Translate this page into:
Inverse correlation of CD3 & vascular endothelial growth factor in incisional oral squamous cell carcinoma biopsies predicts nodal metastasis & poor survival of patients
For correspondence: Dr Dominic Augustine, Department of Oral Pathology & Microbiology, Faculty of Dental Sciences, M.S. Ramaiah University of Applied Sciences, MSR Nagar, Bengaluru 560 054, Karnataka, India e-mail: dominic2germain@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Oral squamous cell carcinoma (OSCC) is widely prevalent in the Indian subcontinent mainly due to habit-associated aetiologies. Immune regulation and angiogenesis are the part of tumourigenesis that play a crucial role in metastasis and survival. However, the concurrent expression of vascular endothelial growth factor (VEGF) and CD3 (immune regulator receptor on T-lymphocyte) in the same OSCC tissue samples has not been reported in the Indian population. The present study evaluated the expression of CD3+ T-cells and VEGF in OSCC tissue samples and studied the clinicopathological correlation and survival analysis in an Indian population.
Methods:
This was a retrospective study conducted on 30 formalin-fixed and paraffin embedded sections which were histologically diagnosed as OSCC cases comprising of 15 metastatic OSCC and 15 non-metastatic OSCC with available clinical data and survival status.
Results:
Reduced expression of CD3+ T-cells and increased VEGF expression were observed in metastatic OSCC samples. The correlation of expression of CD3+ T-cells and VEGF with clinicopathological parameters showed a significant association between these markers with age, nodal status, site of the lesion and survival.
Interpretation & conclusions:
Reduced expression of CD3+ T-cells in OSCC was found to be associated with a significantly poor survival. VEGF was found to be over expressed in metastatic OSCC as compared to that in non-metastatic OSCC. The study findings suggest that the evaluation of CD3 and VEGF in incisional OSCC biopsies can be considered for predicting the survival outcome and metastasis.
Keywords
Angiogenesis
CD3+ T-cells
immune regulation
oral squamous cell carcinoma
poor survival
prognostic indicators
VEGF
Oral squamous cell carcinoma (OSCC) is thought to represent 90 per cent of the head-and-neck malignancies globally1. Over the last few decades, the incidence of head-and-neck squamous cell carcinoma (HNSCC) has reportedly increased by 50 per cent1. The clinical parameters such as age, gender, tumour site, tobacco habit and lymph node metastasis affect the prognosis of the oral cancer patients2. A greater understanding of the prognostic factors and assessing the biological behaviour of oral cancer will facilitate the appropriate management in attaining a higher survival rate3.
Immune surveillance plays a key role in preventing cancer and loss of surveillance often leads to accumulation of mutations and eventually malignancy. The T-lymphocytes have been assumed to play a crucial role in preventing tumour proliferation and tumour metastasis4. The presence of lymphocytes around the tumour is thought to be associated with better prognosis in a diverse group of tumours. CD3 antigen is a receptor glycoprotein located on all T-lymphocytes4. Recent studies have suggested that alteration in the expression and functioning of signal-transducing molecules and immune cells are associated with aberrant CD3 expression. These alterations are responsible for the immune deficiencies in several malignancies of head-and-neck cancer5. Therefore, immune measures could serve as endpoints of clinical responses and could be used as prognostic biomarkers in the field of cancer.
Another essential element for progression and metastasis is angiogenesis6,7. Angiogenesis is a process through which new capillary blood vessels are formed it is controlled by both activator and inhibitor molecules. The newly-formed vessels facilitate tumour cells to leave the primary tumour site and enter circulation. The family of vascular endothelial growth factors (VEGFs), such as VEGF (A-F) are known players in the process of angiogenesis8. VEGF-A is a key angiogenic factor for the regulation of tumour angiogenesis9. An increase in the expression of VEGF in malignant tumours is found to be associated with increased vascularity, cancer cell growth and lymph node metastasis. The inhibition of angiogenesis constitutes an important mechanism in the control of cancer progression10. The present study evaluates the interplay of immune surveillance and angiogenesis in OSCC prognosis.
Few studies in literature have reported the role of immune response and angiogenesis in oral cancer in the same tissue with survival analysis. VEGF disrupts dendritic cell maturation and function and suppresses T-cell development11. The present study aimed to study the relationship between CD3 and VEGF in metastatic and non-metastatic OSCC and the correlation between CD3 and VEGF expression with clinicopathological parameters including the 5-year survival status.
