Translate this page into:
Eco-epidemiology triad to explain infectious diseases
*For correspondence: tjacobjohn@yahoo.co.in
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Every medical student learns the ‘Epidemiology Triad’ as Agent, Host and Environment. The three lend themselves to draw the Epidemiology Triangle (ET), with apices representing Agent, Host and Environment. What keeps agent and host apart avoiding infection, or what brings them together for infection, is the environment.
Epidemiology treatises mention that ET is traditional teaching, without identifying who had designed it. One view is that Rudolf Virchow created it. However, he had taught that microbes did not cause pathology, but they invaded damaged tissue. He wanted to explore why the environment (diseased tissue) was damaged and gave a nidus for microbes to flourish1,2. Another view is that Claude Bernard was instrumental, for he had taught that ‘the agent is nothing, but the terrain is everything’. However, he also did not believe in Louis Pasteur’s teaching that microbes caused diseases.
Lourense Baas-Becking summarised Marcus Beijerinck’s concept that the environment was very important in infectious diseases, saying: ‘everything is everywhere, the environment selects’3. All microbes are not everywhere and the environment does not select, but merely allows. We will probably never know who constructed ET.
Francl4 wrote: ‘The Disease Triangle is one of the first concepts encountered by college students in an introductory plant pathology course’. He credited Stevens RB as its probable author – Stevens had included disease triangle in a book he wrote on plant pathology in 1960. While the interaction amongst plant, pathogen and environment was first explicitly analysed by Gaumann in the 1950s4,5, the disease triangle concept was formalised by George McNew in the 1960s6,7. Scholthof wrote, in 2007, that the disease triangle concept, originally devised to interpret plant disease outcomes was later adapted to public health7.
Wade Hampton Frost8 described the ‘Epidemic Triad’ in 1976, based on his first Cutter lecture on February 3, 1928. Morabia9 mentioned this as the first instance that it was explicitly laid out, although the concept of ET was already in the air in the 19th century. Frost explained epidemic as a disturbance to disease prevalence equilibrium maintained by microorganisms, human hosts and environment. In short, the three factors are necessary to start an infectious disease (disease triangle), for maintaining a stable state of prevalence (epidemiology triad) or for starting an epidemic (Epidemic triad).
The term ‘environment’ was used as a basket to put any and all factors and determinants of infectious diseases, including socioeconomic and demographic. Some pathogens are directly transmitted human-to-human. Here, the infected host is actually ‘environment’ for the uninfected would-be host in the vicinity. In such instances, host and environment blur into each other; this anomaly needs clarification.
Ecology describes and explains the inter-relationships of organisms to one another and to their environment – location and surroundings. Therefore, to fully understand the environment of microbial agents and to identify their sources, their ecology has to be understood. Ecology is missing in the traditional ET. Microbial transmission is another crucial factor for the microbe to reach the host. Environment in ET does not explicitly draw attention to these two elements – ecology and transmission channels – that are essential for microbes to reach the host and establish infection. In other words, ET is incomplete as explanation of the dynamics of infections causing diseases.
Eco-epidemiological triad and triangle
Three distinct systems are essential to explain any and all infectious diseases – systems of microbial amplification, microbial transmission and host-microbe interactions10. These are proposed as the new eco-epidemiological triad – forming the eco-epidemiology triangle (EET) – providing a comprehensive model of infectious diseases (Fig. 1). EET helps us answer the three basic questions about infectious diseases – where do pathogens come from, how do they reach and infect human hosts and how pathogens and hosts exhibit pathology and disease.
