Translate this page into:
Characteristics & outcomes of cancer patients with COVID-19: A multicentre retrospective study from India
For correspondence: Dr Akash Kumar, Department of Medical Oncology, National Cancer Institute, All India Institute of Medical Sciences, New Delhi 110 029, India e-mail: akashjha08@yahoo.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
High mortality has been observed in the cancer population affected with COVID-19 during this pandemic. We undertook this study to determine the characteristics and outcomes of cancer patients with COVID-19 and assessed the factors predicting outcome.
Methods:
Patients of all age groups with a proven history of malignancy and a recent diagnosis of SARS-CoV-2 infection based on nasal/nasopharyngeal reverse transcriptase (RT)-PCR tests were included. Demographic, clinical and laboratory variables were compared between survivors and non-survivors groups, with respect to observed mortality.
Results:
Between May 11 and August 10, 2020, 134 patients were included from the three centres and observed mortality was 17.1 per cent. The median age was 53 yr (interquartile range 39-61 yr) and thirty four patients (25%) were asymptomatic. Solid tumours accounted for 69.1 per cent and breast cancer was the most common tumour type (20%). One hundred and five patients (70.5%) had received chemotherapy within the past four weeks and 25 patients (19.3%) had neutropenia at presentation. On multivariate analysis, age [odds ratio (OR) 7.99 (95% confidence interval [CI] 1.18-54.00); P=0.033], haemoglobin [OR 6.28 (95% CI 1.07-37.04); P=0.042] neutrophil–lymphocyte ratio [OR 12.02 (95% CI 2.08-69.51); P=0.005] and baseline serum albumin [OR 18.52 (95% CI 2.80-122.27); P=0.002], were associated with higher mortality. Recent chemotherapy, haematological tumours type and baseline neutropenia did not affect the outcome.
Interpretation & conclusions:
Higher mortality in moderate and severe infections was associated with baseline organ dysfunction and elderly age. Significant proportion of patients were asymptomatic and might remain undetected.
Keywords
Cancer
COVID-19
infection
mortality – outcome
The first few cases of COVID-19 were reported from Wuhan, China, and initial studies suspected a zoonotic origin1. As of May 1, 2022 the disease had affected 510 million people and almost six million people died, worldwide2. One of the vulnerable populations affected during this pandemic comprises patients with cancer. Not only this group has been at risk of higher morbidity and mortality but also the pandemic has led to significant disruption of cancer-directed services in the country3. Some large studies have reported their observations in this patient population, but more data are needed45. Moreover, as various studies suggest infectivity and fatality rates of COVID-19 is affected by regional variations, information sharing between different regions becomes pivotal67. This study was aimed to demonstrate the experience with COVID-19 in cancer patients in cancer care facilities in India and to identify the factors predicting outcome.
Material & Methods
Study design & participants: This was a retrospective study involving patients from three centres in New Delhi, India, namely All India Institute of Medical Sciences (AIIMS), B.L.K Superspeciality Hospital, New Delhi and Asian Institute of Medical Sciences, Faridabad, Haryana. All these hospitals were designated COVID-19 care centres and all had separate oncology units. Patients with cancer, who were infected with COVID-19 were managed by designated COVID-19 specialty teams in continuous consultation with oncologists. The study period was from May 11 to August 10, 2020. All cases with biopsy-proven malignancy who were admitted or advised home isolation, at any of the participating centres on the diagnosis of COVID-19 by the reverse transcriptase-polymerase chain reaction (RT-PCR) on nasopharyngeal swab were included. Cases diagnosed based on rapid antigen tests only were excluded. Patients of all age groups were allowed to participate.
As a uniform policy among the three centres, all cancer patients with suspected symptoms were tested. Asymptomatic patients were tested only before a planned invasive procedure or as a part of screening if they had a high risk of infection after contact exposure to a known case of COVID-19.
