Translate this page into:
Detection of paroxysmal nocturnal haemoglobinuria clones in cases of deep vein thrombosis in a tertiary care centre, western Rajasthan
For correspondence: Dr Abhishek Purohit, Department of Pathology & Laboratory Medicine, All India Institute of Medical Sciences, Basni Industrial Area Phase-II, Jodhpur 342 005, Rajasthan, India e-mail: purohitabhi80@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Paroxysmal nocturnal haemoglobinuria is a rare acquired disease characterized by bone marrow failure, intravascular haemolysis and thrombophilia. Thrombosis is the deadliest complication of paroxysmal nocturnal haemoglobinuria (PNH). The present study was conducted to study the prevalence of PNH in cases of deep vein thrombosis (DVT) which was previously undocumented from western Rajasthan.
Methods:
In the present cross-sectional study, 61 adult patients with DVT were tested using flow cytometry to detect PNH clones. Blood samples were processed using fluorescein-labelled proaerolysin, CD14, CD24, CD33 and CD45 panels for granulocytes and monocytes and CD59 and CD235a panel for red blood cells.
Results:
Three cases (4.92%) having large clones on monocytes as well as granulocytes, which fulfilled the diagnostic criteria of PNH were detected. Further, three cases (4.92%) showed small clones on both granulocytes and monocytes. Nine (15%) cases showed small clones only on granulocytes, and 11 (18%) cases showed small clones only on monocytes.
Interpretation & conclusions:
The results of the present study suggest that a higher proportion of patients had PNH in western Rajasthan compared to previously reported studies from elsewhere. It is suggested that PNH testing should be added to the procoagulant work-up panel in institutions of this region where it is not routinely done. This provides an otherwise missed opportunity to diagnose this disorder. Eculizumab may be employed, which is effective in reducing thrombophilic events in cases of PNH.
Keywords
Deep vein thrombosis
flow cytometry
fluorescein-labelled proaerolysin
paroxysmal nocturnal haemoglobinuria
thrombophilia
thrombosis
Paroxysmal nocturnal haemoglobinuria (PNH) is characterized by diverse clinical manifestations, including bone marrow failure, intravascular haemolysis and thrombophilia1. It is a rare disease with an estimated prevalence of 0.002 per cent in the western world2. Data pertaining to various epidemiological aspects of PNH from India is negligible. Even though PNH is uncommon, correct and timely diagnosis of suspected patients is vital, as PNH has a chronic course and may have a significant impact on the survival of any individual patient as well as their quality of life3.
Thrombosis is the primary cause of mortality in PNH, and it accounts for 40-67 per cent of all deaths4. Nearly 16-39 per cent of PNH patients from the West have thrombosis5. Higher incidence of thromboembolism is seen in patients that have larger PNH clones6,7; however, patients that have smaller clones also have an increased risk of thrombosis6.
Thrombosis provides a good model to do a population-based screening for PNH since deep vein thrombosis (DVT) occurs in one per cent of the adult Indian population after the age of 40 yr8. However, literature on the diagnosis of PNH in patients with a primary presentation of thrombosis is scarce from India and nil from western Rajasthan.
The present study screened for the presence of PNH clones by flow cytometric immunophenotyping in cases of DVT to study the prevalence of PNH in cases of DVT from western Rajasthan.
Material & Methods
The present cross-sectional study was conducted in the departments of General Medicine and Pathology & Laboratory Medicine, All India Institute of Medical Sciences, Jodhpur, a tertiary care centre of western Rajasthan, from August to October 2019. The study protocol was approved by the Institutional Ethics Committee.
A total of 61 adult patients, diagnosed with DVT, but without any provoking factors such as history of trauma, malignancy, recent pregnancy or hormonal therapy were included in the study after obtaining a written informed consent from all the study participants.
Sample collection and processing: Peripheral blood samples (3 ml) were collected and analyzed as per the protocol described earlier9. Complete blood count was done before processing each sample for immunophenotyping to ascertain the total leucocyte count (TLC).
