Translate this page into:
Placental expression of striatin & endothelial nitric oxide synthase in women with & without pre-eclampsia
For correspondence: Dr Balasubramaniyan Vairappan, Liver Diseases Research Lab, Department of Biochemistry, JIPMER, Dhanvantari Nagar, Puducherry 605 006, India e-mail: balasubramaniyan.v@jipmer.edu.in
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Striatin is a multi-domain scaffolding protein essential for activating endothelial nitric oxide synthase (eNOS). However, its role in pre-eclampsia remains use explored. Hence, this study aimed to investigate the association between striatin and eNOS in regulating nitric oxide (NO) production in the placenta of women with and without pre-eclampsia.
Methods:
Forty pregnant women each without (controls) and with pre-eclampsia (cases) were enrolled in the study. Blood striatin and NO concentrations were detected by the ELISA. Protein expression of striatin, phosphorylated eNOS (peNOS), inducible NOS (iNOS) and phosphorylated NF-κB were measured in the placental tissues by Western blot. Twenty four hour urinary protein and serum urea, uric acid and creatinine were analyzed as an autoanalyzer. Placental histology was analyzed by haematoxylin and eosin staining.
Results:
Compared to normotensive pregnant women, the levels of serum NO and striatin were decreased in pre-eclamptic women. The protein expression of striatin and peNOS was significantly reduced (P<0.05) while p65NF-κB and iNOS were upregulated considerably (P<0.05) in the placenta of cases compared to controls.
Interpretation & conclusions:
Our results show for the first time that decreased striatin expression was associated with decreased peNOS protein expression in the placental tissue of pre-eclamptic women. Interestingly, no significant difference was found in blood striatin or NO levels between controls and cases. Thus, therapies that improve placental striatin expression are attractive possibilities, both for prevention as well as treatment of endothelial dysfunction in pre-eclampsia.
Keywords
Endothelial nitric oxide synthase
inflammation
nitric oxide
pre-eclampsia
striatin
Hypertensive pregnancy disorder affects approximately 7-10 per cent of pregnancies and is considered the most common and significant cause of maternal morbidity and mortality worldwide1. Nearly 5-10 per cent of pregnant women, especially in developing countries, are thought to be at a risk of developing pre-eclampsia, a complication that has a severe impact on both foetal and maternal outcomes2. If left untreated, it can progress to a convulsive state known as eclampsia3. The worldwide and national prevalence of maternal deaths due to pre-eclampsia was 6.8 and 4 per cent, respectively4. Pre-eclampsia is known to be a systemic vascular endothelial disorder. It has been shown that serum from women with pre-eclampsia can induce endothelial dysfunction by way of impairing the monolayer permeability, nitric oxide (NO) production and calcium mediated responses5. In addition, inflammation also contributes to vascular dysfunction and causes maternal endothelial dysfunction which is a hallmark feature of pre-eclampsia progression6.
NO is a major vasodilator in normal health and thought to have a major vasodilatory effect during pregnancy7. It is synthesized from an amino acid, L-arginine by the enzyme nitric oxide synthase (NOS) and plays a substantial role in the normal placental development. The human placenta under normal physiological status does not express inducible or neuronal NO synthases (iNOS or nNOS), but endothelial NOS (eNOS) is present in the umbilical cord endothelium, chorionic plate and villous cytotrophoblasts8,9. A previous study has identified eNOS as one of the susceptible genes of pre-eclampsia10. Moreover, eNOS knockout mice show decreased NO concentrations and associated blood pressure (BP) reduction11. Thus, altered NO bioavailability is one of the major factors in pre-eclampsia development12. Furthermore, endothelial dysfunction due to decreased eNOS activity is an early and relentless event in the development of vascular complications occurring concomitantly during pre-eclampsia. By contrast, iNOS expression was increased in pre-eclampsia and contributing to increased resistance to flow in the foeto-maternal circulation, thereby reduced placental blood flow13.
Striatin, a protein, which belongs to the WD (W – one-letter abbreviation for tryptophan; D - one-letter abbreviation for aspartic acid) (tryptophan-aspartic acid dipeptide) repeat family, is mainly localized in the central and peripheral nervous systems14. In humans, it is encoded by the STRN gene. Interestingly, the human placenta also expresses striatin, a multi-domain scaffolding protein which resides in the membrane caveolae and binds specifically with caveolin-1 and Ca2+-calmodulin, essential for the activation of eNOS14.
