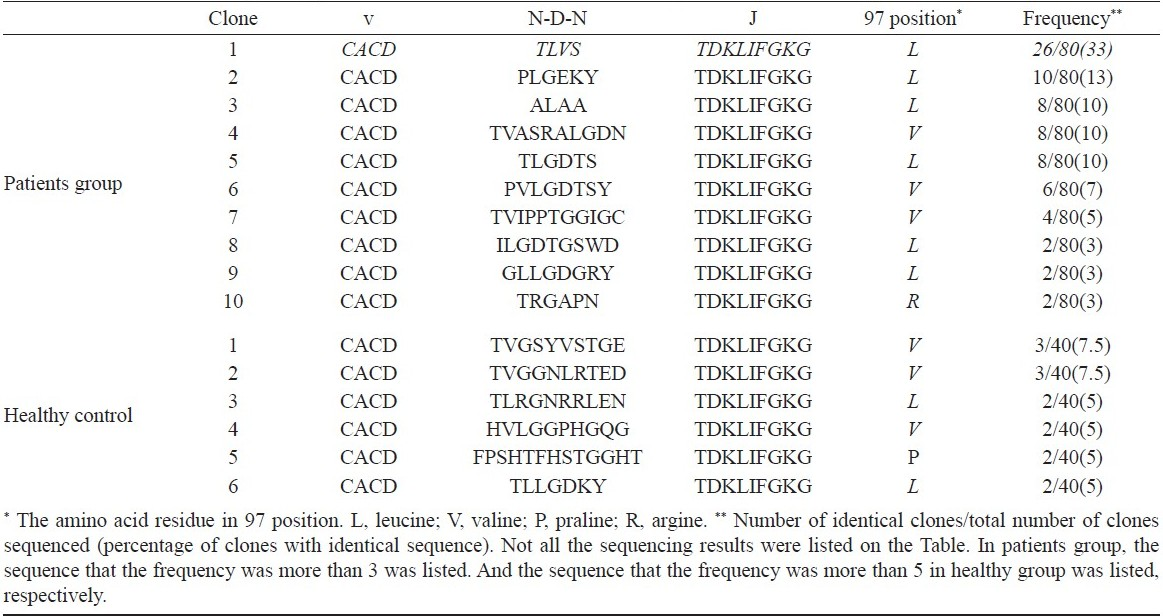
According to previous report10, the difference of δ2 TCR chain was most pronounced presumably because of the increased potential for diverse sequences due to the additional D gene segment rearrangements and nucleotide additions/substitution. The CDR3 regions contained conserved “CA” at the N-terminus and conserved “FGXG” at the C-terminus. In contrast, the inner regions of CDR3 were composed of variable sequences. Here, a common δ2 CDR3 sequence was found in PBMC of patients groups. The common sequence is “CACDTLVSTDKLIFGKG”. No prominent CDR3 sequence was found in control groups and these sequences were not present in patients groups (Table III).
The length of δ2 CDR3 in patients and relation with the disease progress: Further, the difference of CDR3 length of δ2 chain was compared between pulmonary tuberculosis patient and healthy controls. The results were shown in the Fig. 1. From the point of CDR3 length distribution, γδ TCR seems to use shorter CDR3 sequence to recognize M. tuberculosis antigen, which is different from that of healthy control. The CDR3 length of δ2 chain of tuberculosis patients focused on 17 to 19 amino acid residues, while that of healthy controls concentrated on more than 20 amino acid residue.
