Translate this page into:
The Indian registry on current patient profiles & treatment trends in hypertension (RECORD): One year interim analysis
For correspondence: Dr Girish Chandrakant Rajadhyaksha, Department of Medicine, Topiwala National Medical College & B. Y. L. Nair Charitable Hospital, Dr. A. L. Nair Road, Near Railway Station, Mumbai 400 008, Maharashtra, India e-mail: girishraj63@hotmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
In India, hypertension constitutes a significant health burden. This observational, non-interventional, prospective study was conducted in five centres across India to evaluate the current clinical practices for the management of hypertension.
Methods:
Participants were enrolled if they were newly diagnosed with essential hypertension or had pre-existing hypertension and were on the same therapeutic plan for the previous three months. At baseline, three months, six months, and one year, information on the patient and their treatment regimen was documented, and their quality of life (QoL) was evaluated.
Results:
A total of 2000 individuals were enrolled in this study, with a mean age of 54.45 yr. Of these, 55.7 per cent (n=1114) were males, and 957 (47.85%) were newly diagnosed with hypertension, while 1043 (52.15%) had pre-existing hypertension. Stage 2 hypertension (systolic blood pressure (BP) >140 or diastolic BP ≥90 mmHg) accounted for more than 70 per cent of the participants (70.76% of pre-existing and 76.29% of newly diagnosed); the average duration of pre-existing hypertension was 68.72 months. Diabetes (31.6%) and dyslipidaemia (15.8%) were the most common comorbidities. In 43.3 per cent of the participants, monotherapy was used, and in 56.7 per cent (70.55% fixed-dose combination), combination therapy was used. Telmisartan (31.6%), amlodipine (35.2%), and a combination of the two (27.1%) were the most commonly prescribed treatment regimens. At three months, six months, and one year, treatment modifications were observed in 1.4, 1.05, and 0.23 per cent of the participants receiving monotherapy and 2.74, 4.78 and 0.35 per cent receiving combination therapy, respectively. In both groups, the proportion of individuals with controlled hypertension (≤140/90 mmHg) increased by more than 30 per cent after a year. At one year, physical and emotional role functioning, social functioning, and health improved considerably.
Interpretation & conclusions:
Combination therapy for hypertension is increasingly preferred at the time of initial diagnosis. The efficacy, safety, and tolerance of the recommended medications were reflected by improvements in the QoL and the minimal changes in the therapeutic strategy required.
Keywords
Amlodipine
combination therapy
fixed-dose combination
hypertension
India
monotherapy
registry
telmisartan
treatment
Hypertension is a serious medical condition that is associated with a significantly increased risk of brain, heart, kidney and other diseases1. Although a preventable risk factor, hypertension is an important cause of premature death and disability and affects around 1.13 billion people worldwide1. There is a high prevalence of hypertension in India affecting one in three adults with an overall prevalence of 30.7 per cent2. The prevalence of hypertension in India is also common in the younger age groups; with about one in every 10 young Indian adults (18-25 yr) being affected by hypertension3. In 2016, hypertension caused 1.63 million deaths in India3. This hypertension epidemic in the country is further worsened by a lack of awareness among the major proportion of individuals regarding their hypertension status3,4.
The major risk factors for hypertension are age, familial history, comorbidities such as diabetes or kidney disease, sedentary lifestyle, being overweight or obese, high consumption of dietary salt and fat, low consumption of vegetables/fruits and consumption of tobacco and alcohol1. Two studies from India have previously reported a significant association between hypertension and various risk factors such as gender (male predominance), overweight/obesity, increasing age, tobacco and alcohol consumption and history of diabetes mellitus5,6. These findings highlight that the management of hypertension should also involve the prevention and management of other comorbidities, such as diabetes5.
Despite ample information and projected estimates for the hypertension burden in India, the majority of the published literature includes cross-sectional observational studies that describe the prevalence, awareness of, and control of hypertension based on data obtained from surveys, interviews, or questionnaires. Till date, there is no evidence of a prospective cohort study that describes the trends of blood pressure distribution, treatment patterns, treatment modification patterns, and control rates of hypertension in patients with newly diagnosed and pre-existing hypertension with or without comorbid conditions over a follow up duration of two years.
To fill this knowledge gap, a real world registry was planned with two endpoints in mind. The primary endpoint was to document current clinical practices for the treatment of essential hypertension in India. The secondary endpoint was to assess the rate of control of hypertension with various antihypertensive treatment agents in individuals with essential hypertension in India.
Material & Methods
Study design: A prospective cohort, non-interventional, observational and real-world registry was conducted in five primary care centres across India including two private (Apollo Hospitals, Chennai, Tamil Nadu; and the St. John’s Medical College & Hospital, Bengaluru, Karnataka) and three public hospitals (Topiwala National Medical College & B. Y. L. Nair Charitable Hospital, Mumbai, Maharashtra; King George’s Medical University, Lucknow, Uttar Pradesh; and Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh), wherein consecutive patients were enrolled between March 2019 and February 2020. All eligible individuals newly diagnosed with essential hypertension and those with existing hypertension undergoing pharmacological treatment with the same therapeutic regimen for the past three months were enrolled. All participants were followed up at three months, six months, and one year from the initiation of the study. The data from three month, six month, and one year follow up are being reported here. All study participants were followed up for up to two years from study initiation.
This study was approved by the Institutional Ethics Committees of the respective institutes and carried out in compliance with the study protocol, Declaration of Helsinki, Good Clinical Practice, and Indian Council for Medical Research guidelines concerning medical research in human subjects. Written informed consent was obtained from all the participants before the study was initiated.
Diagnosis of essential hypertension: One of the objectives of this study was to determine the type and extent to which international guidelines, such as the American College of Cardiology and American Heart Association (ACC/AHA) or European Society of Cardiology (ESC) guidelines, are preferred in the current clinical practice in India. As this was an observational study, the physicians were free to follow either ACC/AHA or ESC guidelines for the diagnosis and management. Therefore, the diagnosis of essential hypertension was based on the criteria defined by the 2017 ACC/AHA guideline on the prevention, detection, evaluation, and management of high BP in adults7 (or) the 2018 ESC guidelines for the management of arterial hypertension, based on prevailing clinical practices8. In this study, some centres followed the ACC/AHA guidelines for the diagnosis of hypertension, while others followed the ESC guidelines.
Study population: Individuals were enrolled in the study after approval from the study-site institutional review board or ethics committee. Inclusion criteria were as follows: (i) individuals of either sex, aged >18 yr; (ii) individuals with a diagnosis of essential hypertension under the criteria established by the ACC/AHA 2017 or ESC/ESH 2018 hypertension guidelines and those individuals on antihypertensive treatment with the same therapeutic regimen over the past three months; and (iii) those willing to sign the informed consent form.