All the hospitalized cases were followed up till discharge or death during COVID-19 illness. The primary criteria for discharge in hospitalized cases were the resolution of symptoms and a minimum stay of two weeks, in the absence of follow up PCR negativity. Those who became asymptomatic and were PCR negative on repeat swab tests were discharged earlier. All asymptomatic patients and a few of mildly symptomatic cases were kept on home isolation after initial evaluation. All these patients were monitored daily telephonically and followed up till they became asymptomatic for a minimum period of two weeks, since the diagnosis of SARS-CoV-2 infection.
Data recording: The study protocol was reviewed and approved by the Institutional Ethics Committees at all the participating centres. Consent waiver was granted given retrospective nature of the analysis. Confidentiality was maintained by the de-identification of data. All the data were analyzed at the cut-off date of August 24, 2020.
The majority of the data was retrieved from prospectively maintained medical records. The missing data were collected telephonically during the time of analysis. The severity of the disease was defined according to the WHO definition8.
All the demographic data, clinical information, laboratory parameters and complications during the hospital stay were recorded. The staging of the solid tumours was recorded as per TNM (tumour, nodes and metastases) staging9. Haematological tumours were not staged. Among laboratory parameters, neutropenia was defined as an absolute neutrophil count less than or equal to 1500 cells/µl.
Statistical analysis: The differences between the demographic factors, cancer history, clinical findings and laboratory parameters between the survivors and non-survivors groups were analyzed. Descriptive statistics were used to analyze the baseline clinical and treatment characteristics of the overall population. A comparison of baseline and treatment characteristics was performed using t tests and Wilcoxon rank-sum tests for the continuous variables and Chi-squared tests and Fisher’s exact tests for categorical variables, among the two groups.
For continuous parameters, receiver operating characteristic analysis was done to arrive at optimal cut-off values. Thereafter, univariate logistic regression analyses were performed for clinically significant baseline factors to determine the associations with death, while multivariate logistic regression analysis was performed for relevant factors with a significance of P<0.05. All statistical tests were two-sided and the significance level was defined a priori at the conventional level as <0.05. The analyses were conducted using Stata statistical software (StataCorp. 2013. Release 13. College Station, TX).
Analysis of numerical and categorical data from moderate and severe cases was done using standard built-in packages of the R statistical software (v4.1.2; R Core Team 2021). Welch’s two-sided t test was performed for comparing numerical data using the t test function followed by a multiple testing correction using the Benjamini-Hochberg method10. Fisher’s exact test was performed using fisher.test function using default parameter for a two-sided test on 2×2 contingency tables constructed from the categorical parameters or treatment with rows representing patient outcome (survival or death) while columns representing the presence or absence of the treatment.
The Uniform Manifold Approximation and Projection (U-MAP) dimensionality reduction and embedding were calculated using the ‘umap’ package from the CRAN R package repository [arXiv:1802.03426 (stat.ML)] and the U-MAP plot were generated using ggplot2 package in R.
Results
A total of 7297 (including non-cancer patients) SARS-CoV-2-infected individuals were screened during the study period for inclusion and 134 were selected based on confirmed RT-PCR test positivity for SARS-CoV-2 and history of malignancy (Fig. 1). Overall, 23 (17.1%) patients died during the study.
- Flowchart showing patient profile and screening.
The median age was 53 yr (interquartile range 39-61 yr) in the overall population, with non-survivor subset being significantly older than the survivor’s subset, i.e. 59 vs. 53 yr (P=0.03). Eleven patients (8.0%) belonged to the paediatric age group (0-18 yr). The median follow up of all patients was 14 days (range=1-48 days). Gender distribution favoured females in the overall population (53.0%); however, mortality was significantly higher among males (73.9%) (P=0.01). Solid tumours accounted for more than two-thirds (69.4%) of all patients and breast cancer was the most common cancer overall (20.1%) with SARS-CoV-2 positivity. Among 41 patients with haematological cancer, leukaemia was the most common (51.0%) diagnosis.