Striatin also promotes the membrane localization and assembly of Estrogen Receptor alpha (ERα) signaling complexes15. Striatin deficiency has been shown to be associated with elevated BP and decreased vascular relaxation16, suggesting its critical role, in the regulation of vascular function and BP possibly through the modulation of endothelial NO-cGMP. Recently, we showed that decreased hepatic striatin expression was positively correlated with decreased NO in cirrhotic patients17. Despite these reports the association between striatin and phosphorylated eNOS (peNOS) so far has not been studied in context of a human pregnancy with and without pre-eclampsia. Thus, in this study, the aim was to investigate the association between striatin and peNOS in regulating NO synthesis in the placenta of women with and without pre-eclampsia.
Material & Methods
This hospital-based analytical study was carried out in the department of Biochemistry in collaboration with the department of Obstetrics and Gynaecology, Women and Children Hospital, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, from March 2017 to December 2018. The study was approved by the Institutional Ethics Committee (Human Studies) as per the guidelines of the Declaration of Helsinki. The study participants were screened by an obstetrician regularly. They were briefed about the study procedures before enrolling in the study, and written informed consent was obtained from all the study participants.
Study population: A total of 80 pregnant women (40 pre-eclamptic and 40 normal) were included in this study. The inclusion criteria for women with pre-eclampsia (age 20 - 35 yr) were: gestational hypertension and proteinuria commencing after the 20th wk of gestation and with the resolution of both conditions postpartum. Pre-eclamptic women were excluded if they had multiple gestations, prior history of pre-eclampsia or any pre-existing medical conditions including, hypertension, diabetes and renal disease. Moreover, women who underewent emergency delivery were also excluded. Women with normal pregnancy (aged between 20 - 35 yr) without any known pre-existing medical complications were included as a control.
Biological specimen: Urine and 5 ml of venous blood samples were collected from the participants after an overnight fast of at least 6-8 h, immediately after the diagnosis, and from normal pregnant women during their routine visits. Routine biochemical parameters such as urea, creatinine, uric acid and 24 h urinary protein were estimated immediately. The remaining samples were stored at −80°C for the subsequent analysis of NO and striatin levels. Estimation of serum NO and striatin concentrations were estimated by ELISA (Bioassay Technology, Shanghai, China), according to the manufacturer’s instruction. Furthermore, placental tissues (n=20; i.e. control 10 and pre-eclampsia 10) were collected in liquid nitrogen and formalin after delivery. Snap frozen placental tissues were stored at −80°C, until analysis.
Histology and immunohistochemistry: Freshly harvested placental tissues were placed into 10 per cent buffered formalin for 24 h. The specimen was then removed and washed twice with normal saline, dehydrated in alcohol, clarified in xylene and embedded in paraffin wax. Placental sections (3-5-μm thick) were cut using an automated microtome (Leica Biosystems, India) and stained with haematoxylin and eosin using standard procedures.
For immunohistochemistry, sections were dewaxed in xylene and dehydrated in alcohol and then blocked for endogenous peroxidase activity with 10 per cent H2O2. Antigen retrieval was done using citrate buffer (110°C for 10 min). Sections were then incubated with mouse monoclonal anti-striatin (1:100, GeneTex, USA) primary antibody for 1 h (at RT) followed by respective secondary antibody treatment (Vector Lab, UK). Sections were mounted on Histomount, and results were examined using the EVOS cell imaging system (Life Technologies, USA)
Biochemistry: Blood urea was estimated by an enzymatic method18 using urease and glutamate dehydrogenase enzymes; serum creatinine was estimated by Jaffe’s method19; serum uric acid was estimated by uricase/peroxidase method; 24 h urine protein was estimated by pyrogallol red method. The above colorimetric assays were measured using Beckman Coulter AU680 Chemistry Autoanalyzer, USA.