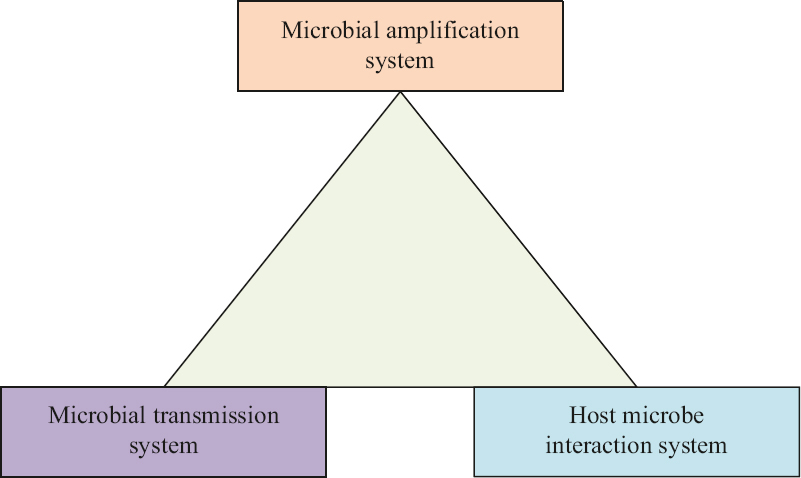
- New eco-epidemiology triangle.
Microbial amplification system
Every microbe has its own unique amplification system. Tuberculosis (TB) bacilli get amplified to the millions and trillions in the lung of the pulmonary TB patient. Malarial parasites and dengue virus get amplified in human host and in Anophelis and Aedes mosquitos, respectively. Shigella dysenteriae get amplified in human colon, but not in the ex vivo environment. Salmonella typhi gets amplified in human intestines, but it can also amplify in stored food containing dairy products.
Microbes of zoonosis amplify in their vertebrate hosts – and shed into the environment. Nipah virus gets amplified in Asian fruit bats (Pteropodidae)11; Marburg virus is amplified in African fruit bats (Rousettus)12. A few zoonotic agents are additionally vector-amplified – in blood-sucking arthropods, namely mosquitoes, ticks, mites and fleas. Japanese encephalitis virus gets amplified sequentially in Culex mosquitos and pigs; viremia in pigs feeds more mosquitoes continuing the cycle. Crimean–Congo haemorrhagic fever virus is amplified in sheep and Hyalomma ticks13. Orientia tsutsugamushi causing scrub typhus is amplified in rodents and larval mites (chiggers)14. Yersinia pestis, the cause of plague is amplified in rodents and their fleas (Xenopsylla cheopis)15.
Saprophytic microbial pathogens – fungi, Burkholderia pseudomallei, Acanthamoeba and Naegleria amplify in their own ecological niches in the environment of earth and water; infection caused by such microbes is called sapronoses16. Vibrio cholerae has two amplification systems – saprophytic (in copepods in water bodies) and anthroponotic (in human intestines). No two agents have truly identical amplification systems – even if apparently similar, subtle but critical differences distinguish the amplification system of individual pathogens.
Microbial transmission system
From where they amplify, microbial pathogens have to reach the host to infect and cause disease. There are only very limited entry doorways for the pathogens to infect the human host. For agents present in air, inhalation is an efficient and unavoidable method of inoculation – such transmission, infection and consequent disease are generally highly contagious.
How do pathogens reach the air? Those that are amplified in the upper respiratory or pharyngeal mucosa, such as influenza, measles, polio, varicella viruses, pneumococci and Haemophilus influenzae are shed in the oral, pharyngeal and nasal fluids that are inevitably expelled while speaking, coughing or sneezing. Pathogens that amplify in the lungs are brought up to the oropharynx by the tracheal mucociliary escalator and thereafter shed via oral fluids or expelled while coughing, particularly in the sputum.
Pulmonary TB is infectious even before cough becomes a symptom. Once the cough begins, the sputum will contain TB bacilli that can survive drying and get carried in the wind. To minimise the risk of respiratory route transmission, therefore, cough and sneeze etiquette – by the way of covering mouth and nose with napkin – and avoidance of spitting in public places, have become the normal behaviour in many countries thereby controlling transmission of TB bacilli.
The severe acute respiratory syndrome (SARS) pandemic of 2002 could be interrupted in 2003 because its transmission system gave public health experts a simple clue for breaking transmission17. Since the upper respiratory airways do not have virus receptors, transmission was possible only after virus was amplified in the lower respiratory airways – but fever set in before the host became infectious to others. Hence, fever screening of travellers from countries known to have infection, and testing and quarantining of potentially infected people were sufficient to break all the chains of transmission.
