Translate this page into:
Delayed adverse reactions in whole blood donors: Importance of active surveillance in identifying the missing gaps in the donor safety
For correspondence: Dr Meenu Bajpai, Department of Transfusion Medicine, Institute of Liver & Biliary Sciences, New Delhi 110 070, India e-mail: meenubajpaii@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The information available regarding delayed adverse donor reactions (D-ADRs) is limited. Proactive follow up of donors for delayed reactions is not done routinely. This study was undertaken to analyze frequency and type of D-ADRs in whole blood donors as also the contributory factors.
Methods:
In this prospective observational study, all eligible whole blood donors were contacted telephonically twice (24 h and 2 wks after donation) and asked about general health and ADR specific questions. The International Society of Blood Transfusion standard guidelines were used to categorize ADRs.
Results:
The ADR data of 3514 donors were analyzed in the study. D-ADRs were more common as compared to immediate delayed adverse donor reactions (I-ADRs) (13.7 vs. 2.9%, P<0.001). The most common D-ADRs were bruises (4.98%), fatigue or generalized weakness (4.24%) and sore arms (2.25%). D-ADRs were more common in first time donors as compared to the repeat blood donors (16.1 vs. 12.5%, P=0.002). Females were more prone to D-ADRs (17 vs. 13.6%). Localized D-ADRs were more frequent as compared to systemic D-ADRs (P<0.001). Repeat donors had a lower incidence of systemic D-ADRs (4.11% vs. 7.37%, P<0.001).
Interpretation & conclusions:
D-ADRs were more common than I-ADRs with a different profile. First time, female and young donors were more prone to D-ADRs. These categories need special care at the time of blood donation. Active follow up of blood donors should be done from time to time to strengthen donor safety.
Keywords
Adverse donor reactions
blood donors
delayed reactions
donor safety
haematoma
vaso-vagal reactions
weakness
A healthy donor can donate 350-450 ml of blood, depending on the body weight1. This amount of blood loss is well tolerated without any serious adverse donor reactions (ADRs). Occasionally, ADRs of variable severity may occur during or after the donation2-4. Most ADRs occur within 30 min of starting a blood donation and are usually managed by simple measures2,3. ADRs contribute to a negative donation experience even though these are usually mild and transient5,6.
ADRs can be classified as immediate and delayed donor reactions7. Immediate adverse donor reactions (I-ADRs) occur before, during or just after donation (on-site, usually within 30 min from the start of blood donation), while delayed adverse donor reactions (D-ADRs) may occur at any time (off-site) up to two-three weeks of donation8-12.
There is an abundance of literature on risk factors and donor characteristics of I-ADRs, but there is limited information regarding D-ADRs8,11. The possible reasons may be that the majority of D-ADRs are usually mild and not reported to the blood transfusion services (BTSs). This study was aimed to analyze the frequency and type of D-ADRs in blood donors and their relation with contributory factors, if any. The study was also aimed to compare I-ADRs and D-ADRs in terms of the nature of reactions, contributory factors and donors at risk.
Material & Methods
This prospective observational study was conducted at the blood transfusion service (BTS) of the Institute of Liver and Biliary Sciences, New Delhi, India, with due approval from the Institutional Ethics Committee. The study was carried out over five months from October 2018 to February 2019. All successive whole blood donors who gave informed written consent for the study were included in the study.
The sample size was decided using data from a pilot study and the studies by others8,11. The incidence of D-ADRs in their studies was 36.1 and 10.3 per cent, respectively. The power of the study was 95 per cent. For a finite population size of one million, with an anticipated per cent frequency of 36.1 per cent8, absolute precision of five per cent and random sample design effect, the sample size was 1396, at a 99.99 per cent confidence level. Larger sample size was included in the study for better results.
The criteria of the standard for surveillance of complications related to blood donation by the working group on donor vigilance of the International Society of Blood Transfusion working party on haemovigilance7 were used for the classification of the ADRs. Documentation of any I-ADRs was done as per the departmental standard operating procedure. Subsequently, telephonic interviews of blood donors were conducted on two occasions to enquire regarding D-ADRs. The first telephonic interview was done after 24 h of blood donation, while the second was done after two weeks. At the start of the interview, donors were asked non-specific questions regarding health and general well-being and any unpleasant experience or discomfort during or after the donation. Blood donors were then asked specific questions regarding any possible D-ADRs experienced by them from a structured questionnaire, which was exclusively generated for the study. Self validation of the questionnaire was done with a pilot study and previous studies8-11. In the case of any D-ADRs, donors were counselled and relevant medical advice was given. A flow diagram of the study design is represented in the Figure.