The following individuals were excluded from the study: (i) those with secondary hypertension; (ii) pregnant women or nursing mothers; (iii) individuals with acute illnesses or having a definite psychiatric diagnosis; (iv) those unlikely to fulfil the study requirements in the opinion of the investigator; (v) individuals currently enrolled in another study or those who had not yet completed at least a month since ending the use of any investigational product or device; and (vi) individuals with other comorbidities that may limit life expectancy to less than one year.
Study parameters: After enrolment in the study, demographics, blood pressure (BP) (as per the ACC/AHA 2017 or ESC/ESH 2018 criteria), other vital signs, medical history, and concomitant medication details of the individuals were recorded. The presence of new/pre-existing comorbidities was recorded based on the medical history provided. Clinical signs and symptoms at presentation, details of electrocardiograms, laboratory investigations, and the prescribed/ongoing treatments were also recorded. The quality of life (QoL) of the participants was assessed using the 36-item short-form (SF-36) health survey questionnaire9.
The parameters assessed at baseline and follow ups were as follows: (i) at baseline: systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse rate, oxygen saturation rate, electrocardiography, laboratory parameters, and QoL; and (ii) at all three follow ups: SBP, DBP, pulse rate, laboratory parameters, and QoL. The compliance with antihypertensive therapy during the follow up period was assessed using a self-reported questionnaire. In addition, patients were instructed to bring back the empty medication strips during follow up visits.
Subgroup analysis: Data were also analysed to assess the influence of factors such as diagnosis of hypertension (pre-existing or newly diagnosed), guidelines used to classify hypertension (ACC/AHA or ESC), type of therapy (monotherapy or combination therapy) and presence of diabetes mellitus on the reduction in SBP and DBP.
Data collection: Data were collected through an electronic case record form, which was hosted on a central server. Access was allowed through a secure website from each participating site. Authorized study personnel from each participating site were responsible for data collection, data entry, and protection of the data being collected from the respective site.
Statistical analysis: The study protocol involved no sampling; therefore, no formal sample size calculations were performed. Categorical variables have been presented as numbers and percentages and continuous data as mean ± standard deviation (SD). Blood pressure readings (for SBP and DBP) between the follow up visits were compared using the paired t test with P<0.05 considered as significant. To evaluate the treatment effect on blood pressure outcomes at follow up visits of three, six months and one year, estimates [least squares mean difference (standard error)] and P values were obtained from mixed model repeated measures (MMRM) analyses with the change in SBP or DBP as a dependent variable and type of therapy at enrolment, follow up visit, the interaction of type of therapy at enrolment and follow up, age, sex and baseline SBP or DBP as fixed effects. Since the comparison was performed at multiple time points (3, 6 months, and one year), the alpha value was adjusted using the Bonferroni correction method by dividing 0.05 by 3. Hence, for the MMRM analysis, P<0.017 was considered as significant. Statistical analysis was done using R-language software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline parameters: A total of 2000 individuals were enrolled, including 1114 males (55.7%) and 886 females (44.3%). The mean age (±SD) of the participants was 54.45 (±11.93) yr, and the mean body mass index (±SD) was 26.24 (±3.42) kg/m2. Majority of the participants were older than 50 yr (61.65%). The mean SBP and DBP were 142.01 and 83.63 mmHg, respectively. Nearly 17 per cent of the participants had a family history of hypertension. The detailed baseline characteristics of the study participants are presented in Table I.
| Characteristics | n (%) or mean±SD |
|---|---|
| Gender | |
| Male | 1114 (55.7) |
| Female | 886 (44.3) |
| Age (yr) | 54.5±11.9 |
| Age predisposition (yr) | |
| 20-30 | 52 (2.6) |
| 31-40 | 239 (11.9) |
| 41-50 | 476 (23.8) |
| >50 | 1233 (61.7) |
| BMI (kg/m2) | 26.2±3.4 |
| Mean SBP (mmHg) | 142.0±19.2 |
| Newly diagnosed (mmHg) | 150.7±14.5 |
| Pre-existing (mmHg) | 146.8±21.3 |
| Mean DBP (mmHg) | 83.6±11.2 |
| Newly diagnosed (mmHg) | 86.7±10.7 |
| Pre-existing (mmHg) | 85.7±11.7 |
| Pulse rate (beats/min) | 82.5±9.8 |
| Addiction history | |
| Smoking | |
| Current | 35 (1.8) |
| Former | 70 (3.5) |
| Never | 1895 (94.8) |
| Alcohol consumption | |
| Current | 42 (2.1) |
| Former | 35 (1.8) |
| Never | 1923 (96.2) |
| Chewing tobacco | |
| Yes | 10 (0.5) |
| No | 1990 (99.5) |
| Family history | |
| Family history of hypertension | 334 (16.7) |
| Family history of other CV conditions* | 90 (4.5) |
| Diagnosis of hypertension | |
| Newly diagnosed | 957 (47.9) |
| Pre-existing | 1043 (52.2) |
| Guidelines used to grade hypertension in newly diagnosed patients | |
| ACC/AHA | 544 (56.8) |
| ESC | 413 (43.2) |
| Guidelines used to grade hypertension in pre-existing | |
| patients | |
| ACC/AHA | 855 (82) |
| ESC | 107 (10.3) |
| UNK | 81 (7.8) |
*Other CV conditions included several factors such as coronary artery disease, heart disease, death due to myocardial infarction, ischaemic heart disease, dyslipidaemia, myocardial infarction, peripheral artery disease, rheumatic heart disease and old ischaemic heart disease. CV, cardiovascular; SD, standard deviation; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; UNK, unknown; ACC/AHA, American College of Cardiology and American Heart Association; ESC, European Society of Cardiology
Diagnosis and assessment of hypertension: The study population comprised almost equal proportions of pre-existing (52.15%) and newly diagnosed (47.85%) hypertension patients.
Diagnosis: The ACC/AHA guidelines on hypertension (56.8%) were more frequently used for the diagnosis and grading of hypertension in the population with newly diagnosed hypertension, as compared to the ESC guidelines (43.2%; Table I).
The use of the ACC/AHA guidelines for grading hypertension was more common in the group of participants with pre-existing hypertension (82%); the ESC guidelines were used only in 10.3 per cent of these participants (Table I).
Mean BP at diagnosis: For newly diagnosed individuals, the mean (±SD) SBP and DBP values at the time of diagnosis were 150.7 (±14.52) and 86.72 (±10.73) mmHg, respectively. For the population with pre-existing hypertension, the median duration of hypertension was 45 months, and at the time of registration in the registry, the mean (± SD) SBP and DBP values were 146.79 (± 21.32) and 85.7 (±11.68) mmHg, respectively.