SARS-CoV-2 amplifies efficiently in upper airways and is therefore highly transmissible even before symptoms set in. As for children, they have very few, if any, virus receptors in the upper airways and have lower viral loads; hence they tend to be inefficient virus transmitters18,19. Face masks became widely recommended to minimise the chances of inhaling droplets or aerosol laden with virus.
Ingestion of microbes is another method of transmission. Where sanitation and hygiene are sub-optimal, bacteria, viruses and protozoa that are amplified in guts of humans and animals and shed via excretion, may find their way to water or food that is later consumed by humans and thus the chain of transmission is completed. An inclusive term to capture all these is faecal-oral transmission.
The global polio eradication programme faces a major challenge currently, namely vaccine-derived wild-like variants of poliovirus causing outbreaks in about twenty countries each year20. Poliovirus amplification occurs in two anatomical sites of the infected person – pharynx and small intestines. Pharyngeal shedding results in respiratory transmission (droplets and aerosol); all evidences pointed to respiratory route21. Assuming faecal-oral transmission, eradication was attempted with the Sabin live oral polio vaccine, resulting in the emergence of mutant vaccine variants with neurovirulence and high transmission efficiency21,22. Clarity on EET of wild polioviruses would have saved the eradication programme from its current imbroglio22.
Mucosa-to-mucosa transmission is another route of pathogen transmission – this way many sexually transmitted agents infect human hosts. Conjunctiva is an open-access mucosal surface, vulnerable to some air-borne microbes (enteroviruses and adenoviruses of conjunctivitis) as well as those transmitted by fomites and flies acting as mechanical vectors such as Chlamydia trachomatis23.
Skin is a barrier to the transmission of many microbes – but spirochetes like leptospira can penetrate the skin, especially when softened by moisture, and enter human epidermal and subcutaneous tissues. Broken skin gives access to many microbes. Brucella in cattle urine may enter the human host through cut or wound on the skin; it may also be inhaled as aerosol or ingested through raw milk. The rabies virus is inoculated by animal bites. Rickettsia in louse faeces is inoculated in the bite site by scratching the skin because of itching.
Skin piercing tools, particularly syringe/needle act as the vehicles of transmission if contaminated with blood/body fluids of patients with viraemia – viruses of hepatitis B, C and AIDS are examples. Penetrating wound contaminated with spores of Clostridium tetani is how infection is introduced – tetanus toxin is produced, causing the disease.
Blood-sucking arthropods transmit blood-borne microbes; malarial parasites and chikungunya, dengue, Japanese encephalitis and zika viruses are vector-transmitted. Visceral leishmaniasis (kala-azar) is a vector-borne parasitic disease caused by Leishmania donovani, transmitted to humans by the sand fly Phlebotomus argentipes biting on kala-azar patients24. Yersinia pestis of bubonic plague is transmitted from rodents to humans by the rat flea vector. Pneumonic plague is contagious – the bacteria are shed via droplets of respiratory fluids, inhaled by those nearby. Ticks transmit Crimean-Congo Haemorrhagic Fever and Kyasanur Forest Disease viruses, Borrelia burgdorferri of Lyme disease, Borrelia recurrentis of Relapsing fever (borreliosis), Rickettsiae of spotted fevers, Francisella tulerensis of Tularaemia and protozoan parasite Babesia microti of Babesiosis25,26. Deer flies (chrysops) can also transmit F. tulerensis27. Tsetse flies transmit Trypanosoma brucei of Sleeping sickness (African trypanosomiasis); and body lice transmit Rickettsia prowazekii, Borrelia recurrentis and Bartonella Quintana causing epidemic typhus, louse-borne relapsing fever, and trench fever respectively28.
Saprophytic microbes may be inhaled – fungi and bacteria such as Legionella pneumophila, Coxiella burnetii are examples. Fungi such as Aspergillus, Blastomyces, Histoplasma, Cryptococcus and Coccidioides are amplified in decaying organic matter in the environment and become airborne when conditions are appropriate. They are inhaled, resulting in lung infection. Acanthameba and Naegleria are directly inoculated from water bodies while swimming in lakes or pools infested with these amebae. B. pseudomallei may be inhaled or inoculated into cuts or wounds on the skin. Filamentous fungi Eumycetes and bacteria such as Actinomycetes, Nocardia and Streptomycetes which cause Eumycetoma (Madura foot)29, and Mycobacterium ulcerans causing Buruli ulcer can be introduced through minor trauma resulting in broken skin30.