The blood samples were processed directly if the observed TLC was within the normal range (TLC - 4000-11,000/mm3). In case of low TLC, the samples were centrifuged at 200 g for five minutes and excess plasma was removed, maintaining the amount of plasma the same as the red cell pellet. Samples were again mixed properly before processing. Two tubes were used: Tube A (for granulocytes and monocytes): fluorescein-labelled proaerolysin (FLAER)-Alexaflour488/CD45-KromeOrange(B36294)/CD33-APC(IM2471)/CD24-PC5.5(B23133)/CD14-PE (A07764); and Tube B (for RBCs): CD235a-APCAlexaF750(A89314)/CD59-PE(B68140). If a positive PNH clone was found on granulocytes as well as monocytes, processing was done for red blood cells as well.
Flow cytometric analysis: Processed samples were acquired on Navios Flow Cytometer (Beckman Coulter, USA) and a minimum of 50,000 events were acquired for each sample. Detailed dot blot analysis was performed on both the stained tubes.
After excluding doublets, cell populations were analyzed on CD45-side scatter population. With the help of the CD33-SideScatter plot, granulocytes and monocytes were gated separately and were analyzed further. Granulocytes were analyzed in the CD24- FLAER (fluorescein-labelled proaerolysin) plot and monocytes were analyzed in the CD14-FLAER plot. The presence of a PNH clone was defined when more than one per cent population was seen in the double-negative quadrants for both granulocytes and monocytes.
Before starting the testing facility, peripheral blood samples from ten normal healthy controls were processed. In these controls, PNH clone (more than 1.0% in size) was not found. At least 50,000 events were acquired for these control cases. Hence, a 50 cell cluster was fixed as a minimum to identify the PNH clone which aided in labeling cases with >0.1 per cent and <1 per cent as small PNH clone. Based on this premise, we did observe a double-negative clone size around 0.02-0.04 per cent in monocytes or granulocytes of some of these ten controls. However, in none of the healthy controls, the clone size crossed 0.05 per cent.
Results & Discussion
Among the 61 cases of DVT recorded, the mean age was 45.21 yr (range: 21-87 yr). Of all the cases, 37 (61%) were male and 24 (39%) were female. All of the patients were from western Rajasthan.
The patients presented with thrombosis at different sites, as shown in Table I. The most common site was found to be the left lower limb, seen in 26 cases (43%), followed by the right lower limb seen in 10 cases (16%). The increased incidence of left lower limb DVT over right lower limb DVT in the present study is in concordance with the existing literature and is hypothesized to be due to compression of the left common iliac vein by the right common iliac artery, leading to stasis of blood10,11.
| Site of deep vein thrombosis | Number of patients (%) |
|---|---|
| Left lower limb | 26 (43) |
| Right lower limb | 10 (16) |
| Cerebral veins | 7 (11) |
| Superior mesenteric vein | 3 (5) |
| Hepatic vein (Budd Chiari syndrome) | 2 (3) |
| Renal vein | 2 (3) |
| Right upper limb | 1 (2) |
| Multiple sites | 10 (16) |
Among the 61 cases of DVT, three cases (4.92%) revealed large clones (size of double-negative population >1%) on monocytes as well as granulocytes, hence fulfilling the criteria for diagnosing PNH, which is, the absence of at least two distinct GPI-anchored proteins, from two different cell lineages9 (Table II and Fig. 1). One of these three cases also had pancytopenia, while the other two had isolated anaemia. Two of the three also had evidence of haemolysis. Since patient A3 showed pancytopenia, hence bone marrow examination was done wherein cellular bone marrow revealed all the haematopoietic components with marked erythroid prominence. Further, three patients (4.92%) showed small clones (size of double-negative population >0.1%, but <1%) on both granulocytes and monocytes (Table II and Fig. 2). Nine (15%) patients showed small clones only on granulocytes, and 11 (18%) patients showed small clones only on monocytes.