Comorbidities: The most common comorbidity observed was diabetes mellitus (n=632, 31.6%), followed by dyslipidaemia (n=316, 15.80%) (Table II).
| Name of conditions | Count, n (%) |
|---|---|
| Diabetes mellitus | 632 (31.6) |
| Dyslipidaemia | 316 (15.8) |
| Previous percutaneous coronary intervention | 83 (4.15) |
| Previous myocardial infarction | 67 (3.35) |
| Previous coronary artery bypass graft | 59 (2.95) |
| Heart failure | 31 (1.55) |
| Bronchial asthma | 22 (1.1) |
| Renal dysfunction | 21 (1.05) |
| Atrial fibrillation | 16 (0.8) |
| Chronic obstructive pulmonary disease | 14 (0.7) |
| Family history of coronary artery disease | 13 (0.65) |
| Stroke | 8 (0.4) |
| Peripheral arterial disease | 3 (0.15) |
| Any other medical condition | 499 (24.95) |
Treatment pattern: In the overall study population (n=2000), 43.3 per cent (n=866) of individuals received monotherapy and 56.7 per cent (n=1134) received combination therapy. Fixed dose combination (FDC) was prescribed to 70.55 per cent (n=800) of all the individuals receiving combination therapy. Based on the diagnosis, 43.26 and 43.34 per cent of newly diagnosed (n=957) and pre-existing (n=1043) hypertension patients received monotherapy, while 56.74 and 56.66 per cent received combination therapy, respectively (data not shown). The percentage change from monotherapy to combination therapy was 1.4 per cent over one year. The different types of antihypertensive medications used by the study population were angiotensin II receptor blockers (ARBs) (telmisartan, azilsartan, losartan, and olmesartan), angiotensin-converting enzyme inhibitors (ACEIs) (ramipril, enalapril, and perindopril), alpha-agonists (clonidine and moxonidine), alpha-blockers (prazosin), beta-blockers (metoprolol, bisoprolol, nebivolol, atenolol, carvedilol, and propranolol), calcium channel blockers (CCBs) (amlodipine, cilnidipine, nifedipine, and diltiazem) and diuretics (torsemide, chlorthalidone, furosemide, indapamide, hydrochlorothiazide, chlorothiazide, eplerenone, indapamide, and spironolactone).
Medication prescribed at the baseline: In the total study population, monotherapy was prescribed in 43.3 per cent of participants, in which amlodipine (35.2%) and telmisartan (31.6%) were most commonly used. Combination therapy was prescribed in 56.7 per cent of the overall population, in which the use of amlodipine + telmisartan combination was most common (27.1%). The medications have been listed based on the proportion of participant prescriptions (Table III).
| Monotherapy | Overall (n=866), n (%) | Pre-existing at the time of enrolment (n=452), n (%) | New diagnosis (n=414), n (%) |
|---|---|---|---|
| Amlodipine | 305 (35.2) | 198 (43.8) | 107 (25.8) |
| Telmisartan | 274 (31.6) | 93 (20.6) | 181 (43.7) |
| Metoprolol | 58 (6.7) | 27 (6) | 31 (7.5) |
| Bisoprolol | 37 (4.3) | 31 (6.9) | 6 (1.4) |
| Ramipril | 37 (4.3 | 13 (2.9) | 24 (5.8) |
| Torsemide | 26 (3) | 1 (0.2) | 25 (6) |
| Enalapril | 22 (2.5) | 17 (3.8) | 5 (1.2) |
| Cilnidipine | 19 (2.2) | 13 (2.9) | 6 (1.4) |
| Nebivolol | 17 (2) | 15 (3.3) | 2 (0.5) |
| Azilsartan | 14 (1.6) | 2 (0.4) | 12 (2.9) |
| Combination therapy | Overall (n=1134), n (%) | Pre-existing at the time of enrolment (n=591), n (%) | New diagnosis (n=543), n (%) |
| Amlodipine + telmisartan | 307 (27.1) | 75 (12.7) | 232 (42.7) |
| Amlodipine + indapamide + perindopril | 106 (9.3) | 97 (16.4) | 9 (1.7) |
| Metoprolol + telmisartan | 81 (7.1) | 38 (6.4) | 43 (7.9) |
| Hydrochlorothiazide + telmisartan | 48 (4.2) | 13 (2.2) | 35 (6.4) |
| Amlodipine + atenolol | 42 (3.7) | 24 (4.1) | 18 (3.3) |
| Amlodipine + hydrochlorothiazide | 41 (3.6) | 2 (0.3) | 39 (7.2) |
| Amlodipine + metoprolol | 35 (3.1) | 25 (4.2) | 10 (1.8) |
| Amlodipine + bisoprolol + indapamide + perindopril | 33 (2.9) | 32 (5.4) | 1 (0.2) |
| Metoprolol + ramipril | 25 (2.2) | 16 (2.7) | 9 (1.7) |
| Amlodipine + telmisartan + torsemide | 22 (1.9) | 9 (1.5) | 13 (2.4) |
Medication prescribed in newly diagnosed stage 2 hypertension participants: Among the 845 newly diagnosed individuals with stage 2 hypertension (≥140 mmHg or ≥90 mmHg), 511 (60.5%) participants were initiated on combination therapy, which was inclusive of FDCs. Amlodipine + telmisartan was the most common combination therapy (n=219, 42.9%). Monotherapy was prescribed in 334 (39.5%) of the newly diagnosed stage 2 participants with telmisartan being the most common monotherapy (n=143, 42.8%), followed by amlodipine (n=93, 27.8%).
Medication prescribed in individuals with pre-existing hypertension at the time of initial diagnosis: At the time of initial diagnosis, the majority of the participants with pre-existing hypertension (n=1043) were on monotherapy (n=689, 66.06%), while the rest (n=354, 33.94%) were on combination therapy. Therefore, the proportion of participants on combination therapy increased from 33.94 per cent at initial diagnosis to 56.66 per cent at the time of study initiation. Among the participants on combination therapy, 46.89 per cent (n=166) were on FDC. Among those on monotherapy, the most common medication used was amlodipine (n=249, 36.1%), followed by telmisartan (n=127, 18.4%). The most commonly used combination therapy was telmisartan + amlodipine (n=70, 19.8%) at initial diagnosis (Supplementary Table I), which increased to 42.7 per cent in newly diagnosed participants.
| Type and list of medications | Count | Per cent |
|---|---|---|
| Monotherapy | 689 | 66.06 |
| Amlodipine | 249 | 36.1 |
| Telmisartan | 127 | 18.4 |
| Metoprolol | 55 | 8 |
| Bisoprolol | 50 | 7.3 |
| Atenolol | 38 | 5.5 |
| Enalapril | 34 | 4.9 |
| Nebivolol | 28 | 4.1 |
| Ramipril | 23 | 3.3 |
| Cilnidipine | 17 | 2.5 |
| Losartan | 14 | 2 |
| Olmesartan | 14 | 2 |
| Carvedilol | 11 | 1.6 |
| Combination therapy (including fixed-dose combinations) | 354 | 33.94 |
| Amlodipine+telmisartan | 70 | 19.8 |
| Amlodipine+atenolol | 34 | 9.6 |
| Metoprolol+telmisartan | 25 | 7.1 |
| Amlodipine+metoprolol | 24 | 6.8 |
| Hydrochlorothiazide+telmisartan | 15 | 4.2 |
| Amlodipine+metoprolol+telmisartan | 12 | 3.4 |
| Metoprolol+ramipril | 11 | 3.1 |
| Chlorthalidone+telmisartan | 9 | 2.5 |
| Amlodipine+enalapril | 7 | 2 |
| Amlodipine+losartan | 6 | 1.7 |
Status of hypertension control: In the overall population, 83.7 per cent of participants receiving monotherapy and 73.2 per cent receiving combination therapy had controlled hypertension (<140/90 mmHg) at one year. Among the newly diagnosed individuals, 76.2 per cent of those who received monotherapy and 64.3 per cent of those who received combination therapy reported controlled hypertension at one year. Similarly, in those with pre-existing hypertension, 90.6 per cent of those who received monotherapy and 81.4 per cent of those who received combination therapy reported controlled hypertension at one year. The proportion of individuals achieving controlled BP was higher in those with pre-existing hypertension than in those with newly diagnosed hypertension for monotherapy (90.6 vs. 76.2%) as well as combination therapy (81.4 vs. 64.3%; Table IV).
