Translate this page into:
Screening & profiling of quorum sensing signal molecules in Pseudomonas aeruginosa isolates from catheterized urinary tract infection patients
Reprint requests: Dr Kusum Harjai, Department of Microbiology, BMS Block, Panjab University, Chandigarh 160 014, India e-mail: kusum_harjai@hotmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Catheter associated urinary tract infections are the second most common nosocomial infections and Pseudomonas aeruginosa is the third most common organism responsible for these infections. In this study P. aeruginosa isolates from catheterized urinary tract infection patients were screened and profiled for the presence of different type of quorum sensing (QS) signal molecules.
Methods:
Screening and quantitation of AHLs was done by using cross feeding assay and by determining β-galactosidase activity respectively using Escherichia coli MG4 as reporter strain. Further, AHL profiles were determined by separating AHLs on TLC coupled with their detection using Chromobacterium violaceum CV026 and Agrobacterium tumifaciens A136 biosensor strains.
Results:
All uroisolates from catheterized patients having urinary tract infections were found to be producers of QS signal molecules. There were differences in amounts and type of AHL produced amongst uroisolates of P. aeruginosa. Several AHLs belonging to C4-HSL, C6-HSL, oxo-C6-HSL, C8-HSL, C10-HSL and C12-HSL were determined in these strains.
Interpretation & conclusions:
Simultaneous use of more than one reporter strain and assay method proved useful in determining the AHLs profile in uroisolates of P. aeruginosa. Observed differences in the amounts and types of AHLs may reflect differences in virulence potential of P. aeruginosa to cause UTIs which can be further confirmed by employing animal model system. The present study speculates that production of QS signal molecules may act as a new virulence marker of P. aeruginosa responsible for causing catheter associated UTIs and can be considered as futuristic potential drug targets towards treatment of UTIs.
Keywords
Agrobacterium A136
AHL
biosensors
Chromobacterium CV026
Pseudomonas aeruginosa
TLC
UTI
Pseudomonas aeruginosa regulates the expression of cell associated and extracellular virulence factors in response to their population density by producing quorum sensing (QS) signal molecules1–11. QS signal molecules belongs to acyl homoserine lactones (AHLs) family in P. aeruginosa. These AHL molecules incorporate a homoserine lactone ring which differs with respect to the length and C3 substitution of the N-linked acyl side chain. Accumulation of the AHL molecule above a threshold level results in its interaction with a member of the LuxR family of activators allowing transcription of target genes7.
Importance of QS system has been demonstrated in various infections like cystic fibrosis, burn wound, respiratory tract infections, microbial keratitis and urinary tract infection caused by P. aeruginosa12–16. Catheter associated urinary tract infections (UTIs) are the second most common nosocomial infections and P. aeruginosa is the 3rd most common pathogen associated with 35 per cent of nosocomial catheter associated UTIs1718. These infections are a major cause of concern because of recurrence and chronicity among several susceptible patient populations. Due to formation of biofilms on the catheter surface which are resistant to antibiotics and host defence mechanism, P. aeruginosa is difficult to eradicate.
Analysis of QS signal molecules can be useful in various infections including UTI caused by P. aeruginosa. Detection and identification of QS signals can give an idea about the type of community, density of population and expression of virulence components of the infecting pathogen. Moreover, these QS regulatory mechanisms are also being proposed as a novel target for developing innovative strategies to control infections19. Since AHLs produced by bacteria differ only in the length of the acyl-chain moiety and substitution at position C3, which can be either unmodified or carry an oxo- or hydroxyl group, screening for production of these compounds may require use of many reporter organisms as well as techniques.
In the present study, analysis of QS signals in the uroisolates of P. aeruginosa has been rendered possible mainly by the use of four bacterial biosensors which were able to detect the presence of exogenous AHLs. These biosensors cannot produce their own AHL but carry functional LuxR family protein cloned together with a cognate target promoter positively regulating the transcription of a reporter gene (e.g. biolumiscence, β- galactosidase, GFP and violacein pigment). Most of the biosensors respond to limited number of acyl-HSLs due to the specificity of their R protein2021. In addition to choice of different biosensor strains, choice of detection method also becomes important and critical. There is a need to use of more than one biosensor strains and different methods for screening and profiling of QS signal molecules in P. aeruginosa isolated from catheterized urinary tract infection patients for evaluation of complete range of AHLs produced by uroisolates of P. aeruginosa, and screen the urine samples of patients directly for the detection of QS signal molecules.
