Translate this page into:
High density lipoprotein heterogeneity & function among Indians with coronary artery disease
For correspondence: Dr Archna Singh, Room No. 3044, Department of Biochemistry, Teaching Block, All India Institute of Medical Sciences, New Delhi 110 029, India e-mail: arch_singh@ymail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Impaired high density lipoprotein (HDL) functionality has been shown to be associated with cardiovascular disease risk. The study was aimed to identify the alterations in HDL function [antioxidative activity (AOA)] and subfraction distribution between acute coronary syndrome (ACS) and stable coronary artery disease (SCAD) individuals and analysing the accuracy of HDL parameters to discriminate between the groups.
Methods:
HDL subfraction distribution analysis was performed in 200 coronary artery disease patients (ACS and SCAD) and 60 control individuals using dextran sulphate, heparin and manganese chloride precipitation method. In terms of HDL function, AOA was evaluated by dihydrorhodamine-based fluorescent cell-free assay and paraoxonase (PON1) enzyme paraoxonase and arylesterase activity.
Results:
We found that higher AOA [odds ratio (95% confidence interval {CI})]: 0.09 (0.02-0.44), P<0.01 for SCAD; 0.008 (0.001-0.07), P<0.001 for ACS and higher PON1 activity [0.22 (0.8-0.59), P<0.01 for SCAD; 0.16 (0.06-0.4), P<0.001 for ACS] were associated with a lower odds of developing coronary artery disease (CAD). AOA of apoB-depleted serum was significantly correlated with HDL2-C/HDL3-C (HDL-cholesterol) ratio in controls (r=−0.31, P=0.01) and ACS (r=−0.18, P=0.04). It was observed that AOA and HDL subfraction distribution together could discriminate between the two groups of CAD with an accuracy of 72.8 per cent (P=0.004).
Interpretation & conclusions:
Impaired AOA and altered subfraction distribution of HDL may be responsible for its diminished anti-athero protective activity and can discriminate between the two groups of CAD individuals.
Keywords
Antioxidative activity
atherosclerosis
coronary artery disease
high density lipoprotein
paraoxonase
Coronary artery disease (CAD) is one of the most common types of cardiovascular disease1. CAD may present as chronic stable angina or acute coronary syndrome (ACS). After years of clinical trials for identifying and mitigating numerous risk factors, CAD remains the major cause of death in many populations, including south Asians. The underlying cause of CAD is atherosclerosis. Atherosclerosis is a chronic inflammatory disease of the intima of arteries that starts developing in childhood2.
Inflammation induced by oxidative stress plays a crucial role in the pathogenesis of atherosclerosis3. Oxidative stress along with other risk factors such as dyslipidaemia and hypertension exacerbates the process of atherosclerotic lesion formation4. Oxidative stress is shown to be positively associated with plaque instability and the process of plaque disruption causing thrombosis and resulting in ACS5.
The high density lipoprotein (HDL) particle is a complex of proteins and lipids with a high protein-to-lipid ratio. HDL exhibits a range of atheroprotective properties apart from its ability to remove excess cholesterol from peripheral tissues to transport it back to the liver (reverse cholesterol transport). These include antioxidative, anti-inflammatory and antiapoptotic properties6-8. Apolipoprotein A-I, the major protein component of HDL along with other associated proteins such as paraoxonase (PON1) and lecithin–cholesterol acyltransferase (LCAT), contributes to the antioxidative activity (AOA) of HDL. HDL-associated AOA prevents the accumulation of primary and secondary peroxidation products on low-density lipoprotein (LDL)9,10. Circulating HDL particles exhibit heterogeneity in terms of their structure, composition and function. They are characterized as HDL2 and HDL3 based on their density with HDL2 being less dense and lipid rich and HDL3 being denser and protein rich. The heterogeneity in terms of protein and lipid cargo on HDL particles provides distinct functionalities to HDL fractions11.
With recent research and evidence available about HDL functionality, it is now being asserted that the quality rather than quantity of HDL is more relevant for its atheroprotective effects. Further, the functionality of HDL could also be influenced by the heterogeneity of HDL particles. There are limited data on HDL characteristics among Indians who typically have low HDL-C and apoA-I levels. Therefore, in this study, the antioxidative property of HDL and HDL subfraction distribution were evaluated in Indian individuals with CAD and compared with control individuals. Further, the potential of HDL AOA and subfraction distribution to discriminate between two groups of CAD [stable CAD (SCAD) and ACS] was assessed, adding to the limited data from a geographical region that contributes a large burden of disease but is underrepresented in terms of research-based evidence.
Material & Methods
The study was conducted by the department of Cardiology, All India Institute of Medical Sciences, New Delhi, India, between December 2014 and January 2019. The study was approved by the Institutional Ethics Committee (IESC/T-380/17 October 2014). Written informed consent was obtained from all the participants prior to their enrolment in the study.
Study participants: This was an exploratory cross-sectional study which included 260 male individuals aged between 20 and 80 yr: comprising 60 healthy controls and 200 individuals with CAD recruited during the study period. Based on the clinical characteristics, CAD individuals were grouped into two categories: Stable coronary artery dissection (SCAD; n=80) and acute coronary syndrome (ACS; n=120). The control group consisted of apparently healthy individuals without any cardiovascular disorders and not receiving any lipid lowering drugs. SCAD patients were diagnosed using computed tomography (CT) angiography.
Inclusion and exclusion criteria: Individuals with more than 50 per cent blockage in any one of the three major coronary vessels were included in the SCAD group. ACS was diagnosed using electrocardiogram (more than 1 mm ST-segment elevation in contiguous limb leads/more than 2 mm ST-segment elevation in precordial leads) and elevated troponin I levels. Individuals with inflammatory or autoimmune disorders (e.g. systemic lupus erythematosus, rheumatoid arthritis or inflammatory bowel disease) or diagnosed with thyroid dysfunction were excluded from the study.