History of fever, cough, headache and myalgia, among symptoms, were more common in non-survivors’ group. Overall, 41 (30.6%) patients were classified as with moderate or severe symptoms, as per the WHO classification and 34 (25%) patients were asymptomatic. Three fourths (n=99) of all cases had an Eastern Co-operative Oncology Group performance status of 2-4 and this proportion was significantly more in non-survivors’ group (P=0.008).
One hundred and four patients (77.6%) had evidence of active disease in the body at the time of inclusion. This set included active disease on treatment, treatment naïve patients as well as patients with refractory and progressive disease. History of treatment within the last four weeks revealed that around 105 (78.5%) received chemotherapy and 21 (15.7%) patients were treatment naïve. Ninety four (70.1%) received cytotoxic chemotherapy. History of chemotherapy, as well as the nature of agents used was not significantly different between the two groups.
Among laboratory parameters, median haemoglobin was significantly lower in non-survivors (8.6 vs. 10.5 g/dl, P=0.001). In response to chemotherapy, 25 (19.3%) patients had neutropenia (ANC ≤1500/µl) at presentation, whereas, the median neutrophil-lymphocyte ratio (NLR), more than 4 (median), was higher among non-survivors (P=0.02). On further analysis, we could not find any significant difference if a cut-off of ANC=500 was taken. Metabolic parameters such as serum creatinine was significantly higher (P=0.001), whereas serum albumin was significantly lower in the non-survivors’ group (P=0.001).
For treatment, steroids were the most common drug used in the overall population (58.0%). Among drugs with potential antiviral activities, azithromycin and hydroxychloroquine were the two most common drugs (39.5 and 31.3 per cent, respectively; Table I). During the hospital stay, 41 (30.6%) patients required oxygen administration and among these, 21 (51.2%) patients died. Intensive care unit (ICU) admission was required in 32 (23.9%), of whom 21 (65.6%) died. Twenty eight patients (n=28) developed acute respiratory distress syndrome (ARDS) and required mechanical ventilation; however, majority of these (n=21) succumbed (Table II).
| Therapy | Total cases (n=134), n (%) | Asymptomatic + mild (n=93), n (%) | Moderate + severe (n=41), n (%) |
|---|---|---|---|
| Steroids | 58 (43.3) | 20 (21.5) | 38 (92.7) |
| Hydroxychloroquine | 42 (31.3) | 22 (23.6) | 20 (48.8) |
| Azithromycin | 53 (39.5) | 32 (34.4) | 21 (51.2) |
| Remdesivir | 14 (10.4) | 2 (2.1) | 12 (29.3) |
| Doxycycline | 12 (9) | 5 (5.4) | 7 (17.1) |
| Favipiravir | 7 (5.2) | 6 (6.4) | 1 (2.4) |
| Ivermectin | 8 (6) | 4 (4.3) | 4 (9.7) |
| Interleukin-6 inhibitors | 3 (2.2) | 0 | 3 (7.3) |
| Plasma | 3 (2.2) | 0 | 3 (7.3) |
| Complications | Intervention (n=134), n (%) | Death, n (%) |
|---|---|---|
| Oxygen administration | 41 (30.6) | 21/41 (51.2) |
| ICU | 32 (23.9) | 21/32 (65.6) |
| ARDS | 28 (20.9) | 21/28 (75) |
| Mechanical ventilation | 25 (18.7) | 19/25 (76) |
| Sepsis | 22 (16.4) | 15/22 (68.2) |
| MODS | 9 (6.7) | 9/9 (100) |
| ARF | 8 (6) | 7/8 (87.5) |
| Cardiac dysfunction | 5 (3.7) | 4/5 (80) |
| CNS event | 4 (3) | 3/4 (75) |
ICU, intensive care unit; ARDS, acute respiratory distress syndrome; MODS, multi organ dysfunction syndrome; ARF, acute renal failure; CNS, central nervous system
On univariate regression analysis, it was found that age more than 60 yr, gender, type of tumour, symptom duration, the severity of disease, haemoglobin <8.9 g/dl, NLR >4.6, albumin ≤3.3 mg/dl and serum creatinine >0.74 mg/dl were associated with higher odds of death (Table III). On multivariate analysis older age, serum albumin, haemoglobin and NLR were found to be associated with significantly high odds of death.
