Translate this page into:
Safety, immunogenicity & effectiveness of the COVID-19 vaccine among healthcare workers in a tertiary care hospital
For correspondence: Dr Padam Singh, Medanta Institute of Education & Research, Sector 38, Gurugram 122 001, Haryana, India e-mail: dr.padamsingh2013@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The COVID-19 pandemic has caused significant global morbidity and mortality. As the vaccination was rolled out with prioritization on healthcare workers (HCWs), it was desirable to generate evidence on effectiveness of vaccine in prevailing real-life situation for policy planning. The objective of the study was to evaluate the safety, effectiveness and immunogenicity of COVID-19 vaccination among HCWs in a tertiary care hospital.
Methods:
This prospective observational study was undertaken on the safety, immunogenicity and effectiveness of the ChAdOx1 nCoV- 19 coronavirus vaccine (Recombinant) during the national vaccine roll out in January-March 2021, in a tertiary care hospital, New Delhi, India.
Results:
The vaccine was found to be safe, with local pain, fever and headache as the most common adverse events of milder nature which generally lasted for two days. The adverse events following vaccination were lower in the second dose as compared to the first dose. The vaccine was immunogenic, with seropositivity, which was 51 per cent before vaccination, increasing to 77 per cent after single dose and 98 per cent after two doses. Subgroup analysis indicated that those with the past history of COVID-19 attained seropositivity of 98 per cent even with single dose. The incidence of reverse transcription (RT)-PCR positive COVID-19 was significantly lower among vaccinated (11.7%) as compared to unvaccinated (22.2%). Seven cases of moderate COVID-19 needing hospitalization were seen in the unvaccinated and only one such in the vaccinated group. The difference was significant between the fully vaccinated (10.8%) and the partially vaccinated (12.7%). The hazard of COVID-19 infection was higher among male, age >50 yr and clinical role in the hospital. After adjustment for these factors, the hazard of COVID-19 infection among unvaccinated was 2.09 as compared to fully vaccinated. Vaccine effectiveness was 52.2 per cent in HCWs.
Interpretation & conclusions:
ChAdOx1 nCoV-19 coronavirus vaccine (Recombinant) was safe, immunogenic as well as showed effectiveness against the COVID-19 disease (CTRI/2021/01/030582).
Keywords
Adverse events
COVID vaccine
effectiveness
hazard ratio
immunogenicity
incidence
seropositivity
In order to mitigate the menace of COVID-19 pandemic, vaccination was considered as one of the major public health initiatives worldwide. In India from January 16, 2021, two vaccines (ChAdOx1 nCoV-19 & BBV152) were approved for emergency use, prioritizing healthcare workers (HCWs) for vaccination. It was important to evaluate the effectiveness of the vaccine under the prevailing real-life situation. This study was undertaken to present evidence on the safety, immunogenicity and effectiveness of the ChAdOx1 nCoV-19 coronavirus vaccine (recombinant covishield) in HCWs in a teriary care hospital in north India. The findings on the immunogenicity and safety after the first dose of vaccination have been published as preprint1. Here we present the comprehensive analysis on safety, effectiveness and immunogenicity after the completion of two doses of vaccination. The comparison on the outcomes after two doses of vaccine vis a vis one dose of vaccine has also been highlighted.
Material & Methods
The study design was prospective, observational study. The study covered three aspects, namely safety, immunogenicity and effectiveness of vaccination in different groups. The study has two groups, first one was whole HCWs and second a subsample in which blood samples and information on adverse events were collected. For the second group, immediately after the announcement of vaccination roll-out and before the start of vaccination, blood samples were collected from each of the enrolled participant to be used to evaluate the outcomes after vaccinations. Thereafter, the participants were followed up after each vaccination. The final second group included participants who gave blood samples at all the three time points (n=976). The information on adverse events after each vaccination was recorded in a predesigned daily diary. Effectiveness evaluation group covered all HCWs in the hospital (n=6550) along with their information on RT-PCR confirmed COVID-19 infection, disaggregating according to those who received both the doses of vaccine, only single dose and those who did not take any vaccine. Those who were currently infected with COVID-19, were not considered eligible for the study. In addition, those who had previously participated in COVID-19 prophylactics or drug trials and those not eligible for vaccination due to allergies or other reasons, were excluded. Written informed consent was taken from each eligible HCW for participation in the study. The study was approved by the Institutional Ethics Committee. For each participant, baseline information was collected on demographic particulars (age and gender), role in the hospital (clinical and non-clinical), nature of exposure (low and high risk) and past history of COVID-19 disease, etc. Unique study identifiers assigned to each participants facilitated linking of participants background information with immunogenicity data and adverse events information. Quality assurance (QA) was undertaken by QA coordinator.