| Follow up | Overall population (n=1987) | Newly diagnosed hypertension (n=955) | Pre-existing hypertension (n=1032) | |||
|---|---|---|---|---|---|---|
| Monotherapy (n=857), n (%) | Combination therapy (n=1130), n (%) | Monotherapy (n=41), n (%) | Combination therapy (n=54), n (%) | Monotherapy (n=44), n (%) | Combination therapy (n=58), n (%) | |
| Controlled (<140/90) | ||||||
| Baseline | 257 (30) | 267 (23.6) | 257 (57.8) | 267 (45.5) | ||
| 3 months | 508 (59.3) | 492 (43.5) | 189 (45.9) | 140 (25.8) | 319 (71.7) | 352 (60) |
| 6 months | 613 (71.5) | 634 (56.1) | 240 (58.3) | 203 (37.4) | 373 (83.8) | 431 (73.4) |
| 1 yr | 717 (83.7) | 827 (73.2) | 314 (76.2) | 349 (64.3) | 403 (90.6) | 478 (81.4) |
| Controlled (<130/80) | ||||||
| Baseline | 94 (11) | 92 (8.1) | 94 (21.2) | 92 (15.7) | ||
| 3 months | 73 (8.5) | 100 (8.9) | 9 (2.2) | 8 (1.5) | 64 (14.4) | 92 (15.7) |
| 6 months | 157 (18.3) | 155 (13.7) | 73 (17.7) | 46 (8.5) | 84 (18.9) | 109 (18.6) |
| 1 yr | 145 (16.9) | 165 (14.6) | 67 (16.3) | 62 (11.4) | 78 (17.6) | 103 (17.6) |
In the overall population, 16.9 per cent of those receiving monotherapy and 14.6 per cent of those receiving combination therapy had controlled hypertension (<130/80 mmHg) at one year. Among the newly diagnosed individuals, 16.3 per cent of those receiving monotherapy and 11.4 per cent of those receiving combination therapy reported controlled hypertension at one year. Similarly, in those with pre-existing hypertension, 17.6 per cent receiving monotherapy or combination therapy reported controlled hypertension at one year. The status of hypertension control in the individuals based on diagnosis and type of therapy received is presented in Table IV.
Treatment outcomes: At all the time points studied (three months, six months and one year), there was a significant decrease in both SBP and DBP in the total study population from the baseline (P<0.001 for each).
Changes in blood pressure based on diagnosis and type of therapy: At all the time points studied (three months, six months and one year), there was a significant decrease in both SBP and DBP in the pre-existing and newly diagnosed group of participants (P<0.001 for each; Supplementary Table II).
| Categorization | BP parameter | Type of hypertension | Follow up | Mean change | P |
|---|---|---|---|---|---|
| Analysis by diagnosis | SBP | Pre-existing (n=1032) | BL | - | - |
| 3 months | −5.49 | <0.001 | |||
| 6 months | −8.52 | <0.001 | |||
| 1 yr | −10.54 | <0.001 | |||
| Newly diagnosed (n=955) | BL | - | - | ||
| 3 months | −9.06 | <0.001 | |||
| 6 months | −13.44 | <0.001 | |||
| 1 yr | −17.19 | <0.001 | |||
| DBP | Pre-existing (n=1032) | BL | - | - | |
| 3 months | −1.03 | 0.00498 | |||
| 6 months | −2.7 | <0.001 | |||
| 1 yr | −2.27 | 0.004 | |||
| Newly diagnosed (n=955) | BL | - | - | ||
| 3 months | −7.49 | <0.001 | |||
| 6 months | −11.99 | <0.001 | |||
| 1 yr | −12.19 | <0.001 | |||
| Analysis by therapy | SBP | Monotherapy (n=857) | BL | - | - |
| 3 months | −6.07 | <0.001 | |||
| 6 months | −9.08 | <0.001 | |||
| 1 yr | −11.56 | <0.001 | |||
| Combination therapy (n=1130) | BL | - | - | ||
| 3 months | −8.07 | <0.001 | |||
| 6 months | −12.26 | <0.001 | |||
| 1 yr | −15.39 | <0.001 | |||
| DBP | Monotherapy (n=857) | BL | - | - | |
| 3 months | −3.38 | <0.001 | |||
| 6 months | −6.23 | <0.001 | |||
| 1 yr | −6.18 | <0.001 | |||
| Combination therapy (n=1130) | BL | - | - | ||
| 3 months | −4.7 | <0.001 | |||
| 6 months | −7.86 | <0.001 | |||
| 1 yr | −7.68 | <0.001 |
BP, blood pressure; SBP, systolic BP; DBP, diastolic BP; BL, baseline
While analysing the BP changes based on the type of therapy, it was observed that combination therapy was associated with higher mean decreases in SBP, and a marginally higher mean decrease in DBP at all time points studied, as compared to monotherapy (Supplementary Table II).
The effect of treatment on SBP denoted by a difference in least squares mean between monotherapy and combination therapy was found to be statistically significant at three month, six month and one year visits (considering P<0.017 as significant). However, for DBP, the effect of treatment denoted by a difference in least squares mean between combination therapy and monotherapy was found to be significant at one year follow up only (considering P<0.025 as; Supplementary Tables III).