Material & Methods
Bacterial strains: A total of 50 uroisolates of P. aeruginosa consecutively isolated from catheterized patients having UTI, attending Government Medical College and Hospital, Chandigarh, India, over a period of two years (November 2006 to December 2008) were used. Study protocol was approved by the Panjab University Ethical Committee. P. aeruginosa standard strain PA01 was also used along with clinical isolates.
For the detection and measurement of AHLs, reporter strain Escherichia coli MG4 (pKDT17) was used (ampicillin-100 μg/ml). AHL biosensors Chromobacterium violaceum CV026 (LB, kanamycin-20 μg/ml) and Agrobacterium tumifaciens A136 (Minimal A media, tetracycline-50 μg/ml and streptomycin-25 μg/ml) were used for TLC. Reporter strain PAO-JP2 (pECP61.5) (carbenicillin-20 μg/ml) was also used as a specific reporter strain for C4-HSL.
Cross feeding assay for AHL detection: Luria agar plates covered with 40 μl of X-Gal (20 mg/ml) were streaked 1 cm apart with reporter strains E. coli MG4 or PAO-JP2 and culture to be tested. AHLs produced and diffused through the agar results in appearance of blue colour in the reporter strain.
Extraction of AHLs: Overnight grown culture supernatants (10 ml) were extracted twice with equal volume of acidified ethyl acetate. Pooled extracts were dried over anhydrous magnesium sulphate and were evaporated to dryness. Residues were re-suspended in 50-100 μl of HPLC grade ethyl acetate.
Analytical thin layer chromatography (TLC): To evaluate the profiles of AHLs, TLC was carried out according to the method of Shaw et al21. Briefly, 4 μl of sample was applied to the silica gel C18RP TLC plates (Merck, Germany). The chromatograms were developed with methanol: water (60:40 v/v). Once the solvent front migrated to within 2 cm of the top, plates were air dried. Plates were then overlaid with a thin film of agar seeded with biosensor strain C. violaceum CV026 or A. tumifaciens A136. In case of A136, agar was supplemented with X-Gal (65 μg/ml). All experiments were done in triplicates. AHLs were identified by comparing the retention factor of synthetic standard AHLs and test AHL spots.
AHLs quantification: Culture supernatant was extracted from overnight grown culture for β-galactosidase activity. Reporter culture was diluted 1:1 in Z buffer and assayed for β-galactosidase activity by using o-nitrophenyl-D-galactopyranoside (ONPG) as a substrate as described by Miller22.
Results & Discussion
Identification of AHLs is becoming important in clinical settings since QS cascades are now becoming futuristic possible drug target factors to combat P. aeruginosa infections6. In the present study, four biosensor strains were employed for screening, identification and quantitation of AHLs in different P. aeruginosa uroisolates. E. coli MG4, used in cross-feeding assay can detect wide range of exogenous AHLs (C8 to C14-HSLs) but cannot detect shorter chain and 3-hydoxy AHLs23. In the presence of exogenous AHLs, lacI:Z gene gets activated and transcripts the reporter gene for the production of blue colouration by the activation of β-galactosidase. Initial screening showed all the uroisolates to be producers of QS signal molecules based on the development of blue colouration in reporter strain (Fig. 1). This method has an advantage of being rapid, and easy to perform. It can directly screen strains isolated from patients for AHL production. All uroisolates of P. aeruginosa have also been found to be producing AHLs earlier using A. tumifaciens reporter strain by cross feeding assay24. These workers made first demonstration of AHL production by biofilms developed on the catheters both in vitro and in vivo in patient's bladder in a clinical setting. However, type of AHL produced by uroisolates of P. aeruginosa has not been reported so far. Biosensor strain PAO-JP2 employed for detection of C4-HSL, contains plasmid pECP61.5 with lacI:Z gene insertion. By employing PAO-JP2 biosensor strain, it was observed that all the uroisolates of P. aeruginosa were C4-HSL producer in the extracts of their supernatants by cross-feeding assay.
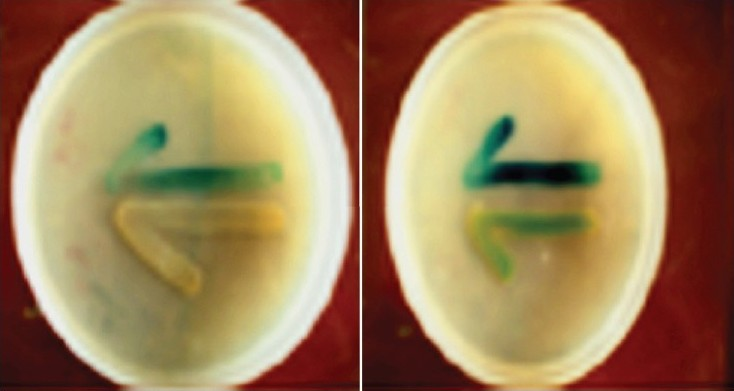
- Agar plate cross-feeding assay for screening of acyl homoserine lactone (AHL) production by uroisolates of P. aeruginosa. Evidence for the production of AHL is indicated by blue colouration of the reporter strain E. coli MG4.