| Parameters | Univariate OR (95% CI) | P | Multivariate OR (95% CI) | P |
|---|---|---|---|---|
| Age (yr) | ||||
| ≤60 | 1 (reference) | Reference | ||
| >60 | 3.32 (1.3-8.46) | 0.012 | 7.99 (1.18-54.00) | 0.033 |
| Sex | ||||
| Male | 1 (reference) | Reference | ||
| Females | 0.25 (0.09-0.68) | 0.004 | 0.56 (0.13-2.39) | 0.438 |
| Severity | ||||
| Asymptomatic + mild | 1 (reference) | |||
| Moderate + severe | 3.21 (1.91-5.38) | 0.001 | - | |
| PS | ||||
| 0-1 | 1 (reference) | |||
| 2-4 | 9.71 (1.25-75.02) | 0.29 | - | |
| Symptom duration (days) | ||||
| ≤3 | 1 (reference) | |||
| >3 | 1.7 (1.07-2.71) | 0.022 | 2.17 (0.62-11.89) | 0.186 |
| Comorbidity | ||||
| 0 | 1 (reference) | Reference | ||
| 1 | 0.83 (0.99-8.61) | 0.793 | 0.23 (0.01-2.89) | 0.254 |
| ≥2 | 2.92 (0.09-0.31) | 0.052 | 2.61 (0.31-21.91) | 0.376 |
| Type of tumour | ||||
| Solid | 1 (reference) | Reference | ||
| Haematological | 0.44 (0.30-0.64) | 0.001 | 1.66 (0.33-8.27) | 0.537 |
| Cancer stage (solid tumours) | ||||
| 1-3 | 1 (reference) | - | ||
| 4 | 1.88 (0.88-4.01) | 0.10 | ||
| Cytotoxic chemotherapy | ||||
| No | 1 (reference) | - | ||
| Yes | 0.76 (0.29-1.97) | 0.57 | ||
| Haemoglobin (g/dl) | ||||
| >8.9 | 1 (reference) | |||
| ≤8.9 | 1.80 (1.12-2.89) | 0.015 | 6.28 (1.07-37.04) | 0.042 |
| NLR | ||||
| ≤4.6 | 1 (reference) | |||
| >4.6 | 4.22 (1.62-10.99) | 0003 | 12.02 (2.08-69.51) | 0.005 |
| Platelets (lacs) | ||||
| >1.39 | 1 (reference) | |||
| ≤1.39 | 1.43 (0.90-2.28) | 0.126 | ||
| Albumin (mg/dl) | ||||
| >3.3 | 1 (reference) | |||
| ≤3.3 | 3.16 (1.74-5.73) | <0.001 | 18.52 (2.80-122.27) | 0.002 |
| Creatinine (mg/dl) | ||||
| ≤0.74 | 1 (reference) | |||
| >0.74 | 3.40 (1.31-8.86) | 0.012 | 4.88 (1.01-23.81) | 0.050 |
P<0.05 was considered significant. OR, odds ratio; NLR, neutrophil-lymphocyte ratio; CI, confidence interval; PS, performance status
Most of the deaths were observed in the group with moderate and severe symptoms at presentation. Hence, the data of 41 patients were analyzed separately who presented with moderate to severe symptoms. A significant association was found in a two-sided t test of patient mortality with higher levels of creatinine (P<0.007) and lower levels of serum albumin (P<0.0051) (Fig. 2A-E). In terms of categorical data (Fig. 2F-J), a marginally significant association of mortality was found with patient gender (P=0.062).