| Follow up | Statistics | SBP | DBP | ||
|---|---|---|---|---|---|
| Monotherapy (n=857) | Combination therapy (n=1130) | Monotherapy (n=857) | Combination therapy (n=1130) | ||
| 3 month | Least square mean (SE) | −8.35 (0.43) | −6.34 (0.38)*** | −3.98 (0.24) | −4.21 (0.21) |
| Treatment effect (monotherapy-combination therapy) | 2.01 | −0.23 | |||
| 95% CI for treatment effect | 0.88-3.15 | −0.85-0.39 | |||
| 6 month | Least square mean (SE) | −11.9 (0.41) | −10.15 (0.36)*** | −6.83 (0.22) | −7.37 (0.19) |
| Treatment effect (monotherapy-combination therapy) | 1.75 | −0.54 | |||
| 95% CI for treatment effect | 0.68-2.8 | −1.11-0.03 | |||
| 1 yr | Least square mean (SE) | −14.58 (0.35) | −12.89 (0.31)*** | −6.79 (0.23) | −7.82 (0.2)*** |
| Treatment effect (monotherapy-combination therapy) | 1.68 | −1.04 | |||
| 95% CI for treatment effect | 0.76-2.6 | −1.62-−0.45 | |||
***P<0.001. P based on the MMRM model with change in SBP or DBP as dependent variable and type of therapy at enrolment, follow up, the interaction of type of therapy at enrolment and follow up, age, sex and baseline SBP or DBP as fixed effects. MMRM, mixed model repeated measure; SE, standard error; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure
On comparing changes in blood pressure between telmisartan (n=180) and amlodipine (n=106) monotherapy in the newly diagnosed hypertensive participants, a significant decrease in both SBP and DBP was noted for both the medications at the three month, six month and one year time points (P<0.001 for each medication at all time points). As compared to telmisartan, the mean decrease in DBP from the baseline was significantly higher for amlodipine (P<0.01) at the one year follow up (Figure and Supplementary Table IV).
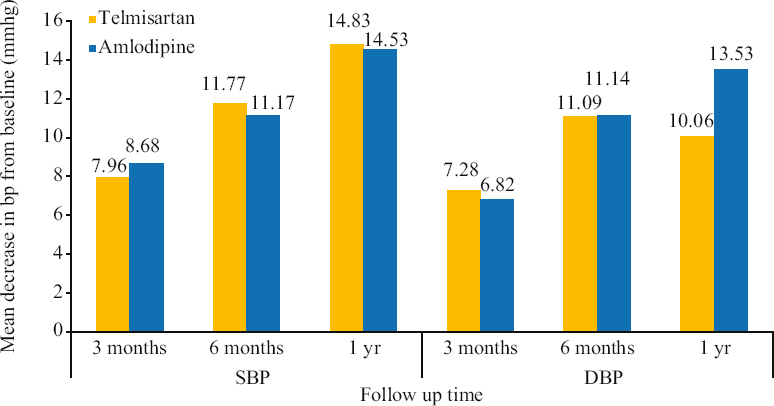
- Changes in SBP and DBP in newly diagnosed patients with telmisartan vs. amlodipine monotherapy. BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure
| BP at different time points (mean±SD) | |||||||
|---|---|---|---|---|---|---|---|
| Medication | SBP | ||||||
| Baseline | 3 months | Mean change | 6 months | Mean change | 1 yr | Mean change | |
| Telmisartan | 146.74±13.11 | 138.79±10.48 | −7.96*** | 134.97±9.06 | −11.77*** | 131.92±7.10 | −14.83*** |
| Amlodipine | 146.83±13.02 | 138.15±14.60 | −8.68*** | 135.66±12.76 | −11.17*** | 132.30±10.39 | −14.53*** |
| DBP | |||||||
| Telmisartan | 85.62±9.66 | 78.33±6.33 | −7.28*** | 74.52±5.10 | −11.09*** | 75.56±6.61 | −10.06*** |
| Amlodipine | 87.17±7.57 | 80.35±6.1 | −6.82*** | 76.03±6.12 | −11.14*** | 73.64±7.65 | −13.53***δδ |
P***<0.001 for mean change in BP at baseline vs. 3 months, 6 months and 1 yr follow up for SBP and DBP groups, respectively. No significant change was observed for the two drugs in SBP group (inter group analysis). δδ<0.01 for telmisartan vs. amlodipine at 1 yr follow up in DBP group
Proportion of participants with controlled hypertension based on the type of therapy: Among the newly diagnosed population, the proportion of participants with controlled BP increased by 30.34 per cent in the monotherapy group and 38.49 per cent in the combination therapy group; this was in comparison with the three month follow up data. For the pre-existing hypertensive population, these values were 32.81 and 35.94 per cent, respectively (from baseline value; Table IV).
The results of the logistic regression analysis of the factors that influenced uncontrolled hypertension are enumerated in Supplementary Table V. Female gender was associated with higher odds of uncontrolled hypertension at all time points. Similarly, participants aged ≥65 yr had higher odds of uncontrolled hypertension as compared to those aged 50-64 yr. The absence of comorbidities, age between 18-49 yr, and the use of combination therapy were found to be associated with lesser odds of uncontrolled hypertension at all time points.
| Characteristics | HTN (130/80) | HTN (140/90) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | One year | Baseline | 3 months | 6 months | One year | |
| Gender (female vs. male) | 1.04 (0.76-1.42) | 0.93 (0.67-1.29) | 0.85 (0.66-1.09) | 0.98 (0.77-1.26) | 1.36 (1.12-1.65) | 1.07 (0.89-1.29) | 1.26 (1.04-1.53) | 1.29 (1.04-1.61) |
| Age category (≥65 vs. 50-64 yr) | 1.18 (0.82-1.69) | 1.38 (0.96-1.99) | 1.1 (0.81-1.5) | 1.14 (0.83-1.57) | 1.1 (0.86-1.42) | 0.94 (0.74-1.20) | 1.07 (0.83-1.39) | 1.16 (0.86-1.56) |
| Age category (18-49 vs. 50-64 yr) | 0.54 (0.35-0.82) | 0.65 (0.42-1) | 0.96 (0.71-1.29) | 1.28 (0.96-1.71) | 0.79 (0.63-0.99) | 0.82 (0.67-1.01) | 0.72 (0.58-0.9) | 0.84 (0.66-1.07) |
| Comorbidity (no vs. yes) | 0.26 (0.18-0.37) | 0.25 (0.17-0.36) | 0.41 (0.32-0.53) | 0.43 (0.33-0.56) | 0.47 (0.39-0.58) | 0.49 (0.40-0.59) | 0.37 (0.30-0.46) | 0.66 (0.53-0.83) |
| Type of therapy at enrolment (combination therapy vs. monotherapy) | 0.65 (0.47-0.89) | 0.95 (0.69-1.31) | 0.66 (0.52-0.85) | 0.81 (0.63-1.03) | 0.53 (0.43-0.64) | 0.5 (0.41-0.6) | 0.47 (0.38-0.57) | 0.52 (0.42-0.65) |
HTN, hypertension
Subgroup analysis for hypertensive participants with diabetes mellitus: The number of participants with diabetes mellitus was 632. Consistent with the overall population, a higher proportion of participants with diabetes were on combination therapy than monotherapy (55.4 vs. 44.6%). Equal proportions of participants with diabetes were on amlodipine and telmisartan monotherapy (29.4%). For combination therapy, amlodipine + telmisartan was most preferred (16%); this was similar to the overall population of hypertensive participants (Supplementary Table VI).