Immunogenicity: Blood samples (3 ml) from HCWs enrolled in the study were collected in Serum Separated Tube (SST, Yellow Top Vacutainer, BD Pvt. Limited, India) aseptically by venepuncture at three time points, i.e., baseline (day 0, day of vaccination), day 14±2 after administration of dose 1 and day 28±7 after dose 2 of the vaccine. The time gap between the two doses was 28 days. The SARS-CoV-2 S1/S2 IgG antibody concentrations (AU/ml) were measured in the samples by the fully automated LIAISON® SARS-CoV-2 S1/S2 analyzer (Dia Sorin S.p.A, USA). The kit was Conformitè Europëenne Mark (CE Mark) which was equivalent to Bureau of Indian Standard (BIS) certification in India. The LIAISON assay detects specific IgG antibodies specific to S1 and S2 spike proteins of SARS-CoV-2 in human serum samples.
Safety: The collection of information on adverse events was for seven days after each vaccination, in a structured diary for passive self-reporting of local and systemic solicited adverse events with their severity. The specific adverse events considered as local were pain at injection site, pruritus associated with injection, erythema, swelling induration, tenderness soreness and warmth, and those under systemic as acute allergic reaction, fatigue, fever, generalized pain, headache, joint pain, muscle pain/myalgia, nausea, rash and vomiting.
Effectiveness: For effectiveness among all HCWs those who developed COVID-19 infection were identified through electronic hospital information system alert. This was done separately for those who received both the vaccines, only single dose and not vaccinated at all.
Statistical analysis: The participants were classified according to demographic characteristics, role in hospital, nature of exposure, and past history of COVID-19 disease. The data on immunogenicity were analyzed in terms of seropositivity rate at different time points, calculated by using the cut-off of IgG levels of 15.0 AU/ml or more2. The results on seropositivity rate are presented in terms of absolute number and percentage along with 95 per cent confidence interval (CI). The disaggregated results on these are presented according to background characteristics. Cochran’s Q test with McNemar as a post hoc test was applied for testing the change in seropositivity over three time points. For testing the difference in seropositivity rate between groups, a two sample Z test was used. The immunogenicity was also analyzed in terms of mean±SD of IgG titre levels.
The data on adverse events were analyzed in terms of day of onset and severity. This was done separately for each adverse events as well as any of the local and systemic adverse events or both. The results are presented in terms of absolute numbers and percentage. McNemar-Bowker test was used to test the difference in paired proportion of adverse events after dose one and two. This was done separately for local or systemic adverse events.
The incidence of COVID-19 for fully, partially and unvaccinated HCWs was calculated separately. A two sample Z-test was used for testing of differences in incidence rates between the two groups. Based on the estimated incidence rate, the risk ratio was computed for fully vaccinated group in comparison to unvaccinated group. It was observed that the profile of HCWs by gender, age and role in the hospital was different in fully vaccinated and unvaccinated groups, which required adjustment for computation of RR. In view of this, hazard rate was calculated using Cox-proportional hazard model after adjusting for age, gender and role in the hospital. The effectiveness was calculated as compliment of hazard/risk ratio3. Kaplan-Meier test was used to depict the difference in the incidence of COVID-19 infection over time for fully, partially and unvaccinated groups. The duration of follow up for all the three groups was from February 16 to May 31, 2021. IBM SPSS Statistics Software for Windows, Version 24.0. (IBM Corp. Armonk, NY, USA) was used for the analysis.
Results
Immunogenicity: The information on IgG titres was available for 976 participants who received both the vaccines and gave blood sample at the three time points. This sample was adequate for disaggregated analysis according to gender, age, role in hospital, nature of exposure and past history of COVID-19. Seropositivity rate was 51 per cent at baseline, 77 per cent after first dose and 98 per cent after both the doses. Those with a past history of COVID-19 attained 98 per cent seropositivity rate with one dose of vaccination as compared to others getting the same level after two doses (Table I). Cochran’s Q with McNemar as a post hoc test revealed significant improvements in seropositivity with vaccinations across all categories. The mean IgG titre for COVID-19 infected and COVID-19 protected (IgG level ≥15 AU/ml) revealed the same trend. The distribution of IgG levels among participants which was left skewed before vaccination completely shifted to a right skewed after the second dose. To be specific it was L shaped before vaccination, changed to U shaped after first dose and J shape after two vaccination (Fig. 1).