Host-pathogen interaction system
Host-pathogen interactions in the human body are (i) microbial multiplication and spread within body organs and tissues, (ii) inflammatory and related host responses and pathology (tissue damage/destruction) resulting in symptoms and signs, and (iii) immune responses that occur irrespective of the severity of disease – from sub-clinical to severe.
Microbial presence in tissues or fluids is direct evidence of infection. One important issue to note is if pathogen amplification in the host results in shedding into the environment, in which case the chain of transmission continues. In extra-pulmonary TB, the agents remain in the host as ecological ‘dead end’ from the perspective of chain of transmission. In Japanese encephalitis viremia in humans is of such low titre that mosquitoes do not get infected; hence epidemiologically human JE is ‘dead end’, similar to Western equine encephalitis virus and West Nile virus. On the other hand, viraemia in humans in dengue, chikungunya, yellow fever and rift valley fever is a component of the amplification system allowing mosquitoes to continue the chain of transmission31.
Tissue pathology can be helpful in diagnosis – caseating granuloma is characteristic of TB; non-caseating granuloma is typical of melioidosis32. Inflammatory cell responses in peripheral blood and cerebrospinal fluid are expressions of tissue inflammation and also clues for diagnosis – the latter is sine qua non of meningitis or encephalitis.
Immune responses occur irrespective of the severity of symptoms/signs; infection remaining sub-clinical or progressing to pathology and disease. In some infectious diseases, immune responses contribute to pathology – either directed against pathogens and infected cells/tissues, or directed against ‘self’ in autoimmune processes triggered by infections. On the other hand, antigenic variation by infectious agents such as a protozoa (T. brucei, malarial parasite), bacteria (Neisseria, Mycoplasma and Borrelia burgdorferi) or viruses (influenza, HIV and SARS-CoV-2) may thus escape host immune responses (immune evasion) resulting in repeated infections, longevity of agent survival/replication and transmissibility33.
Grouping pathogens by amplification & transmission systems
Amplification and transmission systems function in tandem for successful entry of a pathogen into the host. Public health functionaries and infectious disease epidemiologists are concerned with pathogen amplification (to identify the reservoir/source of infectious agents) and pathogen transmission (to identify the routes, channels and methods of transmission) so that further spread can be interrupted with appropriate interventions. Ecological and environmental elements of amplification and transmission lend themselves to be captured in the diagrammatic presentation of the ‘universe’ of all human pathogens – their origins and transmission pathways are shown in a simple Venn diagram34 (Fig. 2).
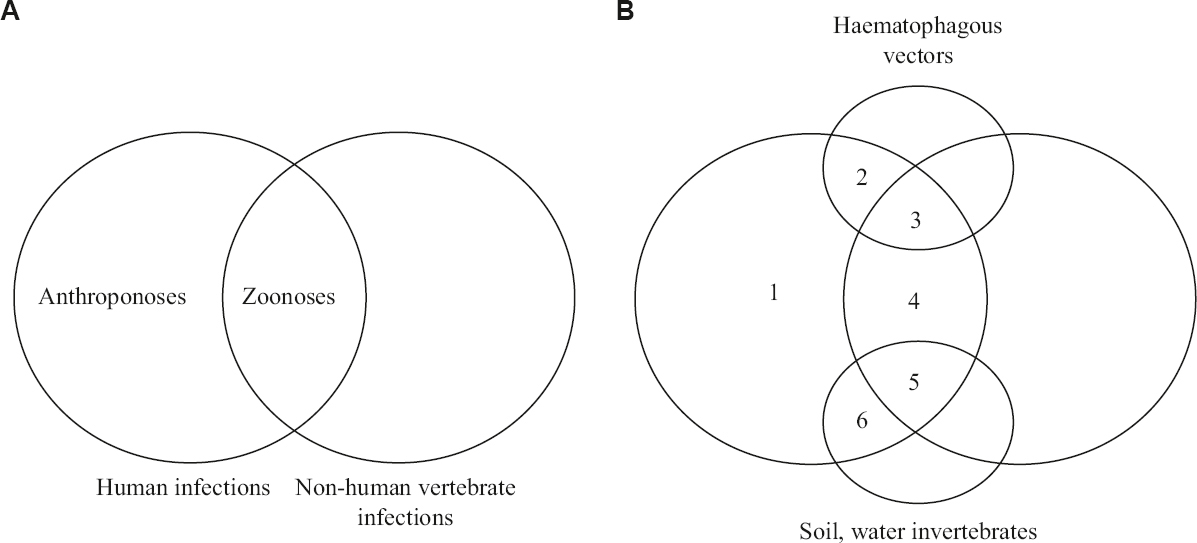