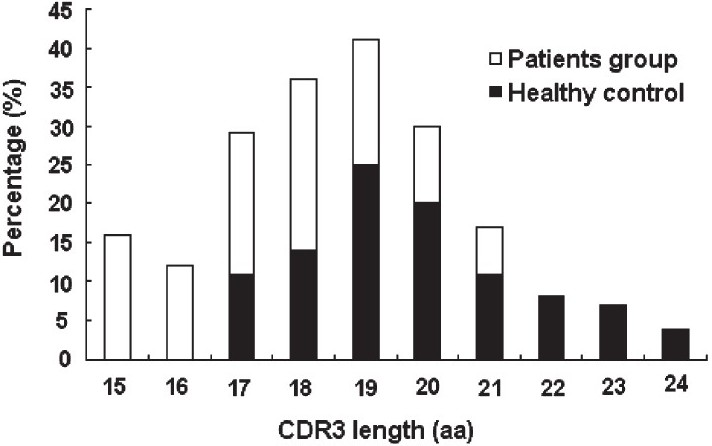
- The δ2 chain CDR3 length distribution in pulmonary tuberculosis patients and healthy controls. For patient and healthy control groups, the percentage of a CDR3 length in total sequence results was calculated. The CDR3 length of δ2 chain of tuberculosis patients focused on 17 to 19 amino acid (aa) residues, while that of healthy controls concentrated on more than 20 amino acid residues.
Conserved hydrophobic acid residue in 97 position in the γδ T cell reactive to M. tuberculosis: Our sequence analysis revealed that most Vδ2 T cell isolated from tuberculosis patients and healthy controls also carried a hydrophobic amino acid residue (isolecuine/leucine/valine) at conserved position 97 (Table III). The results suggested that the hydrophobic amino acid residue is not a prerequisite for γδ T cell reactive to M. tuberculosis.
Discussion
M. tuberculosis is an intracellular pathogen, and cell-mediated immunity plays a key role in the control of the bacterial replication and the subsequent protection against tuberculosis. It is becoming increasing recognized that γδ T cells, especially Vγ9/δ2 subset, are relevant for both innate and adaptive protective immunity against γδ T cells, especially Vγ9/δ2 subset, are relevant for both innate and adaptive protective immunity against M. tuberculosis . Phosphate antigen of M. tuberculosis was regarded as main antigen recognized by γδ T cell. But Spencer et al13 demonstrated that phosphoantigen-activated γ9/δ2 T cells display a restricted TCR diversity. Recently, protein antigen of M. tuberculosis recognized by γδ T cell has been identified1415. These antigens could effectively activate γδ T cell, which could induce innate and adaptive immunity to M. tuberculosis. Meanwhile, γδ T cell could participate in the anit-tuberculosis immune response elicited by other immune cells. The interaction net composed by many immune cells might play an important role in the infection of M. tuberculosis.
The sequencing of CDR3 is a simple method to master the specificity of γδ T cells in disease and healthy condition16. Our results showed that predominant γ9 CDR3 sequence was not specific for tuberculosis patients, but predominant δ2 CDR3 sequence was specific. In recognizing antigen, δ chain seems to be more important than γ chain. Xu et al8demonstrated that the primary sequence of CDR3 in γδ TCR, especially CDR3δ, due to similarity to CDR3δ and VH CDR3 in gene composition, could serve as the key determinant for the specificity of antigen binding. Therefore, although predominant γ9 CDR3 sequence is not specific for tuberculosis patients, specific δ2 CDR3 sequence could represent the sequence specificity.
Our sequence results revealed that most Vδ2 T cell isolated from pulmonary tuberculosis patients carried a hydrophobic amino acid residue (isolecuine/leucine/valine) at conserved position 97. Crystallographic structure of Vγ9δ2 TCR demonstrated that the TCR have a pocket structure formed by CDRs of TCR γ and δ chains1718. Almost all non-peptide antigen reactive clones have a conserved hydrophobic residue at position 97 of CDR3 δ with leucine, isoleucine and valine1920. This conserved hydrophobic residue may interact with hydrophobic parts of antigenic molecules and contribute the antigen recognition of γδ T cells. Dacodeau et al21 have reported that almost all phosphoantigen reactive clones carry a distinctive junctional motif containing strongly hydrophobic amino acids at position 97, which participate in the recognition of γδ TCR to antigen. But as per our previous data11, the hydrophobic amino acid residue at position 97 plays little role in recognizing protein antigen. In the present study, our sequence results showed that healthy individual also carried hydrophobic amino acid residue at position 97. It seems that the role of CDR3δ97 in the recognition of tuberculosis antigen was not specific and needs to be further investigated.