| Characteristics | n=976 | COVID-19 seropositivity (IgG≥15.0 AU/ml), per cent (95% CI) | Cochran’s Q | P | ||
|---|---|---|---|---|---|---|
| Before first dose of vaccination (baseline) | After first dose of vaccination | After second dose of vaccination | ||||
| Gender | ||||||
| Male | 721 | 50.3 (46.6-54.1) | 78.2 (75-81.2) | 98.6 (97.5-99.3) | 518.737 | <0.001 |
| Female | 255 | 53.3 (47-59.6) | 75.3 (69.5-80.5) | 97.6 (94.9-99.1) | 168.018 | <0.001 |
| P | 0.412 | 0.336 | 0.296 | |||
| Age (yr) | ||||||
| ≤50 | 880 | 51.5 (48.1-54.8) | 78.8 (75.9-81.4) | 98.6 (97.6-99.3) | 621.599 | <0.001 |
| >50 | 96 | 47.9 (37.6-58.4) | 65.6 (55.2-75) | 95.8 (89.7-98.8) | 67.625 | <0.001 |
| P | 0.508 | 0.003 | 0.040 | |||
| Role in the hospital | ||||||
| Clinical HCW | 236 | 44.1 (37.6-50.6) | 73.3 (67.2-78.8) | 98.3 (95.7-99.5) | 189.431 | <0.001 |
| Non-clinical HCW | 740 | 53.4 (49.7-57) | 78.8 (75.6-81.7) | 98.4 (97.2-99.2) | 496.315 | <0.001 |
| P | 0.013 | 0.079 | 0.938 | |||
| Nature of exposure | ||||||
| Low risk exposure | 793 | 50.8 (47.3-54.3) | 78.1 (75-80.9) | 98.1 (96.9-98.9) | 557.906 | <0.001 |
| High risk exposure | 183 | 52.5 (44.9-59.9) | 74.9 (67.9-81) | 99.5 (97-99.9) | 129.093 | <0.001 |
| P | 0.689 | 0.351 | 0.196 | |||
| Past history of COVID-19 disease | ||||||
| No | 838 | 45.3 (41.9-48.8) | 74 (70.9-76.9) | 98.1 (96.9-98.9) | 655.732 | <0.001 |
| Yes | 138 | 86.2 (79.3-91.5) | 98.6 (94.8-99.8) | 100 (97.4-100) | 34.421 | <0.001 |
| P | <0.001 | <0.001 | 0.102 | |||
| Severity of COVID-19 (n=138) | ||||||
| Mild | 128 | 85.9 (78.7-91.4) | 98.4 (94.5-99.8) | 100 (97.2-100) | 32.444 | <0.001 |
| Moderate/severe | 10 | 90.0 (55.5-99.7) | 100 (59-100) | 100 (59-100) | 2.000 | 0.368 |
| P | 0.720 | 0.690 | 1.000 | |||
| Past history of COVID-19 disease (time since onset)# n=138 (months) | ||||||
| <3 | 54 | 88.9 (77.4-95.8) | 96.3 (87.2-99.5) | 100 (93.4-100) | 9.333 | <0.001 |
| >3 | 84 | 84.5 (75-91.5) | 100 (95.7-100) | 100 (95.7-100) | 26.000 | <0.001 |
| P | 0.468 | 0.076 | 1.000 | |||
High risk exposure: Doctors, nurses and ground duty assistance providing care to a COVID-19 patients in the hospital and lab worker handling specimens; Low risk exposure: Others, #Duration is time in months between date of enrolment in the study and date of past COVID-19 infection. HCW, healthcare worker; CI, confidence interval
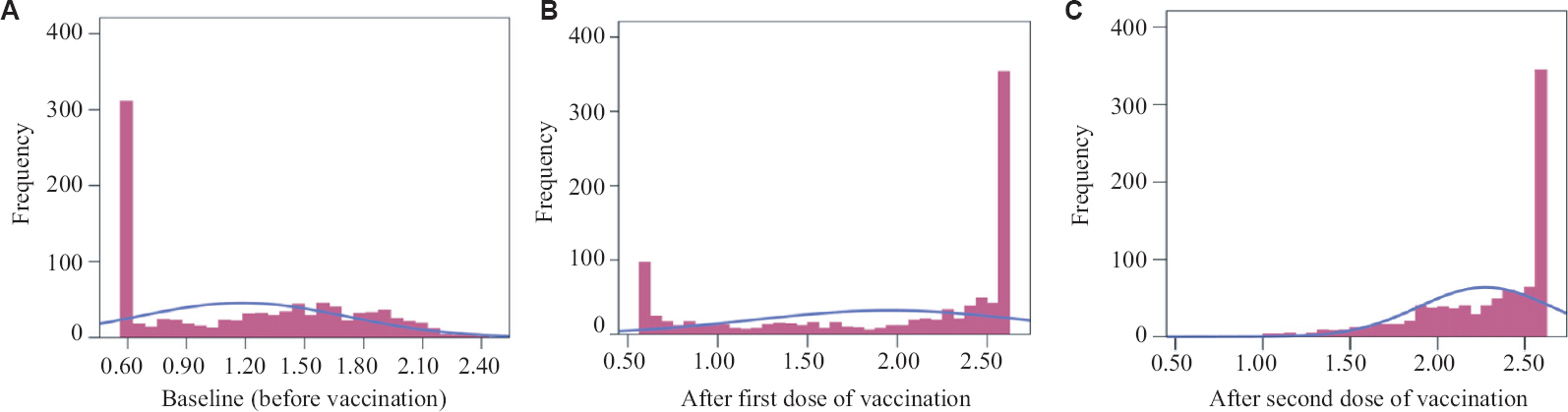
- Distribution of log IgG titre levels of healthcare workers at baseline (A) before vaccination, (B) after the first, and (C) after the second dose of vaccination (n=976).