- Venn diagram depicting infectious diseases according to microbial amplification and transmission. (A) anthroponoses and zoonoses, (B) six categories of human infectious diseases; 1, directly human to human transmitted anthroponoses (TB, measles, AIDS); 2, vector transmitted anthroponoses (malaria, dengue, leishmaniasis); 3, vector transmitted zoonoses (Crimean-Congo haemorrhagic fever, Zika, KFD, Lyme disease, plague); 4, directly animal to human transmitted zoonoses (rabies, brucellosis, tularemia, cat scratch disease); 5, zoonotic pathogens transmitted via the environment (leptospirosis, hantavirus pulmonary syndrome, cutaneous anthrax, salmonellosis); 6, sapronoses amnd all non-zoonotic pathogens transmitted via the environment (melioidoses, legionellosis, Q fever, buruli ulcer disease, anthrax, cryptococcosis, primary amoebic meningeoencephalitis). TB, tuberculosis; KFD, Kyasanur forest disease. Source: Ref 34 (adapted with permission).
Helminths as agents-causing disease
The principles of EET - apply to helminthic infestations as well. Their amplification takes place through complex life cycles starting with ova and progressing as larvae and adult worms. Many helminths have definitive and intermediate hosts; humans may function as either. When non-human vertebrates function as definitive hosts, human infection comes under the definition of zoonoses. When invertebrates are the partner hosts – such as snails in schistosomiasis, crabs in paragonimiasis and water crustaceans in dracunculiasis, the human infection is akin to sapronoses, hence, in the Venn diagram, they are included in category 6 (Fig. 2).
Transmission occurs through the ingestion of ova (e.g. Ascaris), skin penetration of filarial form of larva of ancylostoma (hookworm) or introduction of larva by biological vector (mosquito-transmitting microfilariae). Larval forms may be ingested causing transmission – as in cysticercosis, dracunculiasis, paragonimiasis, trichinellosis and schistosomiasis35. In some cases, ingestion of ova may result in humans becoming intermediate hosts, with the larvae causing disease, as in cerebral cysticercosis.
Host-parasite interactions may be simple worm infestation in the gastrointestinal system or very complex as in pulmonary paragonimiasis, cerebral cysticercosis and fascioliasis35,36.
Conclusion
Epidemiology is the foundation science of public health. Epidemiology itself is incomplete without incorporating ecology – thus forming the concept of eco-epidemiology37. We adapted the term eco-epidemiology to highlight the ecology of microbial amplification, ecology and epidemiology of their transmission and the epidemiology of diseases with eco-epidemiology as the determinants of disease distribution10.
The EET has been designed to provide a complete and dynamic model of infectious diseases. It comprehensively guides the infectious disease physician as well as the public health functionary through the three questions: where do infectious agents come from, how do they reach and enter the human host and how do they lead to pathogenesis.
Financial support and sponsorship
None.
Conflicts of interest
None.
Acknowledgment:
Authors acknowledge Drs CE Eapen, department of Gastroenterology and VP Verghese, department of Pediatrics, CMC, Vellore, for reading the drafts of this paper and encouraging us to progress.
References
- Richert L, ed. Nature's path: A history of naturopathic healing in America (1st ed). Baltimore (MD): Johns Hopkins University Press; 2016.
- 'Everything is everywhere, but, the environment selects';what did Baas Becking and Beijerinck really say? Environ Microbiol. 2006;8:755-8.