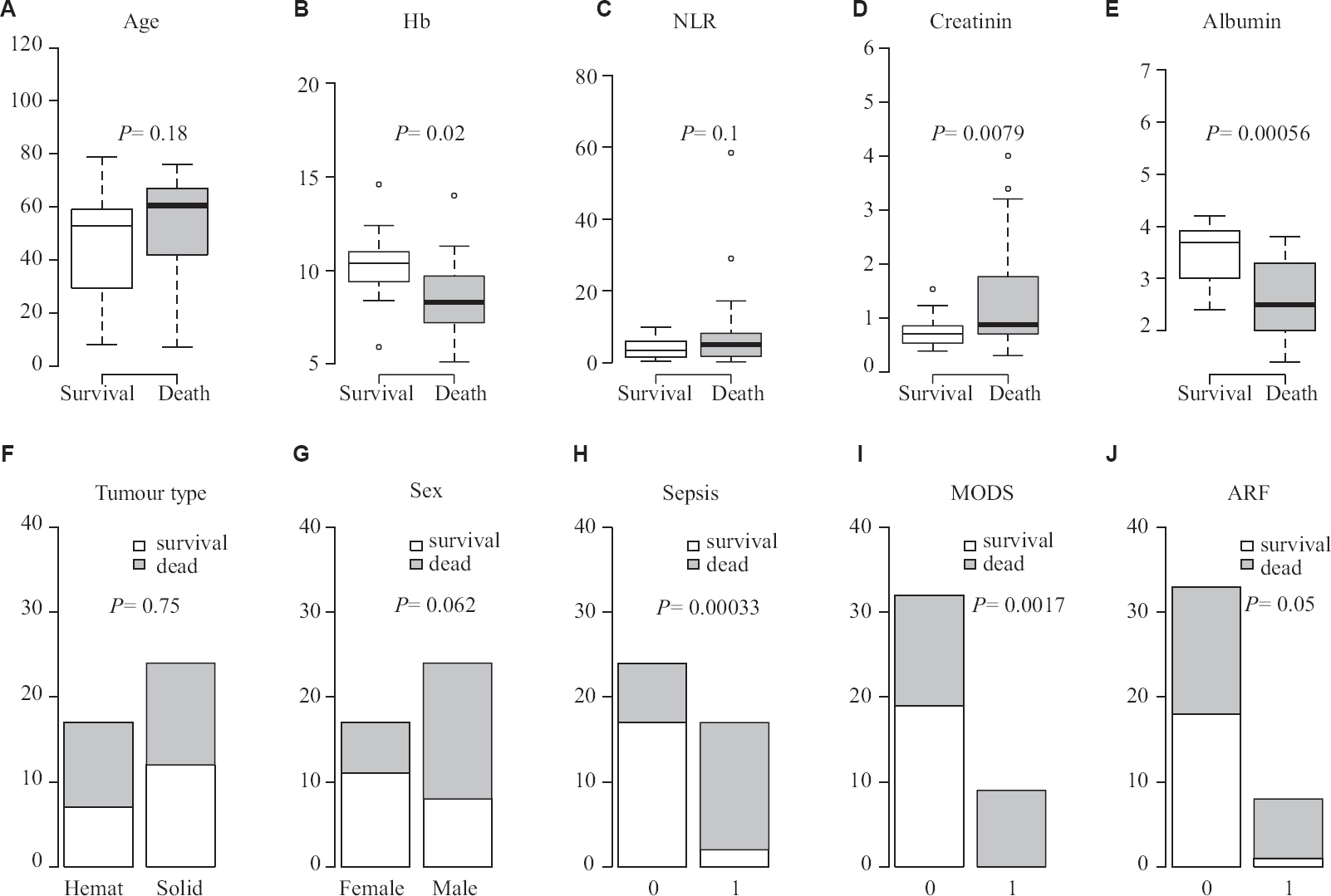
- Factors determining mortality in moderate/severe disease, (A) age; (B) haemoglobin (Hb); (C) neutrophil-lymphocyte ratio (NLR); (D) creatinin; (E) albumin; (F) tumour type; (G) Sex; (H) sepsis; (I) multiorgan dysfunction syndrome (MODS); (J) acute renal failure (ARF).