| List of medication | Count | Per cent |
|---|---|---|
| Monotherapy | 282 | 44.6 |
| Amlodipine | 83 | 29.4 |
| Telmisartan | 83 | 29.4 |
| Bisoprolol | 17 | 6 |
| Metoprolol | 17 | 6 |
| Ramipril | 16 | 5.7 |
| Enalapril | 15 | 5.3 |
| Cilnidipine | 10 | 3.5 |
| Atenolol | 6 | 2.1 |
| Nebivolol | 6 | 2.1 |
| Losartan | 5 | 1.8 |
| Torsemide | 5 | 1.8 |
| Azilsartan | 4 | 1.4 |
| Olmesartan | 4 | 1.4 |
| Perindopril | 4 | 1.4 |
| Carvedilol | 3 | 1.1 |
| Diltiazem | 1 | 0.4 |
| Furosemide | 1 | 0.4 |
| Nifedipine | 1 | 0.4 |
| Prazosin | 1 | 0.4 |
| Combination therapy | 350 | 55.4 |
| Amlodipine+telmisartan | 56 | 16 |
| Amlodipine+indapamide+perindopril | 55 | 15.7 |
| Metoprolol+telmisartan | 31 | 8.9 |
| Amlodipine+bisoprolol+indapamide +perindopril | 24 | 6.9 |
| Metoprolol+ramipril | 12 | 3.4 |
| Bisoprolol+losartan | 11 | 3.1 |
| Amlodipine+metoprolol | 10 | 2.9 |
| Amlodipine+ramipril | 8 | 2.3 |
| Hydrochlorothiazide+telmisartan | 8 | 2.3 |
| Chlorthalidone+telmisartan | 5 | 1.4 |
At one year, the proportion of participants with controlled BP (<140/90) increased from 36.62 per cent at baseline (comprising pre-existing hypertension patients) to 82.96 per cent (an increase of 46.34%). In participants with diabetes on monotherapy (n=279), the increase was 45.16 per cent, and in those on combination therapy (n=349), the increase was 47.27 per cent (
Treatment modifications: The number of individuals requiring treatment modification in the overall population was the highest at six month follow up (overall 3.2, 1.05% for monotherapy, and 4.78% for combination therapy) and lowest (0.3%) at one year follow up (0.23% for monotherapy and 0.35% for combination therapy). As compared to monotherapy, higher numbers of treatment modifications were observed in participants receiving combination therapy at all follow ups. A similar trend was observed in newly diagnosed individuals (Table V).
| Overall population | |||
|---|---|---|---|
| Follow up | Monotherapy (n=857), n (%) | Combination therapy (n=1130), n (%) | Overall (n=1987), n (%) |
| Baseline | |||
| 3 months | 12 (1.4) | 31 (2.74) | 43 (2.2) |
| 6 months | 9 (1.05) | 54 (4.78) | 63 (3.2) |
| 1 yr | 2 (0.23) | 4 (0.35) | 6 (0.3) |
| Newly diagnosed individuals | |||
| Follow up | Monotherapy (n=412), n (%) | Combination therapy (n=543), n (%) | Overall (n=955), n (%) |
| Baseline | |||
| 3 months | 1 (0.24) | 3 (0.55) | 4 (0.4) |
| 6 months | 2 (0.49) | 20 (3.68) | 22 (2.3) |
| 1 yr | 1 (0.24) | 2 (0.37) | 3 (0.3) |
Adverse events: Adverse events were reported by 138 individuals (6.9%). The total number of events reported was 150 (7.5%); of these, 135 events (90% of total events) were adverse events and 15 (10% of total events) were serious adverse events. Of the total 150 adverse events reported, the most common events were fever (14.7%), headache (12%), and weakness (6.7%) (Table VI). Among the total adverse events, 18, 10, and 11.3 per cent of events were reported by participants who received telmisartan, amlodipine, and a combination of amlodipine + telmisartan, respectively (data not shown).
| Events | Count | Per cent of total population (n=2000) | Per cent of adverse events (n=150) |
|---|---|---|---|
| Total number of events reported | 150 | 7.5 | |
| Adverse events | 135 | 6.75 | 90 |
| Serious adverse events | 15 | 0.75 | 10 |
| List of events | |||
| Fever | 22 | 1.1 | 14.7 |
| Headache | 18 | 0.9 | 12 |
| Weakness | 10 | 0.5 | 6.7 |
| Pain in abdomen | 6 | 0.3 | 4 |
| Cough and sneezing | 5 | 0.25 | 3.3 |
| Dry cough | 5 | 0.25 | 3.3 |
| Pain in joints | 5 | 0.25 | 3.3 |
| Cough | 4 | 0.2 | 2.7 |
| Giddiness | 4 | 0.2 | 2.7 |
| Atypical chest pain | 3 | 0.15 | 2 |
| Death | 3 | 0.15 | 2 |
| Heart attack | 3 | 0.15 | 2 |
| Loose motion | 3 | 0.15 | 2 |
Quality of life: In the overall population, emotional role functioning, emotional wellbeing, pain, general health, and health change parameters were significantly improved from baseline at the three months follow up. Significant improvements from baseline were noted in all parameters at the six months and one year follow up visits (P<0.001, respectively), except in physical functioning (P=0.096) at six months follow up (Supplementary Table VII).
| Scales | BL | 3 months | Mean change from BL | P | 6 months | Mean change from BL | P | 1 yr | Mean change from BL | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Physical functioning | 52.20 | 53.00 | 0.8 | 0.067 | 51.1 | −1.1 | 0.096 | 53.90 | 1.8 | 0.01 |
| Role functioning/physical | 47.30 | 48.60 | 1.3 | 0.108 | 52.6 | 5.3 | <0.001 | 64.10 | 16.8 | <0.001 |
| Role functioning/emotional | 46.90 | 52.30 | 5.4 | <0.001 | 63.2 | 28.8 | <0.001 | 72.70 | 25.8 | <0.001 |
| Energy/fatigue | 50.40 | 51.10 | 0.7 | 0.014 | 51.7 | 1.3 | 0.0001 | 56.50 | 6.1 | <0.001 |
| Emotional well-being | 59.30 | 60.70 | 1.4 | <0.001 | 61.3 | 2 | <0.001 | 67.70 | 8.4 | <0.001 |
| Social functioning | 60.10 | 60.90 | 0.9 | 0.022 | 63.2 | 3.1 | <0.001 | 72.50 | 12.5 | <0.001 |
| Pain | 64.40 | 70.10 | 5.7 | <0.001 | 68.7 | 4.3 | <0.001 | 74.20 | 9.8 | <0.001 |
| General health | 47.00 | 50.70 | 3.7 | <0.001 | 54.9 | 7.9 | <0.001 | 54.90 | 7.9 | <0.001 |
| Health change | 42.20 | 49.50 | 7.3 | <0.001 | 53.1 | 10.9 | <0.001 | 53.80 | 11.6 | <0.001 |
BL, baseline
Discussion
Despite an abundance of information and projected estimates for the burden of hypertension in India, there is currently no evidence of a prospective cohort study that describes the distribution and treatment patterns of hypertension in patients with newly diagnosed and pre-existing hypertension with or without comorbid conditions over a follow up duration of two years. To fill this knowledge gap, a real-world prospective study on hypertension in India, involving 2000 adults from five centres across the country, was planned and conducted. In contrast to the most frequently published cross-sectional studies, this study included individuals with both new and old diagnoses of hypertension, allowing for the observation of changes in treatment trends. In addition, the study’s design supported the prospective evaluation of treatment outcomes in terms of reductions in BP, control of hypertension, and QoL.