| Patient number | Age/sex | Site of deep vein thrombosis | Clone size detected | Other findings in haemogram | Evidence of haemolysis# | |||
|---|---|---|---|---|---|---|---|---|
| Per cent granulocytes FLAER and CD24 negative | Per cent monocytes FLAER and CD14 negative | Per cent type 2 RBC (RBC with partial deficiency of GPI-APs)9 | Per cent type 3 RBC (RBC lacking expression of GPI-APs)9 | |||||
| A1 (Fig. 1) | 44/male | Left lower limb | 94.17 | 92.90 | 18.76 | 47.52 | Anaemia (Hb-5.2 g/dl) | Present |
| A2 | 21/female | Hepatic vein | 93.12 | 83.10 | 3.12 | 6.53 | Anaemia (Hb-6.1 g/dl) | Absent |
| A3 | 50/female | Left lower limb | 95.79 | 90.73 | 3.45 | 8.24 | Pancytopenia (Hb-7.2 g/dl, TLC-3330/µl, platelets-23,000/µl) | Present |
| B1 (Fig. 2) | 38/male | Right lower limb | 0.13 | 0.15 | 0.02 | 0.00 | All values within normal range | Absent |
| B2 | 54/male | Left lower limb | 0.12 | 0.21 | 0.00 | 0.00 | All values within normal range | Absent |
| B3 | 22/male | Left lower limb | 0.11 | 0.11 | 0.01 | 0.00 | All values within normal range | Absent |
*Large clone size-size of double-negative population >1 per cent on both granulocytes and monocytes; **Small clone size-size of double-negative population >0.1 per cent, but <1 per cent on both granulocytes and monocytes; #Evidence of haemolysis-indirect hypebilirubinaemia, high reticulocyte index and lactate dehydrogenase >1.5 times upper limit of normal. FLAER, fluorescein-labelled proaerolysin; CD, cluster of differentiation; Hb, haemoglobin; TLC, total leucocyte count; GPI-APs, glycosylphosphatidylinositol-anchored proteins; RBC, red blood cells
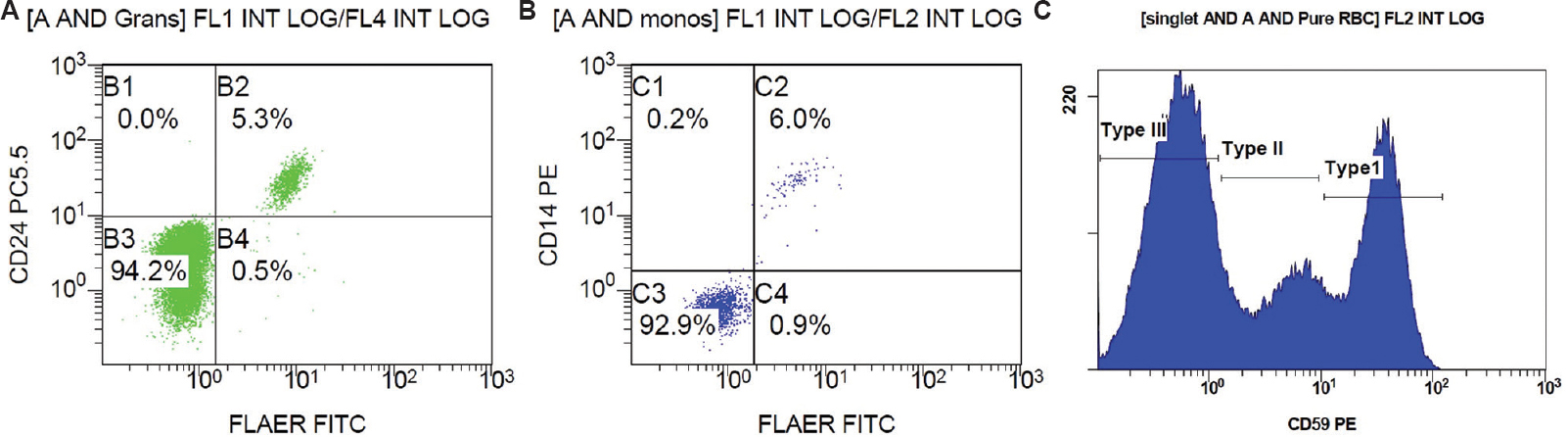
- Scatter plot of patient A1: (A) FLAER and CD24 on granulocytes, (B) FLAER and CD 14 on monocytes, and (C) RBC histogram for expression of CD59 on CD235 a positive RBC. FLAER, fluorescein-labelled proaerolysin; CD, cluster of differentiation; RBC, red blood cells
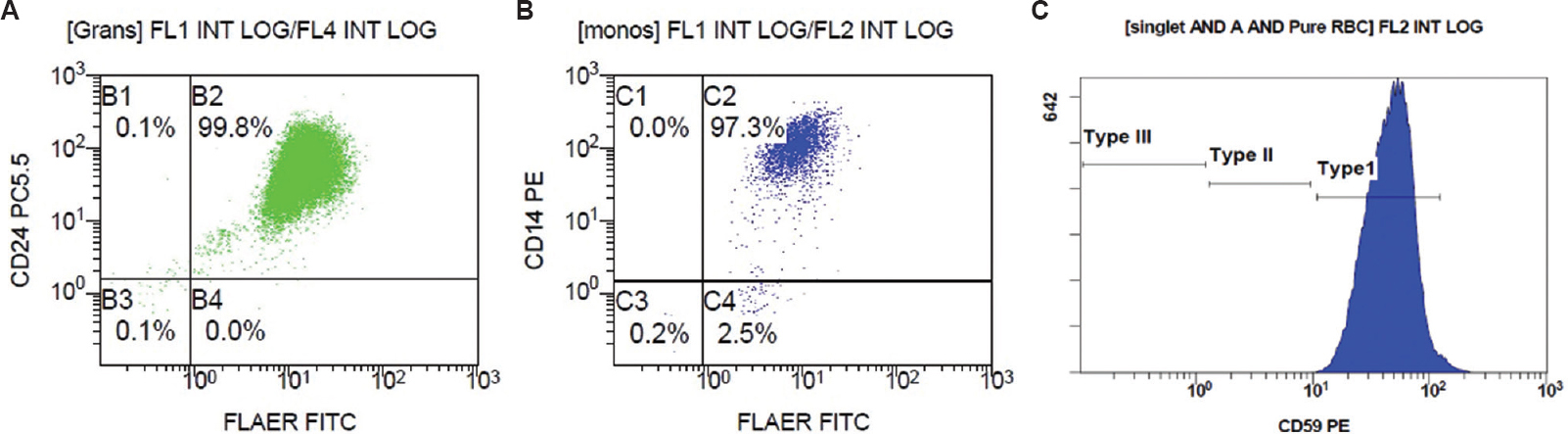