Separation of AHLs by TLC coupled with their detection by AHL biosensor strains gives an identifying index of the AHLs produced by the test bacteria. Keeping this in view, we tried to identify the types of AHLs produced by uroisolates using TLC and two biosensor strains, C. violaceum CV026 and A. tumifaciens A136. C. violaceum CV026 is a violacein and AHL negative double mini Tn5 mutant strain. Transposons are inserted into the CviI AHL synthase gene and violacein repressor gene. This strain can produce pigment violacein after the use of exogenous AHL. C. violaceum CV026 can detect C4 to C8-HSLs but most strongly C6-HSL25. Identification of AHLs was done by separating bacterial extracts by TLC (C18RP Silica gel plates, Merck, Germany) and subsequently development with biosensor C. violaceum CV026. Synthetic AHL standards C4-HSL, C6-HSL, oxo-C6-HSL, C8-HSL, C10-HSL and C12-HSL were also run simultaneously. Purple colour spots parallel to the position of synthetic standards C4 and C6-HSL on plates were observed (Fig. 2). Relative retention factor (Rf) was calculated and compared with that of standards. A total of 74 per cent isolates showed the presence of C6-HSL. Although, C. violaceum CV026 biosensor is reported to detect C4-HSL, C6-HSL and C8-HSL2627, but other than C6-HSL no other AHL was detected in the present study. Non-detection may be due to low production of C4-HSL and C8-HSL which are reported to be detected by C. violaceum CV026 when produced at higher concentrations only25. Negative results from P. aeruginosa isolates from ICU patients for AHLs with C. violaceum CV026 has been reported earlier and it was suggested that the isolates either failed to produce short chain AHLs or the level of signals was very low28. The oxo-C6-HSL and C8-HSL were detected with a 6-fold and C4-HSL with a 30-fold less activity7. However, detection of C4-HSL and C8-HSL was made possible only by using violacein inhibition assay by C. violaceum CV026272930.
![Separation and detection of acyl- HSL standards [mixture of C4 (BHL), C6 (HHL), C8 (OOHL)] (lane 1) and acyl- HSLs produced by uroisolates of P. aeruginosa (lane 2-6) by employing thin layer chromatography. Spots were visualized with biosensor Chromobacterium violaceum CV026. Tentative identification of spots, based on migration of standards, is indicated.](/content/175/2011/134/2/img/IJMR-134-208-g002.png)
- Separation and detection of acyl- HSL standards [mixture of C4 (BHL), C6 (HHL), C8 (OOHL)] (lane 1) and acyl- HSLs produced by uroisolates of P. aeruginosa (lane 2-6) by employing thin layer chromatography. Spots were visualized with biosensor Chromobacterium violaceum CV026. Tentative identification of spots, based on migration of standards, is indicated.
Since C. violaceum CV026 cannot detect AHLs with longer acyl side chains, broad range biosensor, A. tumifaciens A136 was employed thereafter for TLC. A. tumifaciens A136 contains a plasmid with traR promoter and traG::lacZ transcriptional fusion. traG::lacZ gets activated in the presence of exogenous AHL and results in appearance of blue colour. This reporter strain can detect C8 to C12-HSLs including oxo-C6-HSL and is quite sensitive to even low level of longer acyl side chain AHLs31. All the isolates showed production of different types of AHLs on the basis of relative retention factor (Fig. 3). Only 26 per cent isolates were positive for the production of oxo-C6-HSL, 90 per cent isolates for C8-HSL, 58 per cent isolates for C10-HSL and 54 per cent isolates for C12-HSL.
![Separation and detection of acyl- HSL standards [mixture of oxo C6 (OHHL), C8 (OOHL), C10 (ODHL) and C12 (OdDHL)] (lane 1), acyl- HSLs produced by uroisolates of P. aeruginosa (lane 2-5) and wild type PAO1 and mutant JP2 (lane 6-7) by employing thin layer chromatography. Spots were visualized with biosensor A. tumifaciens A136. Tentative identification of spots, based on migration of standards, is indicated.](/content/175/2011/134/2/img/IJMR-134-208-g003.png)
- Separation and detection of acyl- HSL standards [mixture of oxo C6 (OHHL), C8 (OOHL), C10 (ODHL) and C12 (OdDHL)] (lane 1), acyl- HSLs produced by uroisolates of P. aeruginosa (lane 2-5) and wild type PAO1 and mutant JP2 (lane 6-7) by employing thin layer chromatography. Spots were visualized with biosensor A. tumifaciens A136. Tentative identification of spots, based on migration of standards, is indicated.