Material & Methods
Sample collection and study setting: This retrospective study was undertaken on formalin-fixed paraffin embedded (FFPE) tissue samples of OSCC. Histopathologically diagnosed cases (n=30, 15 metastatic and 15 non-metastatic) of OSCC were retrieved from the archives of the department of Oral Pathology and Microbiology, Faculty of Dental Sciences, Ramaiah University of Applied Sciences, Bengaluru, India. Demographic details were collected using a proforma designed for this study (such as site, smoking status, alcohol status, lymph node status and prognosis). The present study was time bound from 2018 to 2019. The details of clinical progress and survival with follow up data of five years were obtained from the hospital records. All the patients with metastasis had undergone surgery with cervical lymph node dissection and had not received any preoperative antitumour therapy. Staging of tumour was done as per the TNM classification by the Union for International Cancer Control 8th edition12.
Inclusion criteria: Incisional biopsies from individuals who were histopathologically confirmed for primary OSCC were included in the study.
Exclusion criteria: Individuals with recurrent OSCC, who had undergone both chemotherapy as well as radiotherapy, patients on corticosteroids and patients with metabolic and other systemic disorders were excluded.
Tissue processing and immunohistochemistry: Immunohistochemistry analysis was performed using Streptavidin-biotin procedure by following standard protocol as described previously13.
Interpretation and scoring: Two investigators (WK and DA) scored the stained and blinded slides independently. The staining interpretation was done: based on the criteria by Naruse et al8 for VEGF and Helal and Wahba14 for CD3 using semi-quantitative method. VEGF-A protein expression was assessed by evaluating the total immunostaining score (brown-coloured reaction in the cytoplasm) as the product of the proportion of cells and the intensity scores. Scoring was based on the proportion of the positively-stained tumour cells (0, none; 1, <10%; 2, 10-50%; 3, 50-80% and 4, >80%). The intensity scores signified the estimated staining intensity (0 = no staining; 1 = weak; 2 = moderate and 3 = strong). Hence, the total immunostaining score ranged between 0 and 12. A total score of >4 was defined for positive cases. The calculation of immunohistochemistry-stained sections for CD3 was considered as positive depending on the presence of brown coloured reaction in the nucleus. The intensity of immunostaining was categorized as either negative, weak or strong. A scale of 0 to + + was used. The location of staining was assessed at the tissue as well as the cellular level. The areas were graded based on the intensity of staining as either (0) = absence of staining, (+) = weak positive staining or (+ +) = strong positive staining.
Statistical analysis: The comparison between the groups and subgroups of metastatic and non-metastatic cases of OSCC was done using the Chi-square test. The CD3 and VEGF scores were evaluated and clinicopathologically correlated to parameters and patients’ survival. For each patient, a follow up time of a period of five years was considered from the date of diagnosis until death by any cause or until the date of last patient contact, cumulative survival was measured. Univariate survival curves were obtained using the Kaplan-Meier method and compared using the log-rank test. The univariate log-rank (Mantle-Cox) and multivariate analyses were used to compare the clinical variables with the cumulative survival. The statistical tests were carried out using the Statistical Package for the Social Sciences (SPSS) software version 22 (IBM Corp., Armonk, NY, USA).
Results
A total of 30 incisional biopsies comprising of 15 metastatic and 15 non-metastatic OSCC cases were evaluated for the immunohistochemical expression of CD3 and VEGF.
CD3 expression in oral squamous cell carcinoma (OSCC): Among the 15 cases of metastatic OSCC, 37 per cent showed high expression along with nuclear localization for CD3, whereas 63 per cent cases showed weak staining (Fig. 1A-C). However, the expression of CD3 in non-metastatic OSCC showed intense staining for the 73 per cent cases while 27 per cent cases showed weak staining (Fig.1A-D). The expression of CD3 in metastatic and non-metastatic cases of OSCC was found to be significant (P<0.004) (Table I).