Biochemical analysis: Blood was collected after overnight fast from participants with SCAD and controls. For ACS patients, blood samples were collected within 8 h of the diagnosis or confirmation of the disease. Serum samples were isolated from the blood by centrifugation, aliquoted and immediately stored at −80°C until further analysis. The lipid profile including HDL-C, LDL-C, triglyceride and total cholesterol was measured using Randox enzymatic (Randox Laboratories, Crumlin, UK) assays on Beckman AU480 autoanalyser (Beckman Coulter Inc., Indianapolis, USA). Apolipoprotein A-I and apolipoprotein B measurement was done by an immunoturbidimetric method using Randox kits according to the manufacturers’ instructions.
Cholesteryl ester transfer protein (CETP) activity was determined using a fluorescence-based activity assay kit (Roar Biomedicals, New York, USA). Results were expressed in terms of pmol of fluorescent substrate transferred. Rabbit serum and torcetrapib were used as positive control and negative control for CETP activity assay validation.
High density lipoprotein (HDL) subfraction analysis: HDL subfraction separation was performed using single-step precipitation according to the procedure described previously12. The precipitation reagent containing heparin, manganese chloride and dextran sulphate (8.25 mg/ml, 98.7 mg/ml and 12 mg/ml, respectively) was used simultaneously to precipitate both the apoB-containing lipoproteins and HDL2. HDL3 was separated by adding 40 µl of precipitation reagent to 0.2 ml of serum with gentle mixing. The mixture was incubated at room temperature for 30 min. Thereafter, it was centrifuged at 10,000 rpm for 10 min at 4°C. An aliquot of the supernatant obtained after centrifugation was used to measure HDL3-C levels using HDL estimation kit. The measured value for HDL3-C was multiplied by 1.2 to correct for dilution by the reagents. HDL2-C was calculated as the difference between the total HDL-C and HDL3-C.
HDL antioxidative activity (AOA): HDL AOA was measured using a time-dependent oxidation of a fluorogenic probe dihydrorhodamine 123 (DHR) to fluorescent rhodamine. The assay was performed as described previously13. Briefly, apoB-depleted serum was prepared by precipitation method using dextran sulphate and magnesium chloride. DHR solution (50 μM) prepared in HEPES buffer and apoB-depleted serum (5 µg cholesterol) were mixed with HEPES buffer in a 96-well plate in a way such that the final volume of the mixture was 175 μl. The plate was incubated in dark for 10 min at 37°C. After the incubation, fluorescence intensity (emission 485 nm and excitation 538 nm) was recorded at every 2 min intervals for an hour. DHR in buffer only was used as sample blank. The change in fluorescence intensity between 10 and 50 min was calculated which represented the oxidation rate of DHR in the presence of apoB-depleted serum. The AOA of HDL was represented in arbitrary units. All samples and blank were run in triplicates. The inter-assay coefficient of variation (CV) for the assay was 10.2 per cent and intra assay CV was 7.5 per cent.
Paraoxonase (PON1) activity: Paraoxonase-hydrolyzing activity of PON1 in serum was measured using paraoxon as a substrate for the enzyme14. Paraoxon was used as a substrate which, in the presence of PON1, is hydrolyzed to diethyl phosphate and p-nitrophenol, which is yellow in colour. The amount of p-nitrophenol formed was measured which reflects the paraoxonase activity of PON1. Paraoxonase activity of PON1 was expressed in U/ml.
Arylesterase activity of PON1 was estimated using phenyl acetate, and phenyl acetate is hydrolyzed to phenol and acetic acid in the presence of PON1 enzyme. The amount of phenol produced indicates the arylesterase activity of PON1. Arylesterase activity was calculated using an extinction coefficient of phenol at 270 nm. Hydrolysis of phenyl acetate by PON1 (E270=1310/M/cm). All the samples were tested in triplicates. Arylesterase activity was expressed in U/ml.
Statistical analysis: Statistical analysis was performed using STATA/SE 12.1 software (StataCorp LP, TX, USA) and GraphPad Prism 6 (GraphPad Software, CA, USA) software. The normality of the data was analyzed using Smirnov-Kolmogorov test. Quantitative variables were expressed as mean±standard deviation (SD). Chi-squared test was used to analyse the differences between the categorical variables in three groups. Student’s t test and one-way ANOVA with Bonferroni correction were performed to analyse the difference between biochemical and HDL parameters between the groups. Pearson correlation coefficient was used to analyse the correlation between HDL2-C/HDL3-C and AOA and their significance. Multinomial logistic regression was performed using ACS/SCAD as dependent variables and AOA as independent variables to assess the association between AOA and CAD. The adjusted odds ratio was analysed after controlling for age, smoking, diabetes, hypertension and LDL-C. Binary logistic regression was used to examine the percentage accuracy of HDL2-C/HDL3-C, AOA and PON1 activity to discriminate ACS from control, ACS from SCAD and SCAD from control. Since this was an exploratory study to investigate the antioxidative function of HDL in Indian population, we did not perform any sample size calculation before starting the study. Post hoc power calculation was performed after the study, which is mentioned in the results section.