Two to six different AHLs were detected in most of the test isolates by employing two biosensor strains in TLC. Presence of at least four AHLs like C12-HSL, C6-HSL, oxo-C6-HSL and C4-HSL was also detected earlier by employing TLC using C. violaceum CV026 and A. tumifaciens A136 as reporter strains in microbial keratitis isolates27. Erickson et al14 showed the production of different AHLs like C8-HSL, C10-HSL and C12-HSL in sputum samples of cystic fibrosis patients indicating their production during lung infections. Recently, we have also shown the production of QS signal molecules qualitatively in renal homogenates of mouse model of UTI indicating their role in pathogenesis of UTIs16.
Results of quantitation of β-galactosidase activity showed production of variable levels of AHLs by P. aeruginosa uroisolates (Fig. 4) indicating that P. aeruginosa isolated from the same source also produces different amounts of AHLs. These findings point towards strain level differences in AHL production. Involvement of different phenotypes in infections among numerous strains of P. aeruginosa has also been indicated previously32. Various phenotypes of isolates from same source have been reported to produce different levels of AHLs in microbial keratitis. Invasive isolates from keratitis patients produced high levels of AHLs whereas cytotoxic isolates produced low levels of AHLs27.
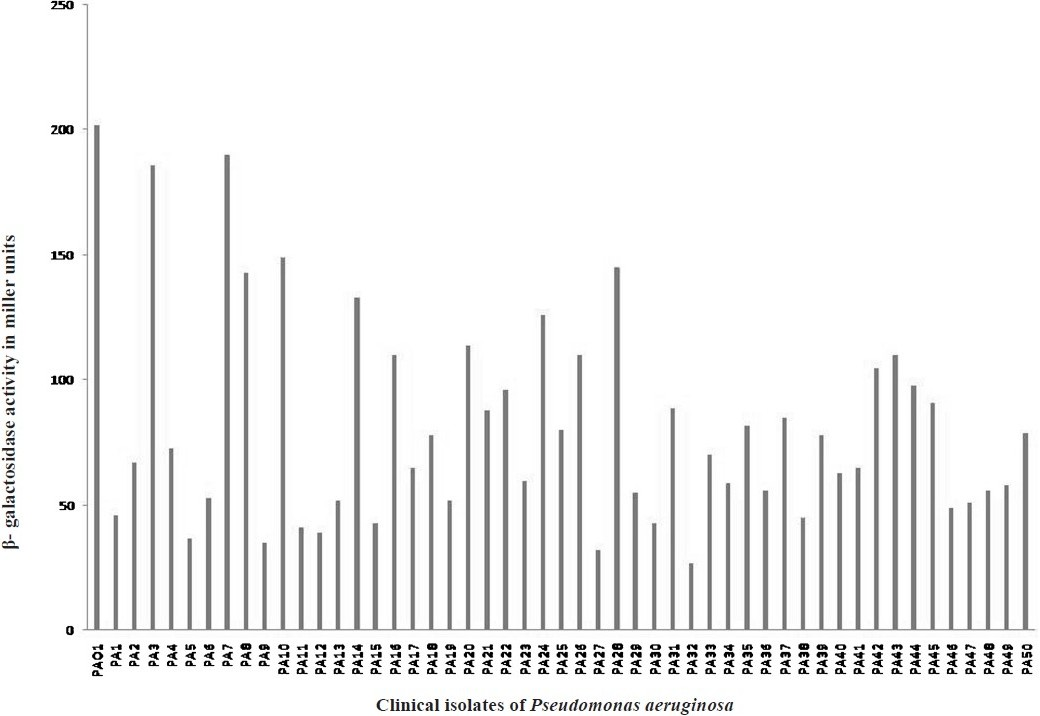
- Quantitative β- galactosidase activity expressed in miller units (MU) for determining the levels of quorum sensing signal molecules in the extracts of culture supernatants of Standard strain PA01 (bar 1) and uroisolates of P. aeruginosa (bar 2-50).
In conclusion, results of the present study indicated that use of more than one biosensor strain and assay methods was useful in determining the profiles of AHLs. All the methods proved to be useful for the detection of AHLs directly from pure cultures. Further, production of AHL was one of the important properties possessed by uroisolates of P. aeruginosa and hence indicating a definitive association of QS with urinary tract infection. This approach can further be exploited for detection of QS signal molecules directly in the urine samples of patients indicating presence of pathogen.