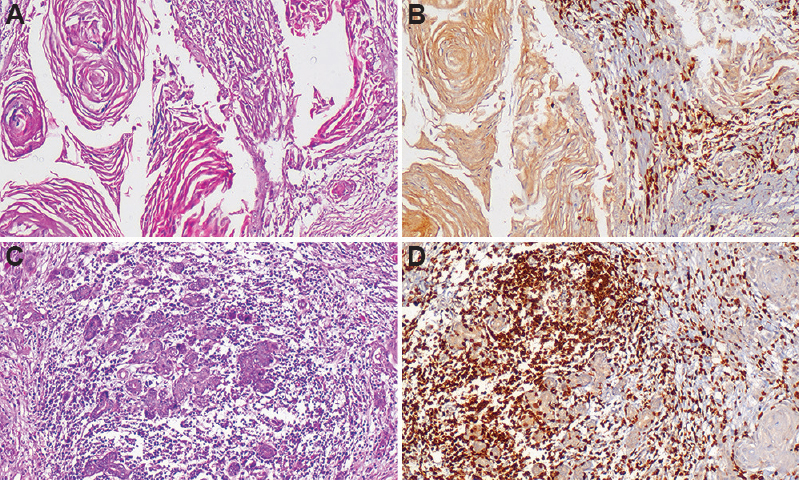
- Photomicrographs of oral squamous cell carcinoma (OSCC). Section of metastatic OSCC tissue showing (A) invading tumour islands and keratin pearls (H & E, ×100), (B) staining with CD3 antibody showing weak nuclear staining (IHC, ×100). Section of non-metastatic OSCC tissue showing (C) small invading tumour islands (H & E, ×100), (D) staining with CD3 antibody showing strong nuclear staining (IHC, ×100).
| Cases | CD3 scoring (%) | P | |
|---|---|---|---|
| Low | High | ||
| Metastatic | 63.3 | 36.7 | 0.004 |
| Non-metastatic | 26.7 | 73.3 | |
| VEGF scoring (%) | |||
| Metastatic | 46.7 | 53.3 | 0.003 |
| Non-metastatic | 83.3 | 16.7 | |
VEGF, vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) expression in OSCC: Among the 15 cases of metastatic OSCC, 53 per cent showed a strong cytoplasmic staining intensity for VEGF, whereas 47 per cent cases showed weak staining. However, the expression of VEGF in non-metastatic OSCC showed intense staining for only 17 per cent of cases while 83 per cent cases showed weak staining (Fig. 2A-D). The expression of VEGF in metastatic and non-metastatic cases of OSCC was also found to be significant (P<0.003) (Table I).
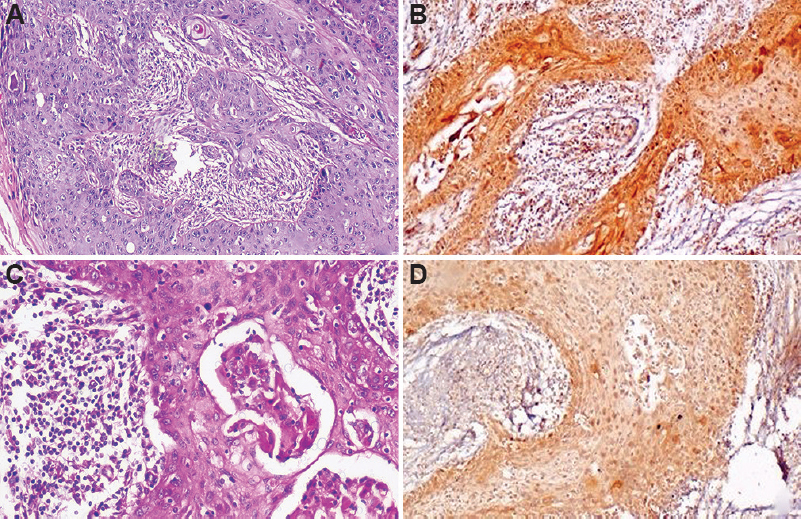
- Photomicrographs of OSCC. Section of metastatic OSCC tissue showing (A) invading sheets of tumour (H & E, ×100). (B) stained with anti-VEGF antibody showing strong cytoplasmic staining (IHC, ×100). Section of non-metastatic OSCC tissue showing (C) invading tumour islands (H & E, ×100). (D) weak cytoplasmic staining (IHC, ×100).
Correlation of CD3 and VEGF in metastatic and non-metastatic OSCC: When analysis of coefficient of correlation was executed, the expression of CD3 and VEGF in metastatic OSCC showed a significant correlation (P≤0.001). High correlation of CD3 and VEGF expression was observed in metastatic OSCC cases (r=-0.675, P=0.001) as compared to non-metastatic OSCC (r=-0.337, P=0.069).