Western blotting: Snap-frozen placental tissues from the control and pre-eclamptic women were homogenized in ice-cold RIPA (Radioimmunoprecipitation assay) buffer with protease inhibitors (Sigma Aldrich, USA). Equal amounts of protein extracts were denatured, separated on 3-8 per cent NuPAGE Tris-Acetate Gels (Thermo Fischer Scientific, USA) and transferred onto nitrocellulose membranes (Advantec, Japan). These were probed with rabbit primary antibodies for anti-eNOS (Boster Bio, USA), mouse anti-striatin (GeneTex, USA), mouse anti-4 HNE (hydroxynonenal), rabbit anti-NF-κB (Cell Signaling, USA), mouse anti-iNOS (1:10000; Cat. BS-2072R, BIOSS, USA) and rabbit anti-β-actin (CST, USA) and then with respective horse-radish peroxidase-conjugated secondary antibody. The bands were visualized using an enhanced ECL detection kit (Thermo Fischer Scientific, USA) and quantified by densitometry.
Statistical analysis: All statistical analyses were done using GraphPad Prism version 6.01 (San Diego, CA, USA) software. Results were expressed as mean ± standard deviation or median (interquartile range) based on the normality of data (Shapiro-Wilk normality test). P<0.05 was considered significant. A two-tailed unpaired t test or Mann-Whitney U test was used for comparison between cases and controls, wherever appropriate.
Results
Compared to normal pregnant women, serum concentrations of creatinine, urea and uric acid were significantly elevated in pre-eclamptic women; although within the normal range. Twenty four hour urinary protein concentration was also significantly elevated in pre-eclamptic women as compared to normotensive women (Table). The mean age in the pre-eclampsia and control groups was 25.28 and 24.05 yr, respectively. The average BP was higher in the pre-eclampsia (167/111 mmHg) as compared to the control group (102/70 mmHg). Furthermore, the observed systolic and diastolic BP was also significantly elevated in the pre-eclamptic women as compared to normotensive women (Table). Serum striatin and NO concentrations were not altered between normal pregnant women and pre-eclamptic women (Table).
| Parameters | Normal pregnancy (n=40) | Pre-eclampsia (n=40) |
|---|---|---|
| Age (yr), median (IQR) | 24.0 (21.0-26.75) | 25.0 (23.0-28.0) |
| Gestational age (wks), mean±SD | 31.98±6.16 | 32.98±5.54 |
| Systolic BP (mmHg), median (IQR) | 100.0 (90.0-110.0) | 160.0 (140.0-190.0)** |
| Diastolic BP (mmHg), median (IQR) | 70.0 (67.0-80.0) | 110.0 (100.0-120.0)** |
| Urea (mg/dl), median (IQR) | 13.0 (12.0-15.75) | 18.0 (16.0-22.75)** |
| Creatinine (mg/dl), median (IQR) | 0.61 (0.58-0.65) | 0.72 (0.66-0.81)** |
| Uric acid (mg/dl), median (IQR) | 3.20 (2.42-3.60) | 4.55 (3.57-5.20)** |
| 24 h urinary protein (g/day), median (IQR) | 76.0 (69.0-84.0) | 233.0 (188.3-672.8)** |
| Striatin (ng/ml), median (IQR) | 9.91 (8.46-11.25) | 9.33 (8.23-10.47) |
| Nitric oxide (μmol/l), median (IQR) | 15.12 (14.07-19.41) | 14.17 (12.42-16.34) |
P **<0.001 compared to normal pregnancy. Based on the normality of data (Shapiro-Wilk normality test) a two-tailed unpaired t test or Mann-Whitney U test was used wherever appropriate for comparison between cases and controls with P<0.05 considered significant. SD, standard deviation; IQR, interquartile range, BP, blood pressure
Placental pathological changes were observed in normotensive as well as pre-eclamptic women, which are shown in Figure 1. Placenta in normal pregnant women (Fig. 1A) showed mature term villi with an increased number of villous vessels and formation of vasculosyncytial membranes. In the placenta of pre-eclamptic women, (Fig. 1B) the villi appeared smaller for the gestational age and lesser in number with reduced villous vessels and fibrotic stroma. Furthermore, there was a significant increase in the number of trophoblastic cell hyperplasia.