Results
Subject characteristics: The clinical characteristics of the participants enrolled in the study are summarized in Table I. The study groups included only male individuals. The mean age of the control group was 42 yr, which was significantly lower when compared to the mean age of individuals with SCAD and ACS. A significant difference was observed between the mean age of participants with SCAD and those with ACS. The body mass index of all the three groups was similar. The control group had a smaller number of individuals with diabetes, hypertension and smokers. The ACS group had more individuals with diabetes and smokers than in the SCAD group.
| Parameters | Control (n=60), mean±SD | SCAD (n=80), mean±SD | ACS (n=120) |
|---|---|---|---|
| Age (yr) | 42.8±10.4 | 57.5±10.2*** | 50.7±10.3###,$$$ |
| BMI (kg/m2) | 24.7±3.8 | 24.6±3.3 | 25.6±3.9 |
| Smoking, n (%) | 7 (11.7) | 25 (31.2)** | 70 (58.3)##,$$ |
| Diabetes, n (%) | 2 (3.3) | 26 (32.5)*** | 29 (24.2)### |
| Hypertensive, n (%) | 8 (13.3) | 31 (38.7)*** | 33 (27.5)# |
| HDL-C (mg/dl) | 43.5±8.5 | 38.8±7.7*** | 39.3±7## |
| LDL-C (mg/dl) | 104.6±25.1 | 93.1±33.1 | 108.3±35.4$$ |
| VLDL-C (mg/dl) | 18.1±8.1 | 17.9±11 | 20.1±10.7 |
| Triglyceride (mg/dl) | 123.4±43.4 | 128.3±58.9 | 123±63.1 |
| Total cholesterol (mg/dl) | 160.2±37.9 | 150.7±43.6 | 169.0±42.9$$ |
| ApoA-I (mg/dl) | 136.2±39.2 | 110.4±28.6*** | 109.2±24.2### |
| ApoB (mg/dl) | 77.6±24.8 | 59.1±15.9*** | 82.1±29.5$$$ |
| AOA (AU) | 78.6±11.1 | 67.6±16.2*** | 56.8±13.7###,$$$ |
| PON1 activity (U/ml) | 107.2±56.2 | 71.4±37.6*** | 70.1±41.2### |
| Arylesterase activity (U/ml) | 113.8±38.9 | 80.1±26.8*** | 79.1±27.9### |
| HDL2-C (mg/dl) | 21.1±7.1 | 21.5±4 | 23.0±5 |
| HDL3-C (mg/dl) | 22.4±5.8 | 19.0±3.2*** | 16.9±3.4###,$$ |
| HDL2-C/HDL3-C | 1.0±0.4 | 1.2±0.3* | 1.4±0.5###,$$ |
| CETP (pmol transferred) | 117.9±61.3 | 109.3±50 | 103.1±56.9 |
P *<0.05; **<0.01; ***<0.001 between control and SCAD; P #<0.05; ##<0.01; ###<0.001 between control and ACS; P $<0.05; $$<0.01; $$$<0.001 between SCAD and ACS. ACS, acute coronary syndrome; BMI, body mass index; CETP, cholesteryl ester transfer protein; HDL-C, high-density lipoprotein-cholesterol; HDL2-C, high-density lipoprotein subfraction 2-cholesterol; HDL3-C, high-density lipoprotein subfraction 3-cholesterol; LDL-C, low-density lipoprotein-cholesterol; n, number of individuals; SCAD, stable coronary artery disease; VLDL-C, very LDL-C; SD, standard deviation; AOA, antioxidative activity; AU, antioxidative unit; PON1, paraoxonase; ApoA-I, apolipoprotein A-I; ApoB, apolipoprotein B
Post hoc power calculation was done based on the sample size and the antioxidative values in the three groups in the current study. The power of the study was 96.7 per cent for AOA and 93.5 per cent for HDL2-C/HDL3-C between the three groups.
Biochemical parameters: The lipid profile of controls, SCAD and ACS groups is represented in Table I. HDL-C levels were significantly lower in SCAD (38.8±7.7 mg/dl, P<0.001) and ACS groups (39.3±7.0 mg/dl, P<0.001) compared to controls (43.5±8.5 mg/dl). In comparison to the control group, the SCAD group had lower levels of LDL-C (93.1±33.1 mg/dl vs. 104.6±25.1, P=0.018) and total cholesterol. Total cholesterol levels were significantly higher in the ACS group (169.0±42.9, P=0.003) compared to SCAD group, while no difference was observed between control and ACS group. Triglyceride and VLDL cholesterol levels did not differ between the groups. Apolipoprotein A-I levels were significantly lower in SCAD (110.4±28.6 mg/dl, P<0.001) and ACS group (109.2±24.2 mg/dl, P<0.001) compared to controls. In parallel to the LDL-C levels, apolipoprotein B levels were also significantly lower in SCAD group compared to ACS group (82.1±29.5 mg/dl, P<0.001) and controls (77.6±24.8 mg/dl, P<0.001). Plasma cholesteryl ester transfer protein (CETP) activity was similar in all the three groups.
Characteristics of HDL: HDL2-C subfraction was significantly higher in ACS group (23.0±5.0 mg/dl, P=0.02) compared to SCAD group (21.5±4 mg/dl), while no difference in HDL2-C was observed between controls vs. ACS group and control vs. SCAD group (Fig. 1A). HDL3-C levels were significantly lower in SCAD group (19.0±3.2 mg/dl, P<0.001) compared to controls. ACS group had significantly lower HDL3-C levels (16.9±3.4 mg/dl, P<0.001) compared to SCAD group (Fig. 1B). The ratio of HDL2-C/HDL3-C subfraction in the serum was significantly higher in ACS group (1.4±0.5, P<0.001) compared to both SCAD group (1.2±0.3) and controls (1.0±0.4; Fig. 1C).