CD3 correlation with clinicopathological parameters: Upon correlating CD3 expression with clinicopathological parameters of metastatic OSCC (age, gender, site, smoking status, alcohol status, nodal status, histologic grade and survival), the expression level of CD3 was found to be significantly associated with older age group (P=0.016) and nodal status (P=0.049). Similarly, a significant association of CD3 expression was associated with survival (P=0.001) (Table II). In non-metastatic OSCC, CD3 expression correlated significantly with older age group (P=0.047), site (tongue), (P=0.005) and survival (P=0.001) (Table II).
| Study parameters | Metastatic | Pearson’s χ2 | P | Non-metastatic | Pearson’s χ2 | P | ||
|---|---|---|---|---|---|---|---|---|
| CD3 Low score | CD3 High score | CD3 Low score | CD3 High score | |||||
| Age groups | ||||||||
| ≤50 | 16.7 | 83.3 | 7.033 | 0.016 | 7.7 | 92.3 | 4.224 | 0.047 |
| >50 | 75 | 25 | 41.2 | 58.8 | ||||
| Gender | ||||||||
| Male | 66.7 | 33.3 | 0.215 | 0.466 | 37.5 | 62.5 | 2.058 | 0.154 |
| Female | 58.3 | 41.7 | 14.3 | 85.7 | ||||
| Site | ||||||||
| Tongue | 70 | 30 | 3.732 | 0.154 | 66.7 | 33.3 | 10.540 | 0.005 |
| GBS | 80 | 20 | 11.1 | 88.9 | ||||
| Buccal mucosa | 40 | 60 | 8.3 | 91.7 | ||||
| Smoking status | ||||||||
| Yes | 52.6 | 47.4 | 2.556 | 0.113 | 30 | 70 | 0.341 | 0.452 |
| No | 81.8 | 18.2 | 20 | 80 | ||||
| Alcohol status | ||||||||
| Yes | 75 | 25 | 0.271 | 0.530 | 50 | 50 | 0.597 | 0.469 |
| No | 61.5 | 38.5 | 25 | 75 | ||||
| Nodes | ||||||||
| 1 | 46.2 | 53.8 | 0.591 | 0.049 | ||||
| 2 | 81.2 | 18.8 | ||||||
| 4 | 0 | 100 | ||||||
| Survival | ||||||||
| Dead | 100 | 0 | 30.0 | 0.001 | 66.7 | 33.3 | 16.364 | 0.001 |
| Alive | 0 | 100 | 0 | 100 | ||||
Chi-square test, P≤0.05 considered as significant. GBS, gingival buccal sulcus
Correlation of VEGF with clinicopathological parameters: The correlation between VEGF expression and clinicopathological parameters showed a significant association with survival (P=0.006) in both metastatic and non-metastatic OSCC. The other parameters did not correlate significantly with VEGF expression (Table III).
| Study parameters | Metastatic | Pearson’s χ2 | P | Non-metastatic | Pearson’s χ2 | P | ||
|---|---|---|---|---|---|---|---|---|
| VEGF Low score | VEGF High score | VEGF Low score | VEGF High score | |||||
| Age groups | ||||||||
| ≤50 | 66.7 | 33.3 | 1.205 | 0.261 | 100 | 0 | 4.588 | 0.043 |
| >50 | 75 | 25 | 70.6 | 29.4 | ||||
| Gender | ||||||||
| Male | 44.4 | 55.6 | 0.829 | 0.529 | 75 | 25 | 1.714 | 0.209 |
| Female | 50 | 50 | 92.9 | 7.1 | ||||
| Site | ||||||||
| Tongue | 50 | 50 | 1.875 | 0.392 | 77.8 | 22.2 | 4.00 | 0.111 |
| GBS | 30 | 70 | 66.7 | 33.3 | ||||
| Buccal mucosa | 60 | 40 | 100 | 0 | ||||
| Smoking status | ||||||||
| Yes | 36.4 | 63.6 | 0.741 | 0.317 | 80 | 20 | 0.480 | 0.449 |
| No | 52.6 | 47.4 | 90 | 10 | ||||
| Alcohol status | ||||||||
| Yes | 46.2 | 53.8 | 0.021 | 0.648 | 50 | 50 | 1.714 | 0.310 |
| No | 50 | 50 | 85.7 | 14.3 | ||||
| Nodes | ||||||||
| 1 | 46.2 | 53.8 | 0.591 | 0.049* | ||||
| 2 | 81.2 | 18.8 | ||||||
| 4 | 0 | 100 | ||||||
| Prognosis | ||||||||
| Dead | 21.1 | 78.9 | 13.659 | 0.001** | 58.3 | 41.7 | 9.00 | 0.006 |
| Alive | 90.9 | 9.1 | 100 | 0 | ||||
Chi-square test, P≤0.05 considered significant
Survival analysis: The comparison of age, site, habits gender and grade with cumulative survival was done using the univariate analysis (Table IV). The age range of the OSCC cases considered in this study was 24-80 yr. In the present study the predominant presentation in the OSCC cases included tumours on the buccal mucosa, tongue and gingivobuccal sulcus. The comparison of clinicopathological parameters (age, gender, site, smoking habit, CD3 and VEGF expression) with cumulative survival was done using univariate analysis with log-rank (Mantel-Cox) test and Kaplan-Meier plots (Fig. 3). When age was correlated with survival, an insignificant result was obtained (P≤0.829). The comparison of gender with survival rate did not show a significant result either (P≤0.559). The correlation of site with the survival rate in OSCC cases showed a significant correlation (P≤0.001). A statistically insignificant result (P= 0.757) was obtained on the comparison of smoking habit with the survival rate of OSCC patients.