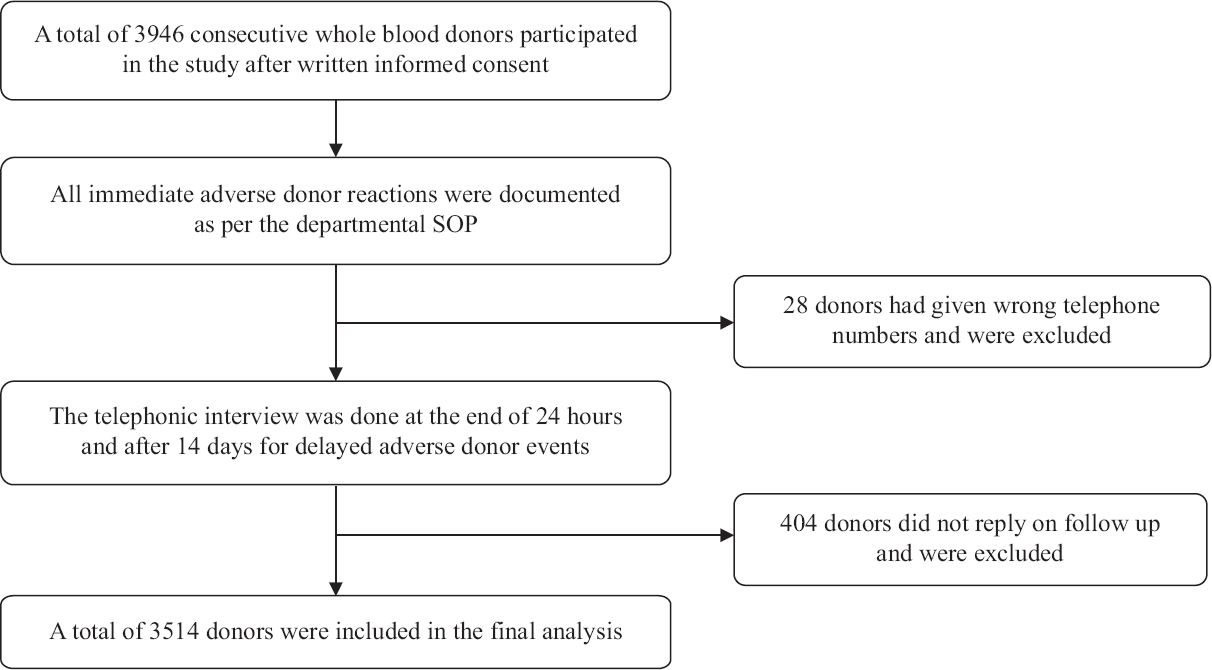
- Schematic flow diagram of the study design. SOP, standard operation procedure
Statistical analysis: The data were computerized using Microsoft Excel spreadsheet, and statistical analysis was carried out using statistical software SPSS for Windows, Version 23.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics such as mean, standard deviation and range values were calculated for normally distributed continuous variables. Categorical data were expressed as frequency and per cent values. Frequency data across categories were compared using Chi-square/Fisher’s exact test as appropriate. Unadjusted odds ratios (ORs) with 95 per cent confidence limits were calculated to assess the possible risk factors for both I-ADR and D-ADR.
For the purpose of analysis, blood donors were grouped in the following categories: first-time vs. repeat donors, male vs. female donors, age groups (18-24, 25-35, 36-50 and ≥51 yr), haemoglobin groups (12.5-14, 14.1-16 and ≥16.1 gm/dl), weight groups (≤60, 61-75, 76-90 and ≥91 kg) and duration of blood donation (≤8 or ≥9 min).