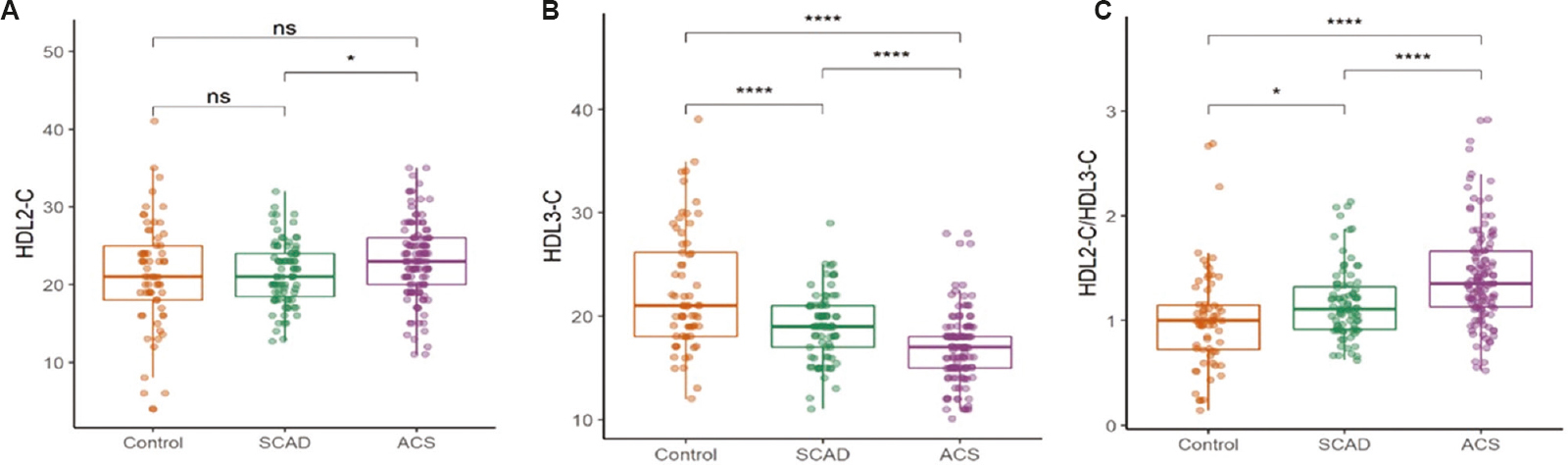
- Box and whisker plot showing HDL subfraction distribution in controls, SCAD and ACS. (A) HDL-2C, (B) HDL-3C and (C) ratio of HDL-2C to HDL-3C. P *<0.05, ****<0.001. HDL, high density lipoprotein; SCAD, stable coronary artery disease; ACS, acute coronary syndrome; ns, not significant
ApoB-depleted serum isolated from both SCAD (67.6±16.2 vs. 78.6±11.1 AU, P<0.001) and ACS groups (56.8±13.7 vs. 78.6±11.1 AU, P<0.001) had a significantly lower capacity to inhibit the oxidation of dihydrorhodamine compared to serum from control individuals (Fig. 2A). Furthermore, apoB-depleted serum from ACS patients had a significantly lower antioxidative potential compared to SCAD patients (56.8±13.7 vs. 67.6±16.2 AU, P<0.001).
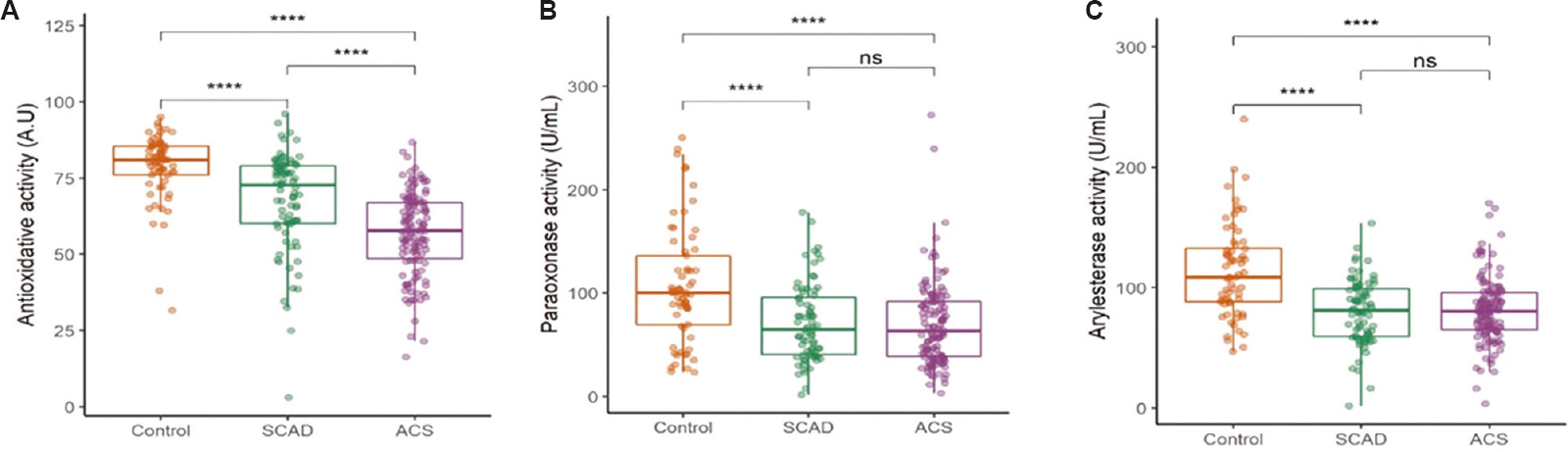
- Box and whisker plot showing comparison of HDL functions in controls, SCAD and ACS groups. (A) HDL antioxidative activity, (B) paraoxonase activity, and (C) arylesterase activity. ****P <0.001. ns, not significant
Compared to the sera of controls, PON1 activity was significantly lower in serum of ACS (70.1±41.2 vs. 107.2±56.2 U/ml, P<0.001) and SCAD groups (71.4±37.6 vs. 107.2±56.2 U/ml, P<0.001). Arylesterase activity of PON1 was significantly impaired in ACS (79.1±27.9 vs. 113.8±38.1 U/ml, P<0.001) and SCAD (80.1±26.8 vs. 113.8±38.1 U/ml, P<0.001) patients compared to controls (Fig. 2B and C).