- Scatter plot of patient B1: FLAER and CD24 on granulocytes (A), FLAER and CD 14 on monocytes (B) and RBC histogram for expression of CD59 on CD235a positive RBC (C). FLAER, fluorescein-labelled proaerolysin; CD, cluster of differentiation; RBC, red blood cells.
The three cases having large clones on granulocytes and monocytes were also tested for other thrombophilia markers that are protein C, protein S, activated protein C resistance, lupus anticoagulant, anticardiolipin antibody, anti-beta-2 glycoprotein antibody, fibrinogen and antithrombin. However, none of the above patients showed positivity for any of these markers.
Previous studies have associated the risk of thrombosis with PNH clone size <50 per cent6 and <20 per cent12. However, no studies have associated the risk of thrombosis with small clone size <1 per cent. Moreover, thrombophilia is more commonly associated with the presence of haemolysis12 (laboratory evidence of LDH >1.5 times the upper limit of normal) in cases of PNH, which none of the patients B1, B2 or B3 showed. Hence, small clones in patients B1, B2 and B3 may not be responsible for thrombosis. This could be reflective of the existence of small clones of PNH in the general population, the exact prevalence of which, however, is unknown5.
Only a few studies worldwide have used FLAER-based markers to screen for PNH in patients presenting with thrombosis: A Canadian study conducted among patients with idiopathic venous thromboembolism showed a PNH clone size of >0.02 per cent in the neutrophil population in only one out of 388 patients5. An Italian study conducted on patients with thrombosis of the splanchnic vein showed small PNH clones (<0.2%) in only two out of 202 patients13. A study from Chandigarh, India, conducted in 2014, showed two out of 142 patients with intra-abdominal thrombosis as having large PNH clones14. Another study from Chandigarh, India, published in 2020 did not detect PNH phenotype in any of the 180 patients with cerebral sinovenous thrombosis15. A study from Turkey published in 2020 conducted on patients with idiopathic portal vein thrombosis showed PNH clone size between 3.02 and 4.62 per cent in four out of 112 patients16.