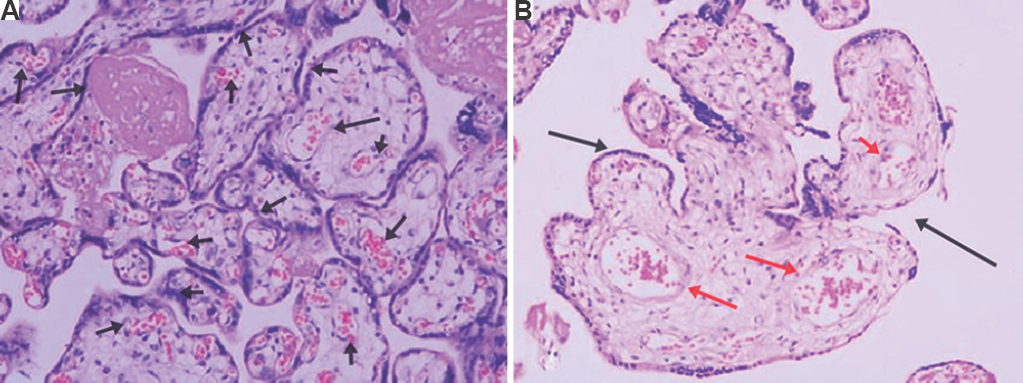
- Histopathological findings of the placenta of controls (normal pregnancy) and cases (pre-eclampsia). Haematoxylin and eosin stain (×20 magnification). (A) Normal women’s placenta shows mature term villi with an increased number of villous vessels and formation of vasculosyncytial membranes (arrows). (B) Pre-eclamptic women’s placenta show smaller villi for the gestational age and lesser in number with reduced villous vessels (black arrow) and fibrotic stroma (red arrows). A significant increase in the number of cytotrophoblastic cell hyperplasia was also seen (n=10).
The cellular expression of striatin was lower in the placenta of pre-eclamptic women (Fig. 2Ab) as compared to that in normal pregnant women (Fig. 2Aa). Furthermore, striatin positivity appeared both in the syncytiotrophoblast as well as perivascular layer of the placenta of normal pregnant women, whereas striatin expression was mostly observed in the perivascular region and was absent in syncytiotrophoblast layer of pregnant women with pre-eclampsia. Furthermore, the placental striatin protein expression was significantly (P<0.05) lower in pre-eclamptic women compared to normal pregnant women (Fig. 2B).
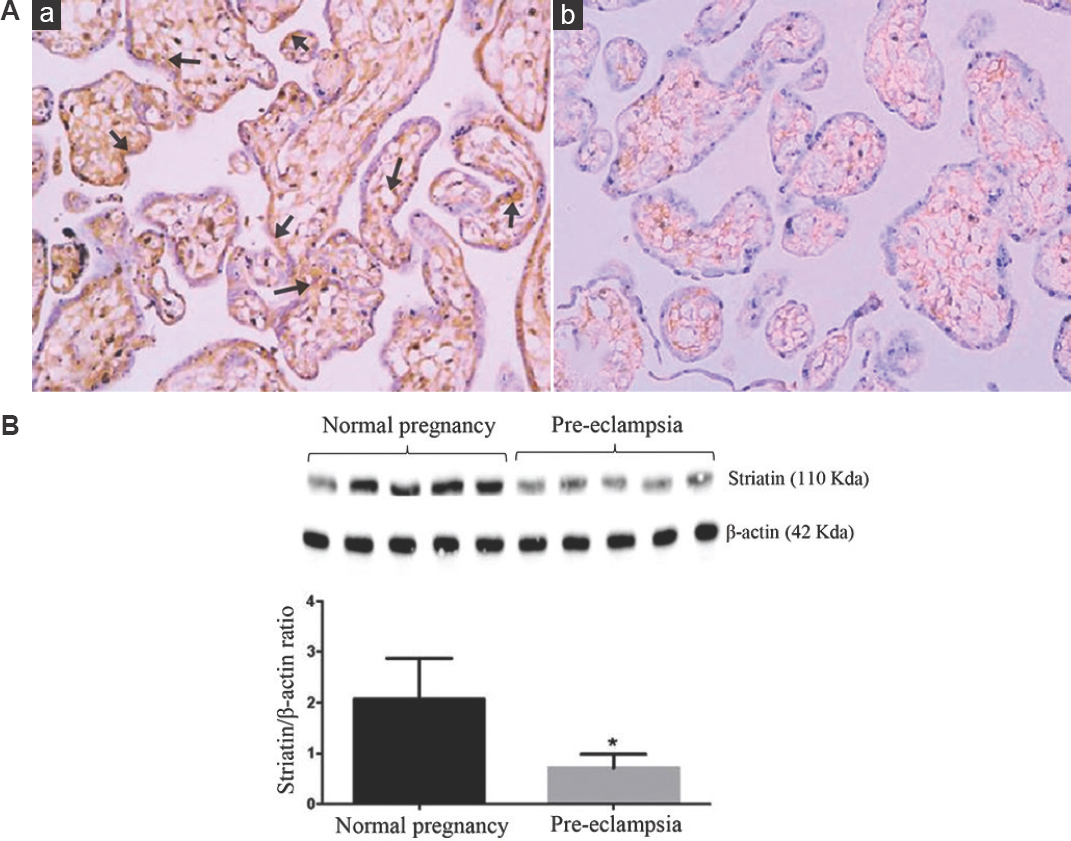