A significant correlation was observed between AOA of apoB-depleted serum and HDL2-C/HDL3-C ratio in controls (r=−0.31, P=0.01) and ACS (r=−0.18, P=0.04). No significant correlation was found between AOA and HDL2-C/HDL3-C in the SCAD group (r=−0.21, P=0.056) (Fig. 3A). Further, no significant correlation was observed between PON1 and HDL subfractions (Fig. 3B and C) in any of the three groups.
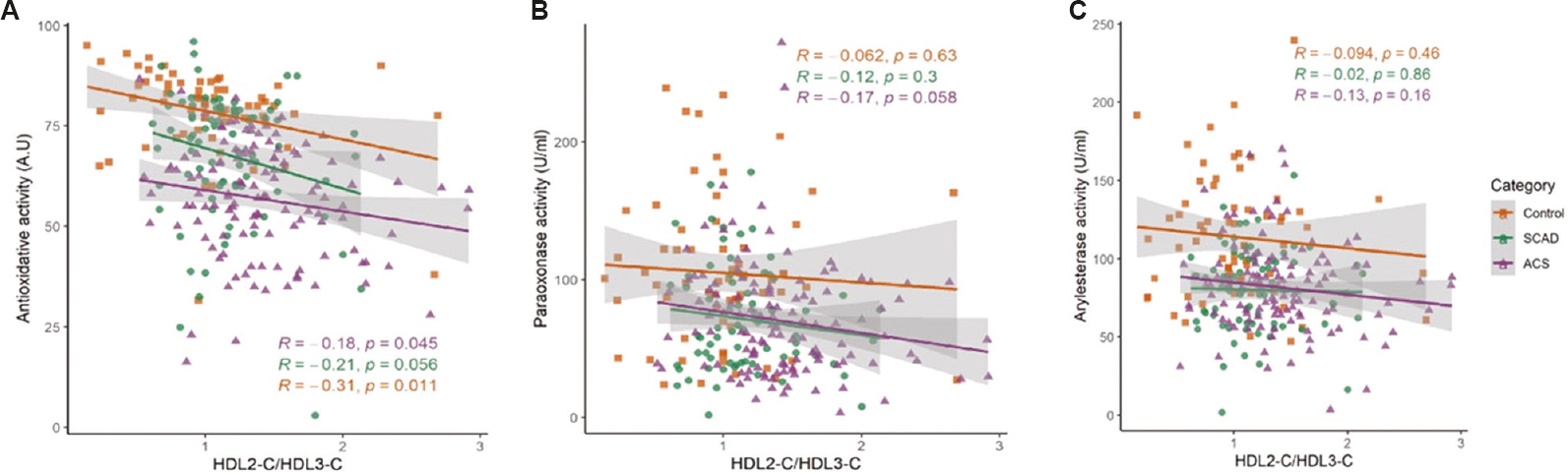
- Correlation of HDL subfraction distribution with antioxidative activity of HDL in the three groups. (A) antioxidative activity, (B) paraoxonase activity, and (C) arylesterase activity. Line represents the regression line.
To further support a relationship between HDL AOA and CAD, parameters were divided into four quartiles and odds ratios for CAD were calculated by comparing the reference highest risk quartile with the three lower risk quartiles. The association between AOA and odds of having cardiovascular disease expressed as AOA quartiles is represented in Table II. Increased AOA (quartiles 3 and 4) was significantly associated with lower odds of development of CAD. The association remained significant even after controlling for traditional risk factors (age, smoking, diabetes, hypertension and LDL-C). The multivariate modelling revealed that the PON1 activity in the fourth quartile was associated with lower odds of having CAD relative to lower PON1 values (quartiles 2 and 3) after adjusting for age, smoking, diabetes, hypertension and LDL-C (Table II). The overall association of the AOA with the odds of developing CAD is shown in Supplementary Table.