Results
Initially, a total of 3946 blood donors consented to participate in the study, but 28 donors had given wrong telephone numbers and 404 donors did not respond to both telephonic interviews and were excluded; thus, a total of 3514 donors were included for the final analysis (Figure). The donors were predominantly male (97.15%) and repeat donors (66.39%). The mean age of donors was 30.62 ± 15.64 (range, 18-62) yr. The demographic profile of the donors is shown in Table I.
| Donor characteristics | Age (yr) | Weight (kg) | Hb (gm/dl) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18-24 | 25-35 | 36-50 | ≥51 | Mean±SD | ≤60 | 61-75 | 76-90 | ≥91 | Mean±SD | 12.5-14 | 14.1-16 | 16.1-18 | Mean±SD | |
| Overall donors (n=3514), n (%) | 821 (23.4) | 1865 (53.1) | 762 (21.7) | 66 (1.9) | 30.62±7.82 | 339 (9.7) | 1587 (45.2) | 1212 (34.5) | 376 (10.7) | 75.75±12.20 | 971 (27.6) | 1874 (53.3) | 66 (19.0) | 14.87±1.26 |
| Males (n=3414), n (%) | 792 (22.5) | 1825 (51.9) | 737 (21.0) | 60 (1.7) | 30.61±7.77 | 303 (8.6) | 1538 (43.8) | 1200 (34.2) | 373 (10.6) | 76.03±12.13 | 883 (25.1) | 1864 (53.1) | 667 (19.0) | 14.92±1.24 |
| Females (n=100), n (%) | 29 (0.8) | 40 (1.1) | 25 (0.7) | 6 (0.2) | 31.18±9.52 | 36 (1.0) | 49 (1.4) | 12 (0.3) | 3 (0.1) | 66.13±10.75 | 88 (2.5) | 10 (0.3) | 2 (0.1) | 13.31±0.76 |
| First-time donors (n=1181), n (%) | 422 (12.0) | 577 (16.4) | 169 (4.8) | 13 (0.4) | 28.19±7.33 | 193 (5.5) | 593 (16.9) | 322 (9.2) | 73 (2.1) | 72.01±11.37 | 323 (9.2) | 625 (17.8) | 233 (6.6) | 14.92±1.28 |
| Repeat donors (n=2333), n (%) | 399 (11.4) | 1288 (36.7) | 593 (16.9) | 53 (1.5) | 31.86±7.76 | 146 (4.2) | 994 (28.3) | 890 (25.3) | 303 (8.6) | 77.64±12.18 | 648 (18.4) | 1249 (35.5) | 436 (12.4) | 14.85±1.24 |
Immediate adverse donor reactions: A total of 103 [2.93%, 95% confidence interval (CI) = 2.42%-3.55%] blood donors experienced one or more I-ADRs. The most common I-ADR was haematoma formation, which was observed in 1.91 per cent (n=67) of the donors, followed by vaso-vegal reactions (VVRs) with an incidence of 1.14 per cent (n=40). Two donors had an episode of vomiting during VVRs. Four donors presented with both VVRs and haematoma formation. Haematomas were more common in the higher weight group (3.99%, weight ≥91 kg) donors and lower weight group donors (2.95%, weight ≤60 kg) as compared to the mid-weight range (1.5%, P<0.001).
VVRs were reported by 1.14 per cent (n=40) of the donors. The majority of VVRs (85%, n=34) were not associated with loss of consciousness (LOC), 12.5 per cent (n=4) donors had VVRs with LOC ≤60 sec and only one donor presented with VVRs associated with LOC and convulsions. Younger age, first time, female and low body weight donors were more prone to VVRs (Table II).
| Type of VVRs | Immediate VVRs | Delayed VVRs | ||||||
|---|---|---|---|---|---|---|---|---|
| Without LOC (n=34) | With LOC ≤60 sec (n=5) | With convulsions (n=1) | Overall (n=40), n (%) | Without LOC (n=31) | With LOC ≤60 sec (n=3) | With convulsions/complications (n=0) | Overall (n=34), n (%) | |
| Males (3414) | 29 | 5 | 1 | 35 (1.03) | 27 | 3 | 0 | 30 (0.88) |
| Females (100) | 5 | 0 | 0 | 5 (5) | 4 | 0 | 0 | 4 (4) |
| P | 0.005 | 0.999 | 0.999 | 0.010 | 0.021 | 0.999 | - | 0.030 |
| First time donors (1181) | 14 | 3 | 1 | 18 (1.52) | 19 | 3 | 0 | 22 (1.86) |
| Repeat donors (2333) | 20 | 2 | 0 | 22 (0.94) | 12 | 0 | 0 | 12 (0.51) |
| P | 0.348 | 0.428 | 0.672 | 0.125 | 0.001 | 0.076 | - | <0.001 |
| EBV≤4500 ml (n=561) | 10 | 0 | 0 | 10 (1.78) | 8 | 0 | 0 | 8 (1.42) |
| EBV ≤4500 ml (n=2953) | 24 | 5 | 1 | 30 (1.02) | 23 | 3 | 0 | 26 (0.88) |
| P | 0.031 | 0.838 | 0.999 | 0.117 | 0.133 | 0.999 | - | 0.227 |
VVRs, vaso-vagal reactions; LOC, loss of consciousness; EBV, expected blood volume
Delayed adverse donor reactions: A total of 481 (13.69%, 95% CI = 12.59-14.86%) donors reported 540 D-ADRs. Among them, the majority of donors (87.7%) reported a single D-ADR, while a few donors (12.3%) reported more than one D-ADRs. Among the D-ADRs, bruising (4.98%, n=175) was the most common D-ADR followed by fatigue or generalized weakness (4.24%, n=149), sore arms (2.25%, n=79), haematomas (1.22%, n=43), local allergic reactions or itching at the site of phlebotomy (1.02%, n=36), VVRs (0.97%, n=34) and delayed bleeding (0.68%, n=24). The distribution of D-ADRs is shown in Table III. The D-ADRs were more frequent than I-ADRs (13.69 vs. 2.93%, P<0.001; OR = 5.25, 95% CI = 4.22-6.53).