This study, which is an interim analysis of a real-world registry, reflects the clinical practices for hypertension management in India, along with the extent of hypertension control. The prevalence of hypertension was found to be higher in men than in women. The prevalence also increased with age, with the highest prevalence being observed in the population aged above 50 yr. A higher preference for combination therapy compared to monotherapy was observed. In the case of combination therapies, the use of FDCs was high. While the CCB, amlodipine and the ARB telmisartan were the monotherapies of choice, the combination of amlodipine and telmisartan was most preferred among the combination therapies. At one year follow up, the proportion of participants with controlled hypertension increased by more than 30 per cent in both, newly diagnosed as well as pre-existing hypertension groups. The improvement in the QoL of participants indicated the safety, tolerability, and effectiveness of the prescribed pharmacological treatments. Both, the ACC/AHA and ESC guidelines are followed for hypertension diagnosis across India. However, the use of ACC/AHA guidelines was relatively higher. Majority of the population (>70%) was diagnosed with stage 2 hypertension (≥140 mmHg or ≥90 mmHg).
In general, the prevalence of hypertension increases with age, from 13.7 per cent in the 30s age group to 64 per cent in the 60s age group10. An increasing prevalence of hypertension with age has been observed in Indian studies4. A similar trend was also observed in this study. Several comorbidities, including coronary artery disease, heart failure, chronic kidney disease, chronic obstructive pulmonary disease, and diabetes, are associated with hypertension, which affects treatment strategies11. In this study population, the prevalence of comorbidities was high: nearly one third of the study population had diabetes and one-sixth had dyslipidaemia. Previous studies have also reported a high prevalence of individuals suffering from both diabetes and hypertension in the Indian population12-14. Similarly, the prevalence of comorbid dyslipidaemia with hypertension has been reported to be higher in the Indian population15,16.
The hypertension control rates observed in this study were higher than those reported by previous studies from India4,17. In a recently published report of the India Hypertension Control Effort initiative (IHCI), it was found that 11,274 (51%) of the 21,895 adult hypertensive individuals who were registered from 24 sites across four Indian States returned for a follow up visit18. Among the individuals returning for follow up, 26.3 per cent had well-controlled BP at registration, and 59.8 per cent had well-controlled BP at the follow up18. In contrast to these findings, our study found that at a one year follow up, 83.7 per cent of those receiving monotherapy and 73.2 per cent of the participants receiving combination therapy had well-controlled hypertension (<140/90 mmHg). This study also found that BP was well-controlled in 99.35 per cent (1987 out of 2000) of those returning for follow up. This can be attributed to increased awareness of and adherence to anti-hypertensive medications, as well as a higher rate of patients returning for follow up visits. In the present study, the pharmacologic management of hypertension was as per the recent Indian Guidelines on Hypertension (IGH)10 and the International Society of Hypertension Global Hypertension Practice Guidelines11, where the use of ARB/ACEI, or BB, and CCB combinations is recommended for hypertension management. As per the IGH guidelines, ACEIs/ARBs in combination with CCBs should be considered as a first line combination10; this was found to be the most common combination therapy used in this setting. Both monotherapy and combination therapy were associated with significant reductions in both SBP and DBP in the present investigation. Even in the subpopulation of hypertension patients with comorbid diabetes, the use of combination therapy was higher than the use of monotherapy (55.4 vs. 44.6%). The increase in the proportion (46.34 vs. 51.34%) of patients with controlled hypertension in this subgroup, however, was lower than that of the overall population. Notably, female gender and age ≥65 yr were associated with higher odds of uncontrolled hypertension, while the absence of comorbidities, age between 18-49 yr and combination therapy were associated with lower odds of uncontrolled hypertension.
The findings of this study are consistent with the guideline recommendations of the ACC/AHA as per which the antihypertensive drug therapy is to be initiated with a single drug in adults with stage 1 hypertension and two first-line anti-hypertensives of different classes, administered either as separate agents or in an FDC in adults with stage 2 hypertension. Furthermore, in adults with hypertension, the use of combination pills and once-daily dosing is recommended to improve therapeutic adherence7. According to the ESC guidelines, a combination treatment with two drugs is recommended for most hypertensive patients as an initial therapy. Preferred combinations should be comprised of an ACE inhibitor or an ARB with a CCB or diuretic8. According to a recent systematic review19, major barriers to medication adherence experienced by Indian hypertensive patients were lack of awareness of the disease and the complications of non-adherence, forgetfulness, and lack of family support. Barriers relating to the health system included acceptability, affordability, and accessibility19.
Telmisartan and amlodipine are the most frequently prescribed antihypertensive medications in India, possibly due to their beneficial properties20-26. In our study, the use of telmisartan and amlodipine monotherapy was similar in the subpopulation with diabetes (29.4% each); however, in the overall population, the use of amlodipine was relatively higher than that of telmisartan (35.2 vs. 31.6%).
In a study on Indian hypertensive patients, telmisartan was comparable to amlodipine in effectiveness26. However, telmisartan had beneficial effects on other metabolic parameters; these included its effects on significantly lowering blood sugar levels and altering lipid profiles. Amlodipine, however, did not affect these metabolic parameters24. Moreover, studies have shown that combination therapy with amlodipine/telmisartan effectively lowers BP in individuals with uncontrolled hypertension who had previously been treated with ARB monotherapy27,28. A study in Indian patients also reported that a low dose amlodipine/telmisartan combination caused greater BP reduction than high dose monotherapy of each medication, thereby establishing combination therapy as a better therapeutic option for hypertension management compared to monotherapies29. Our findings also indicate that the antihypertensive effects of telmisartan and amlodipine monotherapies as well as telmisartan + amlodipine combination therapy are significant.
The number of adverse events reported in the present study was low (135 events or 7% of the overall patient population) and serious adverse events were also uncommon (15 events amounting to 0.75% of the overall population).
These findings indicate that in the high renin hypertensive Indian population with a high rate of comorbidities, such as diabetes and dyslipidaemia, the use of ARBs is tolerable and can be effectively coupled with CCBs for combination therapy. Thus, the increased use of combination therapy in accordance with guideline recommendations, selection of effective treatments (including telmisartan, amlodipine, and telmisartan + amlodipine) in a majority of the patients, regular follow ups, and active patient counselling contributed to an improved and a more sustained response among the study participants over the course of one year.