| Clinicopathological parameters | χ2 | Degrees of freedom | P |
|---|---|---|---|
| Age | 0.047 | 1 | 0.829 |
| Gender | 0.341 | 1 | 0.559 |
| Site | 28.466 | 2 | 0.001 |
| Smoking habit | 0.095 | 1 | 0.757 |
| Stage | 8.064 | 1 | 0.005 |
| CD3 | 25.903 | 1 | 0.001 |
| VEGF | 2.344 | 1 | 0.126 |
Kaplan-Meier method, P≤0.05 considered significant
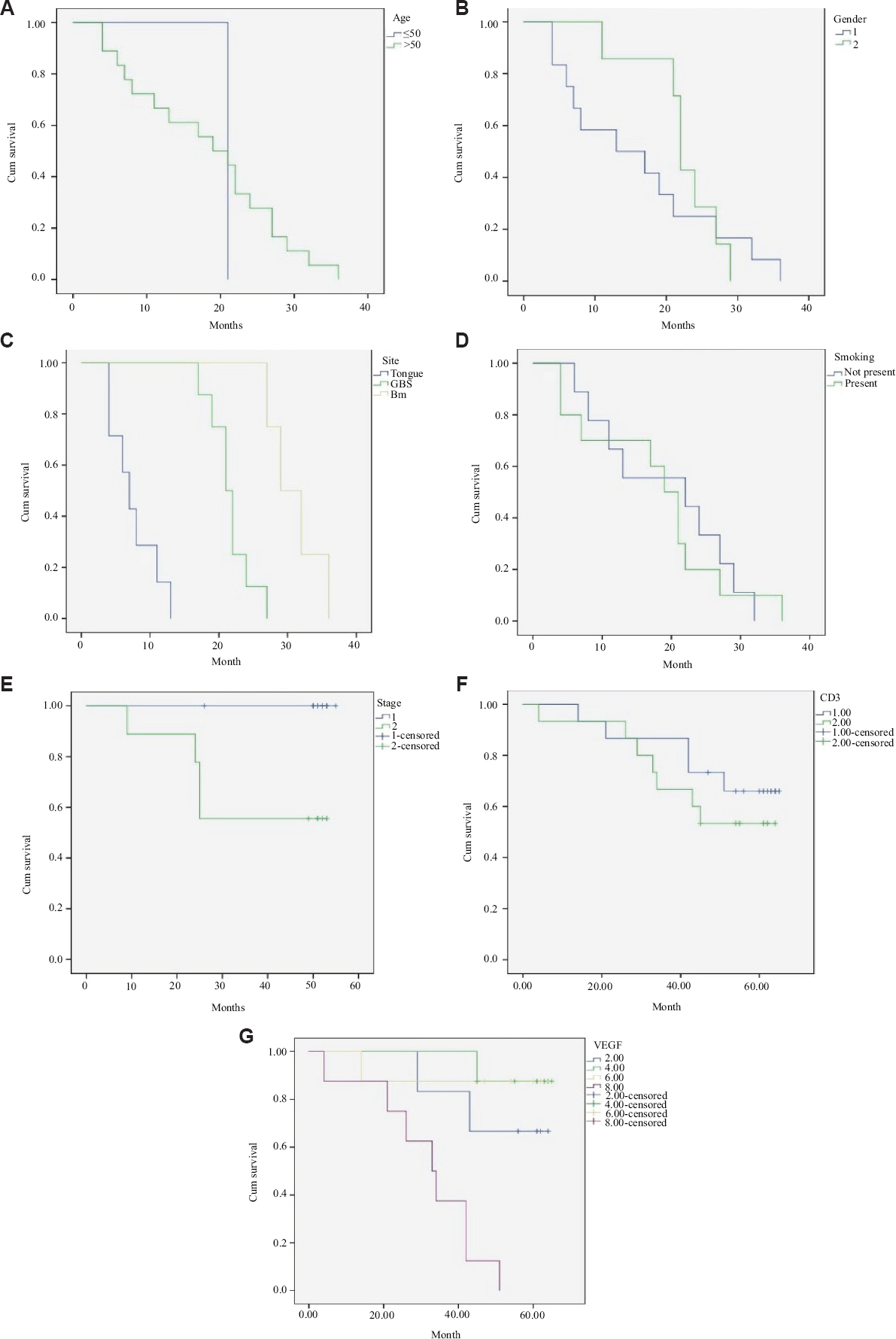
- Kaplan-Meier plots comparing the clinicopathological parameters with cumulative survival. (A) age group, (B) gender, (C) site, (D) smoking, (E) staging, (F) CD3 expression, and (G) VEGF expression.