Our analysis of CDR3 length suggested that γδ T cells reactive to M. tuberculosis were diverse and polyclonal. γδ TCR appears to use shorter CDR3 sequence to recognize M. tuberculosis antigen, which is different from that of healthy control. The CDR3 length of tuberculosis patients focuses on 17 to 19 amino acid residues, while that of healthy controls on more than 20 amino acid residue. Our results showed that γδ T cell response to M. tuberculosis in pulmonary tuberculosis patients using preponderant complementary determinant region 3 sequence, was diverse and polyclonal.
This work was sponsored by grants (2008ZX10003-012) Eleven-fifth Mega-Scientific Project on "Prevention and treatment of AIDS, viral hepatitis and other infectious diseases" from China PR, Grant (30901314) from the National Natural Science Foundation of China PR and grant (2008IPB207) Basic R&D expenses from the Institute of Pathogen Biology, Chinese Academy of Medical Sciences.
References
- Present epidemiology of tuberculosis.Prevention and control programs. Enferm Infecc Microbiol Clin. 2011;29:2-7.
- [Google Scholar]
- A protective role of γδ T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992;175:49-56.
- [Google Scholar]
- T-lymphocytes with γδ+Vδ2+ antigen receptors are present in increased proportions in a fraction of patients with tuberculosis or with sarcoidosis. Am Rev Respir Dis. 1993;148:1685-90.
- [Google Scholar]
- Human NK cells positively regulate γδ T cells in response to Mycobacterium tuberculosis. J Immunol. 2006;176:2610-6.
- [Google Scholar]
- Enhanced secretion of interferon-γ by bovine γδ T cells induced by coculture with Mycobacterium bovis-infected dendritic cells: evidence for reciprocal activating signals. Immunology. 2008;126:201-8.
- [Google Scholar]
- Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci USA. 2009;106:2307-12.
- [Google Scholar]
- γδ T cells recognize tumor cells via CDR3 delta region. Mol Immunol. 2007;44:302-10.
- [Google Scholar]
- Identification for human cell receptor γδ-recognized epitopes/proteins via CDR3δ peptide-based immunobiochemical strategy. J Biol Chem. 2008;283:12528-37.
- [Google Scholar]
- Antigen specificity of gammadelta T cells primarily depends on the flanking sequences of CDR3delta. J Biol Chem. 2009;284:27449-55.
- [Google Scholar]
- The recognition of γδ TCR to protein antigen does not depend on the hydrophobic I97 residue of CDR3δ. Int Immunol. 2010;22:299-306.
- [Google Scholar]
- The NKG2D ligand ULBP4 binds to TCR gamma9/delta2 and induces cytotoxicity to tumor cells through both TCR gammadelta and NKG2D. Blood. 2009;114:310-7.
- [Google Scholar]
- Only a subset of phosphoantigen-responsive γ9δ2 T cells mediate protective tuberculosis immunity. J Immunol. 2008;181:4471-84.
- [Google Scholar]
- Phenotype expression and function of antigen presenting cells in huaman gammadelta T cells activated by peptide antigen from Mycobacterium tuberculosis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2009;25:588-91.
- [Google Scholar]
- CD4+ CD25+ Treg cells inhibit human memory gammadelta T cells to produce IFN-gamma in response to M. tuberculosis antigen ESAT-6. Blood. 2008;111:5629-36.
- [Google Scholar]
- Recognition of nonpeptide prenyl pyrophosphate antigens by human γδ T cells. Microbes Infect. 1999;1:175-86.
- [Google Scholar]
- Contribution of complementarity-determining region 3 of the T-cell receptor Vδ2 chain to the recognition of aminobisphosphonates by human γδ T-Cell. Int J Hematol. 2004;79:369-76.
- [Google Scholar]
- Analysis of mechanism for human γδ T cell recognition of nonpeptide antigens. Biochem Biophy Res Commun. 2005;334:349-60.
- [Google Scholar]
- Peripheral selection of antigen receptor junctional features in a major human γδ subset. Eur J Immunol. 1993;23:804-8.
- [Google Scholar]