Finally, we used unsupervised learning using U-MAP for dimensionality reduction to visualize the correlated structure of our multi-parameter dataset. It was found that the patients data points were distributed in three clusters recapitulating the observations based on the severity of infection and outcome. The first cluster was dominated by asymptomatic patients with no mortality, the second cluster had a majority of mild and a few moderate severity patients with 3.0 per cent mortality, while the third cluster had a majority of severe and a few moderate patients with 68.0 per cent mortality. Moderate patients were split across two clusters, with 12 patients in cluster two and six patients in cluster three.
Discussion
The observed mortality (17.1%) due to COVID-19 in this subset of cancer patients was alarming, but was similar to that reported in two other studies from this region1112. Increased death risk in cancer patients has also been observed in studies reported from China and US1314. Site-specific variation in mortality has been observed in larger studies where patients of lung cancer infected with COVID-19 have experienced higher mortality15. However, due to the small sample size, we could not get similar observations.
It was found that baseline serum albumin could predict higher mortality in the overall population. Low serum albumin, although is a known predictor of poor outcomes in cancer, it may also be a result of the acute-phase response16. It has been recognized as a poor prognostic factor in the non-cancer COVID-19 population1719. It may have a therapeutic role, that requires further investigations20. High NLR was found to be associated with poor outcomes in our study. Being a known marker of immune activation, observed poor prognostic role is similar to other reported studies2122. Lower haemoglobin (≤8.9) was another factor observed to be associated with poor outcomes as reported in past studies23.
Among patients with age more than 60 yr, high mortality was observed. This finding was in concordance with already reported observations4.
Some previous studies suggested lower fatality rates in paediatric cancer patients2425. Our study had 11 paediatric patients and around two-thirds had a diagnosis of haematological tumours (data not shown). There was only one mortality among them, but the sample size was too small to derive any discrete conclusion.
A higher mortality was observed in haematological malignancies as compared with solid cancers (24.4 vs. 13.9 %, respectively). A few other studies from this region have also suggested a higher mortality risk among infected individuals with haematological malignancies2627. Similar findings have been reported in other countries2829. Tumour type is another factor that may affect prognosis variably, as shown in a few studies3031, however, we could not find any differences in outcomes.
Around 30.0 per cent of patients were admitted to the hospital with moderate or severe symptoms and among these, 78.0 per cent of patients required ICU admission. The worrisome trend observed was among sick cases, who developed ARDS. Around three-fourths of such cases could not be salvaged. High fatality rates were also observed in severe cases who developed complications such as sepsis, MODS and ARF. The inability to salvage such cases indicates that the prevention of infection may be the most important strategy. Our study included 19.0 per cent of patients with neutropenia, likely due to recent chemotherapy. The observed mortality in this subset was no different from the non-neutropenic subset.
Distinct clustering of our patients predicting outcome suggested that there could be a potential reclassification of patient severity specifically moderately symptomatic patients, into either critical or non-critical categories, an observation which can further be validated in a larger data set in future. This could help towards optimal utilization of health resources and building a system to triage cancer patients suffering from COVID-19 more effectively.
There were certain limitations in our study. A relatively small sample size and limitation of information available for analysis, were most important. We also could not retrieve data on smoking history and comorbidities like obesity, which may have a bearing on the overall outcome, as reported earlier3233. The characteristics of the non-cancer population infected with COVID-19 could not be evaluated during this period; hence, a comparative analysis between the two groups could not be performed.
In conclusion, our study data add to the existing knowledge of cancer patients with COVID-19. It was observed that baseline organ dysfunction and older age might affect outcomes in such patients. Recent chemotherapy may not affect prognosis adversely. There were a large number of asymptomatic individuals who did well on observation. Hence, chemotherapy in asymptomatic COVID-19 infected cancer patients may be a safe option.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-207.
- [Google Scholar]
- WHO coronavirus (COVID-19) dashboard;2022. Available from: https://covid19.who.int
- Impact of COVID-19 on cancer care in India:A cohort study. Lancet Oncol. 2021;22:970-6.