- (A) Representative immunostaining of striatin (brown) in placental tissues from (a) normal, and (b) pre-eclampsia groups (×20 magnifications). Normal placenta showed increased striatin expression around the syncytiotrophoblast layer and perivascular layer. Pre-eclamptic women’s placenta shows decreased striatin cellular expression, particularly around the perivascular layer, and was absent in the syncytiotrophoblast layer (n=3). (B) Western blot analyses of striatin in placental tissues from normal women and those with pre-eclampsia (n=5). P *<0.05 compared to normal placenta.
NO-synthesizing enzyme such as phosphorylated endothelial nitric oxide synthase (peNOS) protein expression was significantly lower in the placental tissue of pre-eclamptic women as compared to the normal pregnant women (Fig. 3A). By contrast, iNOS protein expression was significantly higher in the placental tissue of pre-eclamptic women as compared to normal pregnant women (Fig. 3B).
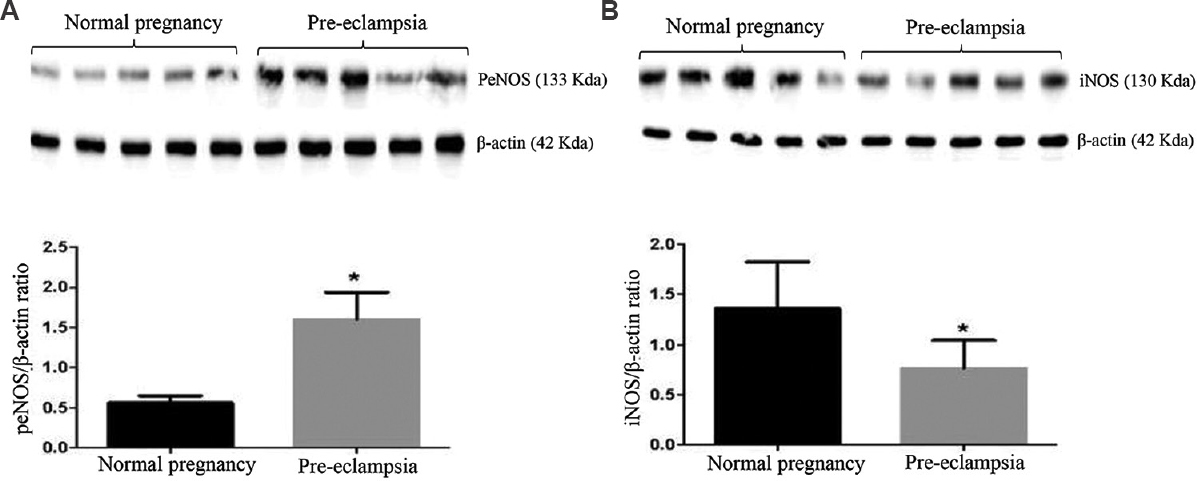
- Western blot analyses of (A) peNOS, and (B) iNOS in placental tissues from normal women and those with pre-eclampsia (n=5). P *<0.05 compared to normal placenta. peNOS, phosphorylated endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase
An inflammatory marker such as p65NF-κB protein expression was significantly higher in the placental tissue of pre-eclamptic women compared to normal pregnant women (Fig. 4).