This work was supported by contingency grant from Department of Biotechnology, New Delhi, India. Authors acknowledge Dr Barbara H. Iglewski for providing standard strain of Pseudomonas aeruginosa, and Dr H. Zhu, NSWU, Australia, for providing biosensor reporter strains for TLC.
References
- Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756-67.
- [Google Scholar]
- Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3127-32.
- [Google Scholar]
- Cell- to cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4:551-60.
- [Google Scholar]
- Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci. 1999;96:11229-34.
- [Google Scholar]
- Quorum sensing: the power of cooperation in the world of Pseudomonas. Environ Microbiol. 2005;7:459-71.
- [Google Scholar]
- Quorum-sensing blockade as a strategy for enhancing host defences against bacterial pathogens. Philos Trans R Soc Lond Biol Sci. 2007;362:1213-22.
- [Google Scholar]
- Detection of quorum-sensing N- acyl homoserine lactone signal molecules by bacterial biosensors. FEMS Microbiol Lett. 2007;266:1-9.
- [Google Scholar]
- Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2008;52:3648-63.
- [Google Scholar]
- Analysis of the hierarchy of quorum-sensing regulation in Pseudomonas aeruginosa. Anal Bioanal Chem. 2007;387:469-79.
- [Google Scholar]
- Quorum sensing inhibition selects for virulence and cooperation in Pseudomonas aeruginosa. PLoS Pathog. 2010;6:e1000883.
- [Google Scholar]
- Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect Immun. 1999;67:5854-62.
- [Google Scholar]
- Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect Immun. 2000;68:4331-4.
- [Google Scholar]
- Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect Immun. 2002;70:1783-90.
- [Google Scholar]
- Pseudomonas aeruginosa with lasI quorum-sensing deficiency during corneal infection. Invest Ophthalmol Vis Sci. 2004;45:1897-903.
- [Google Scholar]
- Quorum sensing is necessary for the virulence of Pseudomonas aeruginosa during urinary tract infection. Kidney Int. 2009;76:286-92.
- [Google Scholar]
- Predominant pathogens in hospital infections. J Antimicrob Chemother. 1992;29(Suppl A):19-24.
- [Google Scholar]
- Risk factors of nosocomial catheter-associated urinary tract infection in a polyvalent intensive care unit. Intensive Care Med. 2003;29:1077-80.
- [Google Scholar]
- Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acal Sci USA. 1994;91:197-201.
- [Google Scholar]
- Detecting and characterizing N- acyl- homoserine lactone signal molecules by thin layer chromatography. Proc Natl Acal Sci USA. 1997;94:6036-41.
- [Google Scholar]
- Experiments in molecular genetics. Plainview, NY: Cold Spring Harbor Laboratory Press; 1972.
- Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant associated bacteria. Mol Plant Microbe Interact. 1998;11:1119-29.
- [Google Scholar]
- Biofilms on indwelling urethral catheters produce quorum sensing signal molecules in situ and in vitro. Appl Environ Microbiol. 1998;64:3486-90.
- [Google Scholar]
- Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143:3703-11.
- [Google Scholar]
- Methods for detecting acylated homoserine lactones produced by Gram-negative bacteria and their application in studies of AHL-production kinetics. J Microbiol Methods. 2001;44:239-51.
- [Google Scholar]
- Determination of quorum-sensing signal molecules and virulence factors of Pseudomonas aeruginosa isolates from contact lens-induced microbial keratitis. J Med Microbiol. 2002;51:1063-70.
- [Google Scholar]
- N- butanoyl-L- homoserine lactone (BHL) deficient Pseudomonas aeruginosa isolates from an intensive care unit. Microbiol Res. 2005;160:399-403.
- [Google Scholar]
- Multiple N- acyl -L- homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:9427-31.
- [Google Scholar]
- The LuxM homologue VanM from Vibrio anguillarum directs the synthesis of N-(3-hydroxyhexanoyl)homoserine lactone and N-hexanoylhomoserine lactone. J Bacteriol. 2001;183:3537-47.
- [Google Scholar]
- Quorum-sensing system of Agrobacterium plasmids: analysis and utility. Methods Enzymol. 2002;358:452-84.
- [Google Scholar]
- Diversity of biofilms produced by quorum-sensing-deficient clinical isolates of Pseudomonas aeruginosa. J Med Microbiol. 2007;56:738-48.
- [Google Scholar]