- [Google Scholar]
- Utilization of COVID-19 treatments and clinical outcomes among patients with cancer:A COVID-19 and Cancer Consortium (CCC19) Cohort Study. Cancer Discov. 2020;10:1514-27.
- [Google Scholar]
- COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments:A prospective cohort study. Lancet. 2020;395:1919-26.
- [Google Scholar]
- Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020;10:1465-74.
- [Google Scholar]
- Clinical management of COVID-19:Interim guidance, 27 May 2020. Geneva: WHO; 2020.
- The eighth edition of AJCC cancer staging manual:Continuing to build a bridge from a population-based to a more “personalized”approach to cancer staging. CA Cancer J Clin. 2017;67:93-9.
- [Google Scholar]
- Controlling the false discovery rate:A practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 1995;57:289-300.
- [Google Scholar]
- COVID-19 in cancer patients on active systemic therapy –Outcomes from LMIC scenario with an emphasis on need for active treatment. Cancer Med. 2020;9:8747-53.
- [Google Scholar]
- COVID-19 mortality in cancer patients:A report from a tertiary cancer centre in India. Peer J. 2021;9:e10599.
- [Google Scholar]
- Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China:A multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904-13.
- [Google Scholar]
- Clinical impact of COVID-19 on patients with cancer (CCC19):A cohort study. Lancet. 2020;395:1907-18.
- [Google Scholar]
- Mortality in adult patients with solid or hematological malignancies and SARS-CoV-2 infection with a specific focus on lung and breast cancers:A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;163:103365.
- [Google Scholar]
- Pretreatment serum albumin as a predictor of cancer survival:A systematic review of the epidemiological literature. Nutr J. 2010;9:69.
- [Google Scholar]
- Hypoalbuminemia, coagulopathy, and vascular disease in COVID-19. Circ Res. 2020;127:400-1.
- [Google Scholar]
- Low albumin levels are associated with poorer outcomes in a case series of COVID-19 patients in Spain:A retrospective cohort study. Microorganisms. 2020;8:1106.
- [Google Scholar]
- Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China:A multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893-903.
- [Google Scholar]
- Albumin administration improves organ function in critically ill hypoalbuminemic patients:A prospective, randomized, controlled, pilot study. Crit Care Med. 2006;34:2536-40.
- [Google Scholar]
- Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762-8.
- [Google Scholar]
- Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19:A report from the Italian front line. Int J Antimicrob Agents. 2020;56:106017.
- [Google Scholar]
- Anemia predicts poor outcomes of COVID-19 in hospitalized patients:A prospective study in Iran. BMC Infect Dis. 2021;21:170.
- [Google Scholar]
- COVID19 in children with cancer in low- and middle-income countries:Experience from a cancer center in Chennai, India. Pediatr Hematol Oncol. 2021;38:161-7.
- [Google Scholar]
- Effect of age, comorbidity and remission status on outcome of COVID-19 in patients with hematological malignancies. Blood Cells Mol Dis. 2021;87:102525.
- [Google Scholar]
- Clinical profile and outcome of COVID-19 in haematological malignancies:Experience from tertiary care centre in India. Ann Hematol. 2022;101:69-79.
- [Google Scholar]
- COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics:A prospective cohort study. Lancet Oncol. 2020;21:1309-16.
- [Google Scholar]
- Clinical outcome of coronavirus disease 2019 in haemato-oncology patients. Br J Haematol. 2020;190:e64-7.
- [Google Scholar]
- COVID-19 in patients with thoracic malignancies (TERAVOLT):First results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914-22.
- [Google Scholar]
- COVID-19 outcomes of patients with gynecologic cancer in New York City. Cancer. 2020;126:4294-303.
- [Google Scholar]
- Features of 20133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol:Prospective observational cohort study. BMJ. 2020;369:m1985.
- [Google Scholar]
- Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-6.
- [Google Scholar]