This prospective multicentre registry provides real world evidence on the clinical practices for hypertension management in essential hypertensives from five premium healthcare institutes across the country. Furthermore this study also had a long follow up period of two years. However, due to the observational nature of this study, its findings may not be representative of the diverse Indian population as a whole. In addition, this study did not explore the influence of different factors that affect BP, such as blood components and serum chemistry. Furthermore, while the baseline BP readings were recorded at the hospital, subsequent readings were recorded either at the clinic/hospital or home due to the COVID-19 pandemic. Therefore, there may be variations in the readings obtained. In addition, this study was largely performed on an urban population, which is considerably more literate and aware of the consequences of hypertension. The findings may not reflect the trends in rural population and may therefore, not be applicable to the entire population of the country. However, the data on current treatment trends, patient outcomes, and patient characteristics yielded by this prospective study may help new and experienced healthcare practitioners gauge their practices in managing hypertension and make informed decisions.
Overall this study highlights the treatment patterns followed in some premium healthcare institutes in India. This is an interim report of the ongoing study, which showed effective hypertension control rates with significant reductions in SBP and DBP at three and six months and at one year. All the patients involved in the study received pharmacologic treatment as per the national and global guidelines. For combination therapy, the use of CCB + ARB was preferred. Telmisartan, an ARB, was included in the majority of the combinations. The QoL also improved with these standard pharmacological interventions.
Financial support and sponsorship
This study has been funded by Glenmark Pharmaceuticals Limited.
Conflicts of interest
The study was sponsored by Glenmark Pharmaceuticals Ltd. across five sites in India. Glenmark Pharmaceuticals Ltd. claims no authorship in the publication and is available to clarify in the event of any disputes in this context.The part of the study was presented in 73rd Annual Conference of Cardiological Society of India (CSI), 2021 and the abstract was published in the Indian Heart Journal 2021; 73: S1-53 (https://www.sciencedirect.com/science/article/pii/S0019483221003515).
Supplementary Figure
Supplementary Figure Changes in controlled BP in diabetics treated telmisartan vs. amilodipine with monotherapy or combination of both.Acknowledgment:
BioQuest Solutions Pvt Ltd for data analysis and providing editorial assistance.
References
- Prevalence of hypertension among Indian adults:Results from the great India blood pressure survey. Indian Heart J. 2019;71:309-13.
- [Google Scholar]
- World hypertension day:Contemporary issues faced in India. Indian J Med Res. 2019;149:567-70.
- [Google Scholar]
- Hypertension in India:A systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J Hypertens. 2014;32:1170-7.
- [Google Scholar]
- Determinants of hypertension among urban adult population in Kancheepuram district of Tamil Nadu determinants of hypertension among urban adult population in Kancheepuram district of Tamil Nadu. IJCMPH. 2017;4:1552-7.
- [Google Scholar]
- Prevalence and associated risk factors of hypertension:A cross-sectional study in Urban Varanasi. Int J Hypertens. 2017;2017:5491838.
- [Google Scholar]
- 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults:Executive summary:A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71:1269-324.
- [Google Scholar]
- 2018 ESC/ESH Guidelines for the management of arterial hypertension:The task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) J Hypertens. 2018;36:1953-2041.
- [Google Scholar]
- The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-83.
- [Google Scholar]
- 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-57.
- [Google Scholar]
- Structural equation modeling to identify the risk factors of diabetes in the adult population of North India. Trop Med Health. 2018;46:23.
- [Google Scholar]
- Diabetes and hypertension in India:A nationally representative study of 1.3 million adults. JAMA Intern Med. 2018;178:363-72.
- [Google Scholar]
- Prevalence of hypertension and diabetes morbidity among adults in a few urban slums of Bangalore city, determinants of its risk factors and opportunities for control –A cross-sectional study. J Family Med Prim Care. 2020;9:3264-71.
- [Google Scholar]
- Dyslipidemia in hypertension- risk and prevention approach in rural population in India. J Hypertens. 2016;34:e272.
- [Google Scholar]
- Study of prevalence of dyslipidemia in newly diagnosed essential hypertension. IJCMSR. 2018;3:D95-8.
- [Google Scholar]
- Hypertension screening, awareness, treatment, and control in India:A nationally representative cross-sectional study among individuals aged 15 to 49 years. PLoS Med. 2019;16:e1002801.
- [Google Scholar]
- India Hypertension Control Initiative-Hypertension treatment and blood pressure control in a cohort in 24 sentinel site clinics. J Clin Hypertens (Greenwich). 2021;23:720-9.
- [Google Scholar]
- Patient and provider's perspective on barriers and facilitators for medication adherence among adult patients with cardiovascular diseases and diabetes mellitus in India:A qualitative evidence synthesis. BMJ Open. 2022;12:e055226.
- [Google Scholar]
- Efficacy and safety of triple therapy with telmisartan, amlodipine, and rosuvastatin in patients with dyslipidemia and hypertension:The Jeil telmisartan, amlodipine, and rosuvastatin randomized clinical trial. Clin Ther. 2019;41:233-48.e9.
- [Google Scholar]
- Efficacy and tolerability of olmesartan, telmisartan, and losartan in patients of stage I hypertension:A randomized, open-label study. J Pharmacol Pharmacother. 2017;8:106-11.
- [Google Scholar]
- Assessment of efficacy, safety and tolerability of fixed dose combination of telmisartan 40 mg and hydrochlorothiazide 12.5 mg in adult Indian patients with mild to moderate hypertension. J Indian Med Assoc. 2004;102:525-7.
- [Google Scholar]
- The antihypertensive efficacy of chlorthalidone and telmisartan in Indian hypertensive patients who were uncontrolled with hydrochlorothiazide and telmisartan combination –A prospective and an open label study. J Clin Diagn Res. 2013;7:687-90.
- [Google Scholar]
- Effect of telmisartan on blood pressure in patients of type 2 diabetes with or without complications. Perspect Clin Res. 2018;9:155-60.
- [Google Scholar]
- Management of hypertension:Insights into prescribing behavior with focus on angiotensin receptor blockers. J Pract Cardiovasc Sci. 2017;3:22-7.
- [Google Scholar]
- Comparative study of telmisartan and amlodipine to assess the effect on blood pressure, lipid profile and blood glucose level in Indian hypertensive patients. Int J Med Res Rev. 2016;4:1693-701.
- [Google Scholar]
- Comparison of telmisartan/amlodipine and telmisartan/hydrochlorothiazide in the treatment of Japanese patients with uncontrolled hypertension:The TAT-Kobe study. Blood Press Monit. 2016;21:171-7.
- [Google Scholar]
- Results of treatment with telmisartan-amlodipine in hypertensive patients. J Clin Hypertens (Greenwich). 2009;11:207-13.
- [Google Scholar]
- Comparative study of high dose mono-therapy of amlodipine or telmisartan, and their low dose combination in mild to moderate hypertension. J Clin Diagn Res. 2014;8:HC08-11.
- [Google Scholar]