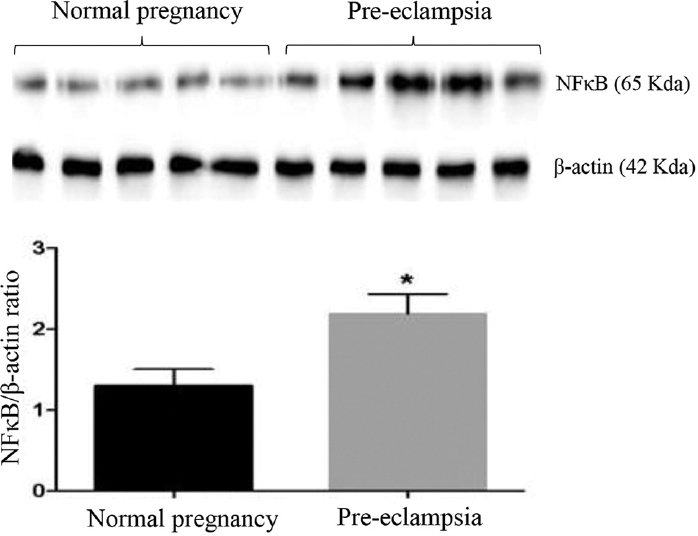
- Western blot analyses of NF-κB in placental tissues from normal women and those with pre-eclampsia (n=5). P *<0.05 compared to normal placenta.
Discussion
An important observation of the present study was the significantly lower placental striatin protein expression in pre-eclamptic women compared to normal pregnant women. This decrease in placental striatin expression was accompanied by a decreased placental peNOS protein expression observed in pre-eclampsia. However, serum striatin and NO concentrations were not altered between normal pregnancy and pre-eclampsia. In this context, a previous study suggested that striatin plays a vital role in both localizing and organizing ER-α complex and thus enabling oestrogen hormone in activating eNOS in a non-genomic manner15. Interestingly, the level of oestrogen in early-onset severe pre-eclamptic mothers was reduced19. In vascular cells, striatin was shown to interact with mineralocorticoid receptors and modulate the diameter of blood vessels, thereby decreasing BP20. Further, it has been reported that mineralocorticoid receptors were found to be significantly reduced in pre-eclamptic women21 and this gave substantial evidence that striatin may be involved in regulating NO bioavailability in pre-eclampsia. Our findings were consistent with the above studies that lower placental striatin expression was accompanied by a decreased placental peNOS expression in pre-eclampsia. This was further confirmed by immunohistochemistry, wherein normal healthy placenta, the appearance of striatin, was found around the syncytiotrophoblast and perivascular layers. Furthermore, cellular localization of striatin occured in pre-eclamptic placenta, particularly in the perivascular layer, and was absent in the syncytiotrophoblast layer. However, serum striatin and NO concentrations were not altered between controls and cases. The current study is also consistent with our earlier observation in human liver cirrhosis that significantly decreased striatin expression in liver tissue and was positively correlated with decreased eNOS and associated NO synthesis17. Moreover, hepatic tissue-mediated eNOS-driven NO has a major role in regulating portal hypertension in cirrhosis. However, increased plasma NO in cirrhotic patients has no effect22. Similarly, in this study, decreased striatin expression may lead to reduced placental eNOS expression and thus increased pressure in pregnant women in particularly pre-eclamptic cases.
Typical villous and vascular histological lesions of the placenta and impaired trophoblastic invasion are the common findings in the placenta of pre-eclamptic women23. Pre-eclampsia is considered a syndrome, more than a disease since it has the potential to affect many organs in the body. It is an obstetric complication characterized mainly by pregnancy-related, new-onset hypertension and significant proteinuria. It can potentially affect other organ systems, compromising normal vascular adaptations of pregnancy, leading to hepatic, renal, cerebral and coagulation dysfunctions24.