| Type of donors | Overall D-ADRs | Localized D-ADRs | Systemic D-ADRs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bruise | Sore arms | Haematoma | Allergic reactions | Delayed bleeding | Overall, n (%) | Fatigue/weakness | VVRs | Overall, n (%) | ||
| Male (3414) | 464 | 171 | 76 | 42 | 36 | 24 | 294 (8.61) | 143 | 30 | 173 (5.07) |
| Female (n=100) | 17 | 4 | 3 | 1 | 0 | 0 | 7 (7) | 6 | 4 | 10 (10) |
| P | 0.328 | 0.874 | 0.784 | 0.999 | 0.704 | 0.997 | 0.570 | 0.497 | 0.030 | 0.029 |
| First time donors (n=1181) | 190 | 59 | 32 | 16 | 12 | 8 | 104 (8.81) | 65 | 22 | 87 (7.37) |
| Repeat donors (n=2333) | 291 | 116 | 47 | 27 | 24 | 16 | 197 (8.44) | 84 | 12 | 96 (4.11) |
| P | 0.003 | 0.975 | 0.190 | 0.615 | 0.972 | 0.977 | 0.717 | 0.008 | <0.001 | <0.001 |
| Overall (n=3514), n (%) | 481 (13.69) | 175 (4.98) | 79 (2.25) | 43 (1.22) | 36 (1.02) | 24 (0.68) | 301 (8.57) | 149 (4.24) | 34 (0.97) | 183 (5.21) |
| Newman et al8 (n=1000) (%) | 36.1 | 22.7 | 10 | 1.7 | - | - | - | 7.8 | 5.3 | - |
| Tiwari et al11 (n=1095) (%) | 10.3 | 3.5 | 2.1 | 1 | 1.1 | 0.5 | - | 1.6 | 0.6 | - |
Cumulative and individual D-ADRs percentage may differ as 12.7% of donors reported more than one D-ADRs. D-ADRs, delayed adverse donor reactions; VVRs, vaso-vagal reactions
Localized D-ADRs: Bruising was reported by 4.98 per cent (n=175) of the donors. This included donors with delayed or immediate haematoma formation. Bruising was more common in donors weighing ≥91 kg (8.36 vs. 4.65%, P=0.009). Sore arm or pain at the phlebotomy site was reported by 2.25 per cent (n=79) of the donors. Delayed haematomas were reported by 1.22 per cent (n=43) of the donors. Delayed bleeding from the phlebotomy site was reported by 0.68 per cent (n=24) of the blood donors. Thirty six (1.02%) blood donors reported an allergic reaction or itching at the site of phlebotomy. Twelve donors (33.33%) were first-time donors, while 24 (66.67%) were repeat donors (Table III).