Kaplan-Meier analysis was used to compare the CD3 expression with the rate of survival in OSCC cases. The CD3 expression in OSCC cases showed decreased survival with a significant difference between IHC (CD3 expression) scores and survival rate (P≤0.001) VEGF expression was not found to be significant as compared to the rate of survival in OSCC cases (P≤0.126).
Decreased expression of CD3+ T-cells in OSCC was related with significantly poor survival. VEGF was found to be over expressed in metastatic OSCC when compared to non-metastatic OSCC.
Discussion
OSCC is thought to be the sixth most common malignancy in the head-and-neck region that is one of the major causes of cancer morbidity and mortality globally. In India, OSCC is believed to stand as the third most common cancer with a high incidence rate of 52,000 annually15. Most deaths from oral cancer result in patients who develop local and distant metastasis that results in poor prognosis, with only few long-term survivors16. For metastasis to occur, the tumour cells need to undergo a metastatic cascade. Metastasis consists of sequential and selective steps including immune cell dysregulation, proliferation and stimulation of angiogenesis17.
The immune system actively prevents tumour formation through immune surveillance. The T-lymphocytes play an essential role in inhibiting tumour proliferation and metastasis. The TILs indicate a good host immune response18. Studies in the past have shown that percentage of tumour-infiltrating lymphocytes in a tumour is correlated with the survival in several types of cancer such as ovarian, pancreatic and breast cancers19. Rajjoub et al20 conducted a study to investigate the number of CD3 tumour-infiltrating lymphocytes in oropharyngeal cancer and its correlation with the survival rates. They concluded that patients with high expression of CD3 have better prognosis and higher rate of overall survival than in patients with low expression. Tiwari et al21 aimed to quantify the tumour-infiltrating CD3 positive T-lymphocytes in the different grades of OSCC. The authors concluded by stating that every individual has a different immune response and CD3 positive T-lymphocytes does not mandatorily correlate with tumour size or stage. The results of the present study were in accordance with the concept of immune dysregulation of T-lymphocytes as low CD3 scores were observed in metastatic tumours, whereas a high CD3 expression was seen in non-metastatic tumours, (P=0.004).
VEGF is an important angiogenic cytokine that is crucial for the progression and invasiveness of most of the tumours including oral cancer22. The probability of metastasis in a tumour is predicted by the intensity of angiogenesis. To metastasise, the tumour cells need to gain access to the vasculature from the primary tumour first, travel through the circulation and survive, then settle down in the resident organ and initiate angiogenesis23. Kapoor and Deshmukh24 investigated the expression of VEGF in OSCC across histological grades, lymph node status and clinical sizes. The authors concluded that VEGF over expression correlates with lymph node metastasis. It was suggested that the expression of VEGF can help in establishing a direct relation between the growth factor and tumour growth. The results of the current study showed that 83 per cent of non-metastatic OSCC cases showed low VEGF expression compared to higher expression in metastatic OSCC cases (P=0.003). This supports the premise that increased expression of VEGF in the metastatic OSCC may indicate that angiogenesis is an essential step in the process of metastasis and can be correlated with tumour aggressiveness. Formation of new blood vessels from pre-existing ones by sprouting provides an essential route for the tumour cells to metastasize leaving its primary site and entering in the circulation. Cell culture studies have also shown that VEGF-neutralizing antibody to the OSCC-conditioned downregulates T-cell proliferation and production of interferon gamma (IFN-γ), perforin and granzyme B25.