Increased BP, proteinuria and systemic and placental inflammation are associated with endothelial dysfunction in pre-eclampsia25. Thus, abnormal placentation and endothelial dysfunction are known to play a fundamental role in the development of pre-eclampsia. The endothelium-derived vasodilator NO is known to play an important role in maintaining vascular tone22. NO directly activates calcium-dependent potassium channels, leading to hyperpolarization of vascular smooth muscle cells, resulting in vasodilatation. In a normal pregnancy, NO production increases by eNOS to potentiate uteroplacental perfusion. Moreover, previous studies have identified diverse results concerning the levels of NO in pre-eclampsia. We found insignificantly decreased systemic NO concentrations in pre-eclampsia, and this may be due to decrease in placental peNOS protein expression. Moreover, lower striatin expression during pre-eclampsia may have an impact on the decrease in eNOS protein expression observed in this study. In this context, a previous study26 also showed that eNOS expression was detectable in the normal healthy placenta, in particular, endothelium of the umbilical cord, stem villous vessels and chorionic plate. However, as expected, increased iNOS expression was found in the placental tissue of pre-eclamptic women. This is in line with a previous study showing increased iNOS expression, which is associated with the pathogenesis of pre-eclampsia27. Moreover, iNOS placental localization was found in the villous stroma and extravillous trophoblasts of pre-eclamptic women28. Furthermore, it was shown earlier that increased iNOS-driven NO may react with the superoxide anion leading to the formation of peroxynitrite resulting placental cellular dysfunction22.
A previous study showed that placental NF-κB was found to be abnormally elevated in pre-eclampsia due to oxidative stress29. Our finding was in agreement with the previous studies, showing significantly elevated NF-κB protein expression in the placenta of pre-eclamptic women. Yang et al30 observed that increased toll-like receptor 4 expression was positively correlated with maternal systemic and placental inflammation, mediated through NF-κB. The upregulation of iNOS expression due to circulating proinflammatory cytokines has also been shown in pre-eclampsia31. The findings of the present study were found to be consistent with the results of previous studies stating that iNOS expression was found to be significantly elevated in the placenta of pre-eclamptic women. Since there is a significant elevation of inflammatory markers in the placenta of pregnant women who suffer from pre-eclampsia, one may speculate that placental inflammation could lead to the downregulation of striatin-eNOS-mediated NO release in the placental tissues of pregnant women and thus pre-eclampsia.
In pre-eclampsia, reduced endovascular trophoblast invasion and impaired vascular remodelling of spiral arteries contribute to the pathogenesis16. Due to ischaemia of spiral arteries, there is reduced uteroplacental perfusion leading to placental hypoxia6. An ischaemic placenta may release several substances, including cytokines and reactive oxygen species (ROS), which could initiate vascular and endothelial dysfunction6. ROS and inflammation synergistically inhibit NO synthesis and may cause endothelial dysfunction.
Overall this study demonstrated that decreased placental tissue striatin expression and decreased peNOS expression may cause a decrease in NO bioavailability in the placentae of pre-eclamptic women. Thus, therapies which increase placental tissue striatin could be considered attractive possibilities for both the prevention and treatment of pre-eclampsia.
Financial support and sponsorship
This work was financially supported by the JIPMER intramural research grant (JIP/RES/INTRA-MD-MS/PHS1/01/2017-18) and partially by the Ramalingaswami Re-entry Fellowship (102/IFD/SAN/22/2013-14) from DBT awarded to author BV.
Conflicts of interest
This abstract was previously published in the 45th National Conference of ACBICON 2018.
References
- Preeclampsia and the future risk of hypertension:the pregnant evidence. Curr Hypertens Rep. 2013;15:114-21.
- [Google Scholar]
- Endothelial dysfunction and preeclampsia: Role of oxidative stress. Front Physiol. 2014;5:372.
- [Google Scholar]
- Prediction of adverse maternal outcomes from pre-eclampsia and other hypertensive disorders of pregnancy: A systematic review. Pregnancy Hypertens. 2018;11:115-23.
- [Google Scholar]
- Sexual dimorphisms of preeclampsia-dysregulated transcriptomic profiles and cell function in fetal endothelial cells. Hypertension. 2019;74:154-63.