| Unadjusted OR (95% CI) | AOR (95% CI) | |
|---|---|---|
| AOA | ||
| Control vs. CAD (quartile) | ||
| Q1 | Reference | Reference |
| Q2 | 0.30 (0.06-1.58) | 0.63 (0.10-3.73) |
| Q3 | 0.09 (0.02-0.42)** | 0.12 (0.02-0.69)** |
| Q4 | 0.02 (0.004-0.09)*** | 0.03 (0.005-0.17)*** |
| Control vs. ACS | ||
| Q1 | Reference | Reference |
| Q2 | 0.32 (0.06-1.7) | 0.73 (0.11-4.8) |
| Q3 | 0.08 (0.01-0.37)*** | 0.20 (0.02-0.95)* |
| Q4 | 0.004 (0.0007-0.02)*** | 0.008 (0.001-0.07)*** |
| Control vs. SCAD | ||
| Q1 | Reference | Reference |
| Q2 | 0.45 (0.15-1.29) | 0.67 (0.15-3) |
| Q3 | 0.26 (0.09-0.75)** | 0.47 (0.10-2.2) |
| Q4 | 0.075 (0.02-0.23)*** | 0.09 (0.02-0.44)** |
| PON1 activity | ||
| Control vs. CAD | ||
| Q1 | Reference | Reference |
| Q2 | 1.50 (0.53-4.24) | 2.00 (0.58-6.69) |
| Q3 | 0.47 (0.2-1.12) | 0.48 (0.16-1.44)* |
| Q4 | 0.21 (0.09-0.49)*** | 0.20 (0.07-0.58)** |
| Control vs. ACS | ||
| Q1 | Reference | Reference |
| Q2 | 1.32 (0.47-3.7) | 1.81 (0.49-6.64) |
| Q3 | 0.43 (0.17-1.08) | 0.40 (0.11-1.42) |
| Q4 | 0.16 (0.06-0.4)*** | 0.14 (0.04-0.5)** |
| Control vs. SCAD | ||
| Q1 | Reference | Reference |
| Q2 | 1.85 (0.63-5.4) | 2.17 (0.47-9.85) |
| Q3 | 0.32 (0.12-0.84)** | 0.09 (0.02-0.43)** |
| Q4 | 0.22 (0.8-0.59)** | 0.14 (0.03-0.62)** |
| PON1 (arylesterase activity) | ||
| Control vs. CAD | ||
| Q1 | Reference | Reference |
| Q2 | 0.57 (0.19-1.71) | 0.57 (0.16-2.02) |
| Q3 | 0.35 (0.13-0.98)* | 0.53 (0.16-1.76) |
| Q4 | 0.07 (0.03-0.2)*** | 0.11 (0.03-0.34)*** |
| Control vs. ACS | ||
| Q1 | Reference | Reference |
| Q2 | 0.63 (0.22-1.8) | 0.65 (0.18-2.39) |
| Q3 | 0.33 (0.12-0.9)** | 0.31 (0.08-1.17) |
| Q4 | 0.080 (0.03-0.21)*** | 0.12 (0.03-0.44)** |
| Control vs. SCAD | ||
| Q1 | Reference | Reference |
| Q2 | 0.32 (0.11-0.93)* | 0.49 (0.12-1.95) |
| Q3 | 0.41 (0.14-1.2) | 0.72 (0.18-2.9) |
| Q4 | 0.04 (0.01-0.14)*** | 0.03 (0.006-0.22)*** |
P *<0.05; ** <0.01; **** <0.001. AOR adjusted for age, smoking status, diabetes, hypertension and LDL. OR, odds ratio; AOR, adjusted OR; ACS, acute coronary syndrome; CAD, coronary artery disease; SCAD, stable CAD; LDL, low-density lipoprotein; AOA, antioxidative activity; CI, confidence interval; PON1, paraoxonase
| Unadjusted OR (95% CI) | AOR (95% CI) | |
|---|---|---|
| AOA | ||
| Control versus CAD | 0.89 (0.86-0.92) | 0.9 (0.86-0.95) |
| Control versus SCAD | 0.93 (0.9-0.96) | 0.95 (0.91-0.99) |
| Control versus ACS | 0.85 (0.81-0.89) | 0.86 (0.81-0.9) |
| PON1 activity | ||
| Control versus CAD | 0.98 (0.97-0.99) | 0.98 (0.97-0.99) |
| Control versus SCAD | 0.98 (0.97-0.99) | 0.97 (0.96-0.99) |
| Control versus ACS | 0.98 (0.97-0.99) | 0.98 (0.97-0.99) |
| Arylesterase activity | ||
| Control versus CAD | 0.97 (0.96-0.98) | 0.97 (0.96-0.98) |
| Control versus SCAD | 0.97 (0.95-0.98) | 0.97 (0.95-0.98) |
| Control versus ACS | 0.97 (0.95-0.98) | 0.97 (0.96-0.98) |
AOR adjusted for age, smoking status, diabetes, hypertension and LDL. OR, odds ratio; AOR, adjusted OR; LDL, low-density lipoprotein; CAD, coronary artery disease; SCAD, stable CAD; ACS, acute coronary syndrome; CI, confidence interval; PON1, paraoxonase; AOA, antioxidative activity
Binary logistic regression model using the ACS and SCAD groups to look for AOA and HDL2-C/HDL3-C individually and together as a marker to differentiate between ACS and SCAD patients (Table III) revealed that HDL2-C/HDL3-C could correctly distinguish between ACS and SCAD with an accuracy percentage of 62.9 (B=1.51, P<0.001) while AOA with an accuracy of 71.1 per cent (B=−0.05, P<0.001). The model comprising AOA and HDL2-C/HDL3-C together showed an accuracy of 72.8 per cent (P<0.001), demonstrating that AOA and HDL2-C/HDL3-C together could discriminate between acute and stable coronary events better than when taken individually (Table III).
| Parameter | Per cent accuracy (%) | Coefficient (B) | SE | P |
|---|---|---|---|---|
| ACS vs. control | ||||
| HDL2-C/HDL3-C | 71.66 | 2.06 | 0.44 | <0.001 |
| AOA | 85.56 | −0.16 | 0.02 | <0.001 |
| HDL2-C/3-C+AOA | 88.24 | 1.03 (HDL2-C/3-C) −0.15 (AOA) | 0.53 (HDL2-C/3-C) 0.02 (AOA) | <0.001 <0.001 |
| SCAD vs. control | ||||
| HDL2-C/HDL3-C | 64.19 | 0.93 | 0.44 | 0.03 |
| AOA | 68.24 | −0.07 | 0.02 | <0.001 |
| HDL2-C/3-C+AOA | 66.89 | 0.5 (HDL2-C/3-C) −0.06 (AOA) | 0.47 (HDL2-C/3-C) 0.01 (AOA) | 0.29 <0.001 |
| ACS vs. SCAD | ||||
| HDL2-C/HDL3-C | 62.93 | 1.51 | 0.4 | <0.001 |
| AOA | 71.17 | −0.05 | 0.11 | <0.001 |
| HDL2-C/3-C+AOA | 72.86 | 1.2 (HDL2-C/3-C) −0.04 (AOA) | 0.43 (HDL2-C/3-C) 0.01 (AOA) | 0.004 <0.001 |
ACS, acute coronary syndrome; HDL2-C, high-density lipoprotein subfraction 2-cholesterol; HDL3-C, high-density lipoprotein subfraction 3-cholesterol; SE, standard error; AOA, antioxidative activity; SCAD, stable coronary artery disease
Analysis of AOA and HDL2-C/HDL3-C showed that HDL2-C/HDL3-C could distinguish ACS from controls with an accuracy of 71.66 per cent, while for AOA, it was 85.56 per cent. HDL2-C/HDL3-C and AOA together can differentiate between controls and ACS patients with an accuracy of 88.24 per cent.