- [Google Scholar]
- The disease triangle: A plant pathological paradigm revisited. The disease triangle: A plant pathological paradigm revisited. Available from: https://www.apsnet.org/edcenter/foreducators/TeachingNotes/Pages/DiseaseTriangle.aspx
- Principles of plant infection: A text-book of general plant pathology for biologists, agriculturists, foresters and plant breeders. New York: The University of Chicago Press Journals; 1950.
- The nature, origin and evolution of parasitism. In: Horsfall JG, Dimond AE, eds. Plant pathology: An advanced treatise. Vol 2. New York: Academic Press; 1960. p. :19-69.
- [Google Scholar]
- The disease triangle: Pathogens, the environment and society. Nat Rev Microbiol. 2007;5:152-6.
- [Google Scholar]
- Some conceptions of epidemics in general by Wade Hampton Frost. Am J Epidemiol. 1976;103:141-51.
- [Google Scholar]
- Snippets from the past: The evolution of Wade Hampton Frost's epidemiology as viewed from the American journal of hygiene/epidemiology. Am J Epidemiol. 2013;178:1013-9.
- [Google Scholar]
- The influence of local changes in the rise of infectious disease Discussion. In: Greenwood B, de Cock K, eds. New and resurgent infections Prediction, detection and management of tomorrow's epidemics. Ch. 1. Chichester, England: John Wiley &Sons; 1998. p. :1-15.
- [Google Scholar]
- Bats: Important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006;19:531-45.
- [Google Scholar]
- Oral shedding of Marburg virus in experimentally infected Egyptian fruit bats (Rousettus aegyptiacus) J Wildl Dis. 2015;51:113-24.
- [Google Scholar]
- Plague gives surprises in the first decade of the 21st century in the United States and worldwide. Am J Trop Med Hyg. 2013;89:788-93.
- [Google Scholar]
- Sapronosis: A distinctive type of infectious agent. Trends Parasitol. 2014;30:386-93.
- [Google Scholar]
- The chronology of the 2002-2003 SARS mini pandemic. Paediatr Respir Rev. 2004;5:262-9.
- [Google Scholar]
- Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol Ther Methods Clin Dev. 2020;18:1-6.
- [Google Scholar]
- Implications of SARS-CoV-2 viral load in children: Getting back to school and normal. JAMA Pediatr. 2021;175:e212022.
- [Google Scholar]
- India's research contributions towards polio eradication (1965-2015). Available from: https://www.indianpediatrics.net/supplaug2016/aug-S38-S43.htm
- Polio: The eradication imbroglio: The malady &its remedy. Mumbai: Notion Press Inc; 2021.
- Epidemiology and control of trachoma: Systematic review. Trop Med Int Health. 2010;15:673-91.
- [Google Scholar]
- Implication of vector characteristics of Phlebotomus argentipes in the Kala-azar elimination programme in the Indian sub-continent. Pathog Glob Health. 2016;110:87-96.
- [Google Scholar]
- Problem of ticks and tick-borne diseases in India with special emphasis on progress in tick control research: A review. J Vector Borne Dis. 2014;51:259-70.
- [Google Scholar]
- Zoonotic encephalitides caused by arboviruses: Transmission and epidemiology of alphaviruses and flaviviruses. Clin Exp Vaccine Res. 2014;3:58-77.
- [Google Scholar]
- Non-caseating granulomatous infective spondylitis: Melioidotic spondylitis. Asian Spine J. 2016;10:1065-71.
- [Google Scholar]
- Common strategies for antigenic variation by bacterial, fungal and protozoan pathogens. Nat Rev Microbiol. 2009;7:493-503.
- [Google Scholar]
- Helminths:pathogenesis and defenses. In: Baron S, ed. Medical microbiology (4th ed). Galveston (TX): University of Texas Medical Branch at Galveston; 1996.
- [Google Scholar]
- Immunity against helminths: Interactions with the host and the intercurrent infections. J Biomed Biotechnol. 2010;2010:428593.
- [Google Scholar]
- Choosing a future for epidemiology: II. From black box to Chinese boxes and eco-epidemiology. Am J Public Health. 1996;86:674-7.
- [Google Scholar]