- [Google Scholar]
- Serum podocalyxin is significantly increased in early-onset preeclampsia and may represent a novel marker of maternal endothelial cell dysfunction. J Hypertens. 2017;35:2287-94.
- [Google Scholar]
- Endothelial eNOS/arginase imbalance contributes to vascular dysfunction in IUGR umbilical and placental vessels. Placenta. 2013;34:20-8.
- [Google Scholar]
- Placental expression of eNOS, iNOS and the major protein components of caveolae in women with pre-eclampsia. Placenta. 2015;36:607-10.
- [Google Scholar]
- Genetic susceptibility to pre-eclampsia and chromosome 7q36. Hum Genet. 1999;105:641-7.
- [Google Scholar]
- The eNOS-NO pathway attenuates kidney dysfunction via suppression of inflammasome activation in aldosterone-induced renal injury model mice. PLoS One. 2018;13:e0203823.
- [Google Scholar]
- The nitric oxide pathway and possible therapeutic options in pre-eclampsia. Br J Clin Pharmacol. 2014;78:244-57.
- [Google Scholar]
- Expression levels of cyclooxygenase-2, tumor necrosis factor-αand inducible NO synthase in placental tissue of normal and preeclamptic pregnancies. J Matern Fetal Neonatal Med. 2012;25:826-30.
- [Google Scholar]
- Cloning of human striatin cDNA (STRN), gene mapping to 2p22-p21, and preferential expression in brain. Genomics. 1998;51:136-9.
- [Google Scholar]
- Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc Natl Acad Sci U S A. 2004;101:17126-31.
- [Google Scholar]
- Critical role of striatin in blood pressure and vascular responses to dietary sodium intake. Hypertension. 2015;66:674-80.
- [Google Scholar]
- Utility of STRIATIN as novel prognostic marker in decompensated cirrhosis. Hepatol Int. 2019;13:S1-266.
- [Google Scholar]
- Fundamentals of clinical chemistry 1987:676.
- The reduction in circulating levels of estrogen and progesterone in women with preeclampsia. Pregnancy Hypertens. 2018;11:18-25.
- [Google Scholar]
- Variants in striatin gene are associated with salt-sensitive blood pressure in mice and humans. Hypertension. 2015;65:211-7.
- [Google Scholar]
- Mineralocorticoid effector mechanism in preeclampsia. J Clin Endocrinol Metab. 1992;74:946-9.
- [Google Scholar]
- Post-transcriptional regulation of hepatic DDAH1 with TNF blockade leads to improved eNOS function and reduced portal pressure in cirrhotic rats. Sci Rep. 2017;7:17900.
- [Google Scholar]
- Placental histopathology associated with pre-eclampsia: Systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50:295-301.
- [Google Scholar]
- Endothelial nitric oxide synthase immunreactivity and the ultrastructure of endothelial cells of umbilical artery in normal and preeclamptic pregnancies. Clin Exp Hypertens. 2010;32:458-63.
- [Google Scholar]
- From pregnancy to preeclampsia: A key role for estrogens. Endocr Rev. 2017;38:123-44.
- [Google Scholar]
- Systemic and fetal-maternal nitric oxide synthesis in normal pregnancy and pre-eclampsia. Br J Obstet Gynaecol. 1996;103:879-86.
- [Google Scholar]
- eNOS/iNOS and endoplasmic reticulum stress-induced apoptosis in the placentas of patients with preeclampsia. J Hum Hypertens. 2017;31:49-55.
- [Google Scholar]
- Nitric oxide synthase activity and localization do not change in uterus and placenta during human parturition. Hum Reprod. 1997;12:2546-52.
- [Google Scholar]
- Activation of NF-κB in placentas of women with preeclampsia. Hypertens Pregnancy. 2012;31:243-51.
- [Google Scholar]
- Causal relationship between obesity-related traits and TLR4-driven responses at the maternal-fetal interface. Diabetologia. 2016;59:2459-66.
- [Google Scholar]
- Antihypertensive effects of inducible nitric oxide synthase inhibition in experimental pre-eclampsia. J Cell Mol Med. 2013;17:1300-7.
- [Google Scholar]