Discussion
The present study describes the antioxidative properties of HDL and its subfraction distribution and how they differ in the case of CAD. In the present study, it was found that the apoB-depleted sera of CAD patients had significantly impaired AOA compared to controls. The lower AOA was significantly negatively correlated with HDL2-C/HDL3-C levels. Higher AOA (total and PON1 activity) was also significantly associated with lower odds of having CAD. AOA and HDL2-C/HDL3-C were able to distinguish between controls and ACS patients significantly with an accuracy of 88.24 per cent and between ACS and SCAD with an accuracy of 72.86 per cent.
It has been suggested that HDL subclasses, including the different measures of HDL particle heterogeneity, are better markers for CVD in comparison to static measures of HDL mass. In comparison to an overall end-point measure like HDL cholesterol, the profile of HDL particles has shown a stronger association with atherosclerosis15. In the present study, HDL3-C levels were significantly higher in controls compared to CAD patients. In a cross-sectional analysis performed on the large MESA cohort, small- and medium-sized HDL particles classified using NMR were found to be strongly and inversely associated with carotid intima thickening16.
A study performed in non-treated SCAD patients reported that a higher large HDL-C fraction was associated with lower cardiovascular risk but not small or medium HDL-C17. In contrast to the above study, we observed that small and dense HDL3-C was lower and the ratio of HDL2-C/HDL3-C was higher in CAD patients. Similarly, a study conducted in a community-based prospective sample observed that baseline HDL3-C levels were an independent protective factor against arterial stiffness, while no association was observed between HDL2-C and carotid pulse wave velocity18.
Our estimation of the antioxidative status of HDL using time-dependent oxidation of a fluorogenic probe dihydrorhodamine 123 (DHR) to fluorescent rhodamine showed that the AOA of HDL was significantly lower in CAD patients compared to controls. Lower antioxidative activity of HDL in CAD patients means decreased ability of HDL to prevent the oxidation of LDL from oxidative stress in the arterial intima. This could result in the increased formation of pro-inflammatory molecules such as lipid hydroperoxides (LOOHs) and LOOH-derived short-chain oxidized phospholipids. Impaired HDL functionality could be due to the high production of myeloperoxidase from macrophages in the arterial intima which induces oxidative modification of apolipoprotein A-I19. Oxidative modification of apoA-I may be responsible for decreased inactivation of LOOH by HDL. Higher antioxidative capacity seen in controls contributes to preventing oxidative modification of LDL by free radicals in the arterial intima and therefore protecting it from atherogenic insults. Dense HDL3 particles have been observed to have potent AOA attributed to the redox status of apoA-I and the surface lipid rigidity of the particles20. Alteration in levels of HDL3-C could be responsible for the diminished activities of HDL3-C and lower level of protection against the development of atherosclerotic plaque.
The lower levels of circulating HDL3-C subfraction and apolipoprotein A-I levels in CAD patients might have resulted in lower AOA observed in CAD patients compared to controls. Apolipoprotein A-I plays a major role in HDL-mediated AOA and HDL3-C carries large number of apolipoprotein molecules and other enzymes that contribute to the antioxidative properties of HDL including PON1, LCAT and PAF-AH21. In the present study, a negative correlation was observed between AOA and HDL2-C/HDL3-C. The negative correlation suggests that the changes in HDL subfraction distribution could be the reason for altered HDL functionality. The lower concentration of PON1 levels in the SCAD and ACS groups could be the reason for lower PON1 paraoxonase and arylesterase activity observed in ACS and SCAD patients in the study. Similar studies conducted in hypertensive and dyslipidaemic patients from India observed a lower HDL-paraoxonase activity associated with oxidative stress22,23.
The impaired HDL AOA observed in ACS patients could be due to the acute inflammatory and oxidative conditions present in ACS condition24. A study on the effect of inflammation on HDL proteome has shown that HDL efflux capacity correlates inversely with the content of serum amyloid A1 (SAA1) and serum amyloid A2 on HDL25. Several studies have shown that high inflammation levels are independently associated with CVD events, and anti-inflammatory intervention studies (CANTOS and COLCOT) have suggested that reducing the inflammatory profile reduces the risk of secondary CVD events26,27. In the present study, AOA was significantly associated with the incidence of ACS when compared with controls and SCAD group, indicating that the HDL properties may be influenced by the inflammatory milieu in individuals with ACS. An important clinical implication of the present investigation was the potential of the HDL functional parameters analyzed to be able to delineate between CAD subtypes and the control group.
According to clinical practice followed, all the suspected SCAD patients were started on statins and antiplatelet drug before they underwent CT angiography. Hence, all the participants in the SCAD group were exposed to statins, albeit for few weeks before they were enrolled in the study. As all the SCAD participants in our study were on statins, we may surmise that statin therapy could be the reason for reduced inflammatory stress28,29. This could explain the better AOA and lower LDL-C and total cholesterol levels in case of SCAD patients compared to controls and ACS patients.