Systemic D-ADRs: Fatigue or generalized weakness was encountered by 4.24 per cent (n=149) of the donors. The incidence of fatigue in first-time donors (5.5%, n=65) was significantly higher (P=0.008) than in repeat donors (3.6%, n=84). Thirty-four (0.97%) donors reported VVRs after they left the BTS. VVRs without LOC were the most commonly reported VVRs (91.18%, n=31), followed by VVRs with LOC ≤60 sec (8.82%, n=3). No VVRs with convulsions or any other complications were reported. Most delayed VVRs occurred within six hours of blood donation (76.47%, n=26), while four VVRs (11.76%) occurred from 6 to 24 h of donation. Two VVRs (5.88%) occurred between 24 and 48 h of donation. First-time donors experienced more delayed VVRs (1.86%, n=22), as compared to repeat donors (0.51%, n=12, P<0.001; OR = 3.67 with 95% CI = 1.81-7.44). Delayed VVRs were more common in donors with expected blood volume ≤4500 ml (1.42% vs. 0.88%, P=0.227; OR = 1.63 with 95% CI = 0.73-3.62) (Table II).
Factors associated with D-ADRs: D-ADRs were more common in first-time as compared to repeat donors (16.1 vs. 12.5%, P=0.003; OR = 1.35 with 95% CI = 1.10-1.64) similar to I-ADRs. The reason for this could be that repeat blood donors were familiar with the donation experience, thus less anxious. Repeat donors had a lower incidence of systemic D-ADRs (4.11 vs. 7.37%, P<0.001; OR = 0.54 with 95% CI = 0.40-0.72), while localized D-ADRs did not show any such predilection (8.44 vs. 8.81%, P=0.717; OR = 0.96 with 95% CI = 0.75-1.23). Female gender was an independent risk factor for both immediate and delayed VVRs (P<0.001); they had more systemic D-ADRs (10 vs. 5.07%, P=0.029; OR = 2.08 with 95% CI = 1.06-4.07), which was probably related to low body weight and a higher percentage of first-time donors among the female donors (44 vs. 33.3%, P=0.010).
A total of nine donors reported fever after blood donation. Fever was reported from the first day to the tenth of the day of donation and was not associated with any further complications. In all the cases, the fever subsided without any diagnosis and need for hospitalization. One donor reported diarrhoea on day three after donation and one donor reported blisters all over the body at day five. All these donors did not report any long-term complications/consequences.
Discussion
The presence of delayed donor reactions was 4-5 times higher than I-ADRs. The D-ADRs in the present study were comparable to the study by Tiwari et al11 (10.3%) in the Indian population and much lower than reported in the study by Newman et al8 (36.1%) in the western population. The profile of D-ADRs was different from I-ADRs. Haematomas (1.91%) and VVRs (1.14%) were the most common I-ADRs, whereas bruising (4.98%) and fatigue or generalized weakness (4.24%) were the most common D-ADRs.
Haematoma formation is usually mild and donors normally do not report or document them to the BTS. As this study was a prospective observational study, all I-ADRs including haematomas were carefully recorded giving a higher incidence of haematoma formation (1.91%) as compared to other similar studies (0.21-0.88%)2-4.
Immediate haematomas were more common in lower (≤60 kg, 2.95%) and higher body weight (≥91 kg, 3.99%) donors compared to mid might donors. One of the reasons for this could be that low weight group donors have lower subcutaneous fat and there are more chances of the needle piercing the vein through and through. On the other hand, high weight group donors have more subcutaneous fat; thus, the veins are situated deeper, which makes identification of veins for phlebotomy more difficult. The cause for the delayed haematomas may be sports activities, strenuous physical activity, lifting of bags/luggage or re-trauma to the phlebotomy site.
VVRs are alarming and negatively impact the donor return rate6. Although the majority of the delayed VVRs had occurred in less than six hours after blood donations, but these were documented up to 48 h after blood donation. Though delayed VVRs are usually mild, the donor is at risk of sustaining injuries due to a fall or they may have serious implications if the donor is working on activities requiring attention and precision such as tasks involving machinery or during driving.
Allergic reactions, sore arms and post-donation fatigue or generalized weakness are some of the other D-ADRs, which are usually not encountered during and post-donation in the blood donation premises. BTS staff must be trained to manage and give advice on D-ADRs and refer them to the BTS doctor. Approximately one per cent of the donors reported allergic reactions at the site of phlebotomy. Donors may be sensitive to the medicated adhesive bands, antiseptics used for disinfection or metal alloy of the needle13. These may be the causes for the delayed allergic reaction in blood donors13.
The post donation weakness or fatigue was more common in first time donors. The lower incidence of fatigue as compared to the study by Newman et al8 in the western population (4.2 vs. 7.8%) may be related to differences in gender proportions (97.2 vs. 44.7% males) and ethnicity of the two study populations8. Despite having same-gender proportion (97.2 vs. 97.1% males), Tiwari et al11 reported a lower incidence of fatigue in the Indian population as compared to the current study (1.6 vs. 4.2%). This may be related to the timing of the telephonic interviews. In these studies8,11, the authors called the donors directly after 21 days as compared to the present study, in which donors were called after 24 h and 14 days of blood donation resulting in better recall of D-ADRs in the donors.