The current study investigated OSCC (metastatic & non-metastatic) for the expression of VEGF and CD3 in an attempt to evaluate the correlation of expression with tumour progression and poor prognosis. In the present study, CD3 expression and VEGF in metastatic OSCC showed a significant (P=0.001) correlation.
In the present study, nodal metastasis was found to be one of the major outcome determinants in OSCC. The specific aim for developing a prognostic method for predicting cervical metastasis in an individual has to be emphasized in establishing the survival outcome. The CD3 protein complex is a defining feature of the T-cell lineage. Hence, anti-CD3 antibodies can reportedly be used effectively as T-cell markers26. Furthermore, the T-cell subset CD4 has been shown to actively participate in shaping anti-tumour immunity and its contribution to the anti-tumour response.
A study conducted by Aggarwal et al27 in 2017 stated that efficient recruitment of T-regulatory cells to the tumour site occurred in cases where increased expression of CCR5 and CCR7 on T-regulatory cells was found.
Increasing evidence also suggests that some patients with cancer also mount an adaptive immune response which is particularly directed against the antigenic proteins expressed in their tumours. T-cells secrete various kinds of cytokines such as IFN-γ and produce acute inflammation that causes expansion of cytotoxic T-cells (CTLs) and tissue destruction, the potential control or suggestively even elimination of cancer28.
Zhou et al29 assessed CD3+ and CD8+ cells in a cohort of 169 cases and stated that high density was significantly associated with increased overall survival. A high expression of CD3 was observed in the non-metastatic cases of OSCC that indicated that enhanced immunity has protective effect in oral cancer patients. Our results are also in accordance with Balermpas et al30 who demonstrated that high levels of CD3+ and CD8+ T-cells showed good prognosis and less metastasis compared with cases with low CD3+ and CD8+ T-cells. It was deduced that T-lymphocytes may have a beneficial role to play in HNSCC.
Higher expression of VEGF in the metastasizing cases of OSCC indicate that VEGF could be used to interfere with the initial development of tumour growth by blocking the angiogenic switch pathway that leads to the progression of an invasive cancer. Naruse et al8 in their study illustrated that the high expression levels VEGF-A and VEGF-C also significantly correlate with lymph node metastasis in oesophageal squamous cell carcinoma8.
A study conducted by Astekar et al31. evaluated the expression of VEGF in OSCC with few clinicopathological parameters without overall survival rate in patients with OSCC. However, in the present study, a broad spectrum of clinicopathological parameters such as age, site, gender, sex, tobacco status and nodal metastasis was included with its correlation with five-year survival. The authors believe that clinicopathological correlation and prognostic significance of immune regulation and angiogenesis in OSCC in the same tissues is so far not reported. The present study is a first of its kind evaluating the interrelationship of these markers (CD3 and VEGF) concurrently in OSCC tissues and comparing it with various clinicopathological parameters along with the survival analysis in the Indian population. The CD3 expression correlated significantly with age, nodal status and survival in metastatic OSCC. The VEGF expression correlated significantly with the nodal status and survival in metastatic OSCC. It was found in the present study that site, staging and CD3 expression had a significant correlation. A significant difference between IHC (CD3 expression) scores and survival rate was also obtained. Due to increased vasculature and lymphatic drainage, tongue cancers have a higher risk of metastasis to cervical lymph nodes. Hence, tumours of the tongue behave more aggressively and show site-specific variation to the expression of markers32.
Future studies, addressing immune response in OSCC will be of help in finding strategies to enhance immune activity in OSCC. These may point to the importance of a combination of chemoradiotherapy with targeted immunotherapies which activate T-cells in HNSCC patients with low levels of CD3+ tumour-infiltrating lymphocytes to enhance treatment outcomes. In conclusion, our results suggest an association of CD3 expression with good prognosis.
Financial support and sponsorship
None.
Conflicts of interest
None.
Acknowledgment
The authors acknowledge Dr Suvi Kanchan for performing the statistical analysis
References
- Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67-76.