The study had a few limitations. The groups being compared were not matched with respect to cardiovascular risk factors such as age, smoking, diabetes and hypertension. We adjusted for these risk factors in the statistical analysis. Only male individuals were included in the study, and the sample size of the control group was less compared to the case groups. Since the control group did not undergo any evaluation for the presence of subclinical atherosclerosis, the presence of subclinical atherosclerosis in these individuals cannot be ruled out. The SCAD group had substantial number of individuals on statin therapy. For assessing the AOA of HDL in the study, only apoB-containing lipoproteins were removed from serum, leaving the rest of the components that is albumin, immunoglobulins and other minor antioxidants such as ascorbates and tocopherol30. It is not clear if the AOA measurement is influenced by the components left in the serum. Although we could not directly estimate the particle size of HDL in this study, we estimated the cholesterol levels in smaller HDL3 and larger HDL2 subfractions.
In conclusion, the results of this study suggested that the lower HDL AOA is significantly associated with higher odds of having CAD. Altered HDL subfraction distribution was negatively correlated with the AOA, suggesting that HDL subfraction distribution could also provide information about the AOA. Both the properties differed with the severity of the disease. Thus, in alignment with currently evolving evidence, improving HDL properties should be a major therapeutic goal for the treatment of CAD patients.
Financial support and sponsorship
None.
Conflicts of interest
None.
References
- A nationwide framework for surveillance of cardiovascular and chronic lung diseases. Washington DC: National Academies Press; 2011.
- Atherosclerotic cardiovascular disease beginning in childhood. Korean Circ J. 2010;40:1-9.
- [Google Scholar]
- Pathogenesis of atherosclerosis:A multifactorial process. Exp Clin Cardiol. 2002;7:40-53.
- [Google Scholar]
- Oxidative stress:Predictive marker for coronary artery disease. Exp Clin Cardiol. 2013;18:e88-91.
- [Google Scholar]
- Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127-35.
- [Google Scholar]
- Native high density lipoproteins (HDL) interfere with platelet activation induced by oxidized low density lipoproteins (OxLDL) Int J Mol Sci. 2013;14:10107-21.
- [Google Scholar]
- Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest. 1995;96:2882-91.
- [Google Scholar]
- Sequence conservation of apolipoprotein A-I affords novel insights into HDL structure-function. J Lipid Res. 2011;52:435-50.
- [Google Scholar]
- Proteomic diversity of high density lipoproteins:Our emerging understanding of its importance in lipid transport and beyond1. J Lipid Res. 2013;54:2575-85.
- [Google Scholar]
- A simple and precise method for measuring HDL-cholesterol subfractions by a single precipitation followed by homogenous HDL-cholesterol assay. J Lipid Res. 2008;49:1130-6.
- [Google Scholar]
- A biochemical fluorometric method for assessing the oxidative properties of HDL. J Lipid Res. 2011;52:2341-51.
- [Google Scholar]
- HDL functions and their interaction in patients with ST elevation myocardial infarction:A case control study. Lipids Health Dis. 2020;19:75.
- [Google Scholar]
- High-density lipoprotein particle concentration and subclinical atherosclerosis of the carotid arteries in Japanese men. Atherosclerosis. 2015;239:444-50.
- [Google Scholar]
- Concentration of smaller High-Density Lipoprotein Particle (HDL-P) is inversely correlated with carotid intima media thickening after confounder adjustment:The multi ethnic study of atherosclerosis (MESA) J Am Heart Assoc. 2016;5:e002977.
- [Google Scholar]
- Large HDL subfraction but not HDL-C is closely linked with risk factors, coronary severity and outcomes in a cohort of nontreated patients with stable coronary artery disease:A prospective observational study. Medicine (Baltimore). 2016;95:e2600.
- [Google Scholar]
- High-density lipoprotein 3 cholesterol is a predictive factor for arterial stiffness:A community-based 4.8-year prospective study. Lipids Health Dis. 2018;17:5.
- [Google Scholar]
- Methionine oxidized apolipoprotein A-I at the crossroads of HDL biogenesis and amyloid formation. FASEB J. 2018;32:3149-65.
- [Google Scholar]
- HDL3-mediated inactivation of LDL-associated phospholipid hydroperoxides is determined by the redox status of apolipoprotein A-I and HDL particle surface lipid rigidity:Relevance to inflammation and atherogenesis. Arterioscler Thromb Vasc Biol. 2009;29:2169-75.
- [Google Scholar]
- Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters:Relevance to antioxidative function. Arterioscler Thromb Vasc Biol. 2009;29:870-6.
- [Google Scholar]
- Correlation of serum paraoxonase activities in known cases of 130 elderly hypertensive South Asian aged 56-64 years –A hospital based study. Asian Pac J Trop Dis. 2014;4:S330-5.
- [Google Scholar]
- Correlation of HDL Associated paraoxonase 1 with oxidative stress markers in hypertensive dyslipidemic patients. 2018. JCDR. 12:BC23-6. Available from: https://jcdr.net/article_fulltext.asp?issn=0973-709x&year=2018&volume=12&issue=10&page=BC23&issn=0973-709x&id=12189
- [Google Scholar]
- Oxidative stress as the leading cause of acute myocardial infarction in diabetics. Cardiovasc Drug Rev. 2006;24:77-87.
- [Google Scholar]
- Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J Lipid Res. 2015;56:1519-30.
- [Google Scholar]
- Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119-31.
- [Google Scholar]
- Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497-505.
- [Google Scholar]
- Strong decrease of high sensitivity C-reactive protein with high-dose atorvastatin in patients with type 2 diabetes mellitus. Atherosclerosis. 2003;166:129-35.
- [Google Scholar]
- Ascorbate and urate are the strongest determinants of plasma antioxidative capacity and serum lipid resistance to oxidation in Finnish men. Atherosclerosis. 1997;130:223-33.
- [Google Scholar]