The present study in western Rajasthan shows a higher proportion of patients with PNH compared to those reported in studies previously done elsewhere5,13–16. Based on the findings it is suggested that PNH screening should be added to the panel of investigations done for patients presenting with thrombosis, if other indications such as coexistent cytopenias, or intravascular haemolysis are noted, or if the patients are young. This may provide an otherwise missed opportunity to diagnose this disorder. Patients with DVT are given anticoagulants17. However, in PNH, anticoagulation needs to be continued for life. In addition, eculizumab may be employed as it has been found to be effective in reducing thrombophilic events in cases of PNH18. A study in the UK showed reduction in thromboembolic event rate in 103 antithrombotic-treated patients from 10.61 to 0.62 events per 100 patient-years with eculizumab treatment18. However, in India and developing countries, the use of eculizumab is limited as it is expensive and not available19. Shorter duration and small sample size were the limitations of this study. Nevertheless, our study opens the avenue for larger studies to find out the pathophysiology of thrombosis in PNH, and to determine its epidemiological aspects in the western Rajasthan region.
Acknowledgment:
The authors acknowledge Dr Gopal Krishana Bohra, Associate Professor, department of Medicine, All India Institute of Medical Sciences, Jodhpur, who helped in enrolling patients, and Ms Jagriti Jha, MBBS student, AIIMS Jodhpur, who helped in sample collection
Financial support & sponsorship: The first author (SP) received ICMR- Short Term Studentship (2019-05492) for conduction of this study.
Conflicts of Interest: None.
References
- Large clones with PNH-type phenotype are not common in patients presenting with intra-abdominal thrombosis –A prospective study. Clin Appl Thromb Hemost. 2013;19:562-9.
- [Google Scholar]
- The incidence and prevalence of paroxysmal nocturnal hemoglobinuria (PNH) and survival of patients in Yorkshire. Blood. 2006;108:985.
- [Google Scholar]
- Fatigue, symptom burden, and health-related quality of life in patients with myelodysplastic syndrome, aplastic anemia, and paroxysmal nocturnal hemoglobinuria. Cancer Med. 2019;8:543-53.
- [Google Scholar]
- Screening of patients with idiopathic venous thromboembolism for paroxysmal nocturnal hemoglobinuria clones. Thromb Res. 2015;135:1107-9.
- [Google Scholar]
- Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2003;102:3587-91.
- [Google Scholar]
- Natural history of paroxysmal nocturnal haemoglobinuria using modern diagnostic assays. Br J Haematol. 2004;126:133-8.
- [Google Scholar]
- Incidence and diagnostic modality for deep vein thrombosis in lower limb surgeries in rural India. Glob J Res Anal. 2016;5:321-2.
- [Google Scholar]
- Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by flow cytometry. Cytometry B Clin Cytom. 2010;78:211-30.
- [Google Scholar]
- Clinical implications of the anatomical variation of deep venous thrombosis. Phlebology. 2018;33:97-106.
- [Google Scholar]
- Predominance of left-sided deep vein thrombosis and body weight. J Thromb Haemost. 2010;8:2083-4.
- [Google Scholar]
- Clinical signs and symptoms associated with increased risk for thrombosis in patients with paroxysmal nocturnal hemoglobinuria from a Korean Registry. Int J Hematol. 2013;97:749-57.
- [Google Scholar]
- Clonal populations of hematopoietic cells with paroxysmal nocturnal hemoglobinuria phenotype in patients with splanchnic vein thrombosis. Thromb Res. 2014;133:1052-5.
- [Google Scholar]
- Paroxysmal nocturnal hemoglobinuria is rare cause for thrombosis of the intra-abdominal veins in the ethnic Indian population –Results from FLAER-based flowcytometry screening. Eur J Haematol. 2014;92:435-43.
- [Google Scholar]
- Screening for paroxysmal nocturnal hemoglobinuria (PNH) in patients presenting with cerebral sinovenous thrombosis (CSVT):Results of a FLAER based flowcytometry study in Indian patients. J Thromb Thrombolysis. 2020;49:584-90.
- [Google Scholar]
- Presence of paroxysmal nocturnal hemoglobinuria in patients with idiopathic portal vein thrombosis:A single-center study. Turk J Med Sci. 2020;50:1344-9.
- [Google Scholar]
- Consensus on management of deep vein thrombosis with emphasis on NOACs (non-vitamin k antagonist oral anticoagulants):Recommendations from inter-disciplinary group of Indian experts. J Assoc Physicians India. 2016;64:7-26.
- [Google Scholar]
- Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110:4123-8.
- [Google Scholar]
- Guidelines on hemolytic uremic syndrome by Indian Society of Pediatric Nephrology:Key messages. Indian Pediatr. 2020;57:744-7.
- [Google Scholar]