- [Google Scholar]
- Squamous cell carcinoma antigen as a prognostic marker and its correlation with clinicopathological features in head and neck squamous cell carcinoma: Systematic review and meta-analysis. J Oral Pathol Med. 2018;47:3-10.
- [Google Scholar]
- CD3+, CD4+&CD8+tumour infiltrating lymphocytes (TILs) are predictors of favourable survival outcome in infiltrating ductal carcinoma of breast. Indian J Med Res. 2014;140:361-9.
- [Google Scholar]
- Head and neck carcinoma immunotherapy: Facts and hopes. Clin Cancer Res. 2018;24:6-13.
- [Google Scholar]
- The contribution of angiogenesis to the process of metastasis. Cancer J. 2015;21:267-73.
- [Google Scholar]
- Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1-29.
- [Google Scholar]
- Immunohistochemical study of vascular endothelial growth factor-C/vascular endothelial growth factor receptor-3 expression in oral tongue squamous cell carcinoma: Correlation with the induction of lymphangiogenesis. Oncol Lett. 2015;10:2027-34.
- [Google Scholar]
- Current challenges of cancer anti-angiogenic therapy and the promise of nanotherapeutics. Theranostics. 2018;8:533-48.
- [Google Scholar]
- Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol Immunother. 2007;56:761-70.
- [Google Scholar]
- Changes in the 8th edition of the American Joint Committee on Cancer (AJCC) staging of head and neck cancer: Rationale and implications. Curr Oncol Rep. 2019;21:52.
- [Google Scholar]
- Immunohistochemistry for pathologists: Protocols, pitfalls, and tips. J Pathol Transl Med. 2016;50:411-8.
- [Google Scholar]
- Immunohistochemical study of CD68, CD3 and Bcl-2 and their role in progression and prognosis of head and neck squamous cell carcinoma. Arch Cancer Res. 2016;4:1.
- [Google Scholar]
- Clinicopathological analysis of oral squamous cell carcinoma among the younger age group in coastal Karnataka, India: A retrospective study. J Oral Maxillofac Pathol. 2018;22:180-7.
- [Google Scholar]
- Distant metastasis from oral cancer: A review and molecular biologic aspects. J Int Soc Prev Community Dent. 2016;6:265-71.
- [Google Scholar]
- The concept of immune surveillance against tumors. The first theories. Oncotarget. 2017;8:7175-80.
- [Google Scholar]
- Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24:311-35.
- [Google Scholar]
- Prognostic significance of tumor-infiltrating lymphocytes in oropharyngeal cancer. Ear Nose Throat J. 2007;86:506-11.
- [Google Scholar]
- Augmentation of TIL-CD3 in oral cancer: Variations in relation to stage and grade of disease. Int J Pharm Sci Res. 2016;7:2476-82.
- [Google Scholar]
- Targeting angiogenesis in cancer therapy: Moving beyond vascular endothelial growth factor. Oncologist. 2015;20:660-73.
- [Google Scholar]
- New paradigm for vessel intravasation by tumor cells. Am J Pathol. 2002;160:1937-9.
- [Google Scholar]
- VEGF: A critical driver for angiogenesis and subsequent tumor growth: An IHC study. J Oral Maxillofac Pathol. 2012;16:330-7.
- [Google Scholar]
- Secretion of vascular endothelial growth factor by oral squamous cell carcinoma cells skews endothelial cells to suppress T-cell functions. Hum Immunol. 2009;70:375-82.
- [Google Scholar]
- CD3: Structure, function, and role of immunostaining in clinical practice. J Pathol. 1994;173:303-7.
- [Google Scholar]
- Dynamics of regulatory T cells (Tregs) in patients with oral squamous cell carcinoma. J Surg Oncol. 2017;116:1103-13.
- [Google Scholar]
- Density and location of CD3+and CD8+tumor-infiltrating lymphocytes correlate with prognosis of oral squamous cell carcinoma. J Oral Pathol Med. 2018;47:359-67.
- [Google Scholar]
- Tumor-infiltrating lymphocytes favour the response to chemoradiotherapy of head and neck cancer. Oncoimmunology. 2014;3:e27403.
- [Google Scholar]
- Expression of vascular endothelial growth factor and microvessel density in oral tumorigenesis. J Oral Maxillofac Pathol. 2012;16:22-6.
- [Google Scholar]
- Oral squamous cell carcinoma:metastasis, potentially associated malignant disorders, etiology and recent advancements in diagnosis. F1000Res. 2020;9:229.
- [Google Scholar]