The “Grading Severity of Blood Donor Adverse Events tool by AABB Donor Haemovigilance Working Group’’ was used for categorization of ADRs14. All the I-ADRs were grade I and all the D-ADRs were grade II. A similar study done on plateletpheresis donors has shown the equivalent results15. The D-ADRs has also resulted in decreased intention for future donations16.
In this study donors were contacted twice, i.e. after 24 h and after 14 days of blood donation. The strategic timing of the ‘post-donation calls’ helped in better recall of information regarding D-ADRs. D-ADRs such as VVRs, fatigue, sore arms and bleeding were detected on the 24 h call and the majority of bruises were detected at the 14 day call. The study had some limitations. The D-ADRs were identified by the subjective interpretation of the blood donor rather than objective assessment by medical personnel. This may have caused false underreporting or overreporting of certain D-ADRs like fatigue. The number of female donors represented a very small proportion of the study; therefore, it was difficult to draw convincing gender-specific conclusions. The blood donations were done in a hospital blood bank setting under highly controlled conditions and results might be different if donations were done at outdoor blood donation camps.
Our results showed that blood donation was a relatively safe procedure without any significant immediate or delayed donor complications. D-ADRs were more common than I-ADRs. First-time, female and younger age donors were more prone to D-ADRs and need special care during donation and post-donation advice. Active follow up of blood donors should be done from time to time to strengthen donor safety. Povidone-iodine and medicated adhesive bands should be avoided in donors with a prior history of allergy to iodine and topical antibiotics, respectively17. Implementation and strengthening of the D-ADRs reporting system should be done at all BTS.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Ministry of Health and Family Welfare, Government of India. Transfusion medicine technical manual (2nd ed). India: DGHS, MoHFW, GoI; 2003.
- Analysis of adverse events and predisposing factors in voluntary and replacement whole blood donors: A study from north India. Asian J Transfus Sci. 2012;6:155-60.
- [Google Scholar]
- On-site and off-site adverse donor reactions in voluntary whole blood donors: A study from a tertiary care oncology center. Glob J Transfus Med. 2019;4:28-32.
- [Google Scholar]
- The influence of adverse reactions, subjective distress, and anxiety on retention of first-time blood donors. Transfusion. 2013;53:337-43.
- [Google Scholar]
- Adverse reactions and other factors that impact subsequent blood donation visits. Transfusion. 2012;52:118-26.
- [Google Scholar]
- Standard for surveillance of complications related to blood donation. Available from: https://www.isbtweb.org/resource/standardfor surveillance of complicationsrelatedtoblooddonation.html
- Adverse effects in blood donors after whole-blood donation: A study of 1000 blood donors interviewed 3 weeks after whole-blood donation. Transfusion. 2003;43:598-603.
- [Google Scholar]
- Adverse effects in blood donors after whole-blood donation: You find what you look for! Transfusion. 2004;44:135-6.
- [Google Scholar]
- Post donation adverse reactions among Greek blood donors: A preliminary report based on phone interviews. Transfus Apher Sci. 2009;41:77-8.
- [Google Scholar]
- Post-donation telephonic interview of blood donors providing an insight into delayed adverse reactions: First attempt in India. Transfus Apher Sci. 2017;56:141-6.
- [Google Scholar]
- Are medicated adhesive bands at the phlebotomy site useful? Indian J Hematol Blood Transfus. 2009;25:86-7.
- [Google Scholar]
- 2018. Donor Hemovigilance Working Group. AABB; Available from: http://www.aabb.org/research/hemovigilance/Documents/Donor-Adverse-Reaction-Severity-Grading-Tool.pdf
- Delayed adverse events in male plateletpheresis donors: Initial insights on donor safety. J Clin Apher. 2020;35:18-24.
- [Google Scholar]
- The impact of delayed and immediate adverse events on the intention of future donations in whole blood and plateletpheresis donors. J Clin Apher. 2021;36:621-7.
- [Google Scholar]
- A comparison of the irritant and allergenic properties of antiseptics. Eur J Dermatol. 2014;24:3-9.
- [Google Scholar]