Safety: The information on adverse event was analyzed for the same 976 participants as in immunogenicity. The results are presented on reporting of at least one (any) of the local or systemic or any of the two categories of adverse events. The proportion of HCWs reporting any adverse event after first dose of vaccination was 59.8 per cent for local events, 58.4 per cent for systemic events and either of these were 69.7 per cent. The corresponding figures after the second dose were 14.2 per cent for local events, 11.6 per cent for systemic events and either of these as 17.7 per cent. There was a sharp decline in adverse events after the second dose as compared to the first dose. About 90 per cent of adverse events were reported on the first day after the vaccination. After testing the difference in proportion of adverse event after the first and the second dose at different time points was the results indicated significant reduction in adverse events after the second dose in comparison to the first dose over time (Table II). In the present study, the adverse events were reported less by individuals of older age >50 (59.4%) yr as compared to ≤50 yr (70.8%), the difference being more than 10 percentage points. As to the individual adverse events, after the first dose of vaccination, pain at injection site was reported highest by 55.9 per cent followed by fever by 39.8 per cent, headache by 36.8 per cent and muscle pain/myalgia by 32.1 per cent. After the second dose of vaccination, only pain at injection site was reported by a significant proportion (12.8%) and other symptoms were reported by less than five per cent. As to the severity of symptoms after the first dose of vaccination, for pain at injection site, it was reported as mild (37.1%), moderate (14.5%) and severe (4.3%). For the same, after the second dose, the severity reduced to mild (9.9%), moderate (2.3%) and severe (0.4%). For fever after the first dose, the severity was mild (9.6%) moderate (10.8%) and severe (6%), the corresponding figures after the second dose being mild (4.2%), moderate (0.5%) and severe (0.0%) (Table III).
| Days of onset | Any local solicited adverse events | Any systemic solicited adverse events | Any local/systemic solicited adverse events | |||
|---|---|---|---|---|---|---|
| After first dose, n (%) | After second dose, n (%) | After first dose, n (%) | After second dose, n (%) | After first dose, n (%) | After second dose, n (%) | |
| 1 | 521 (53.4) | 129 (13.2) | 514 (52.7) | 94 (9.6) | 629 (64.4) | 156 (16) |
| 2 | 58 (5.9) | 8 (0.8) | 49 (5) | 14 (1.4) | 46 (4.7) | 12 (1.2) |
| 3 | 2 (0.2) | 0 | 3 (0.3) | 1 (0.1) | 2 (0.2) | 1 (0.1) |
| 4 | 1 (0.1) | 1 (0.1) | 1 (0.1) | 2 (0.2) | 1 (0.1) | 3 (0.3) |
| 5 | 0 | 0 | 0 | 1 (0.1) | 0 | 0 |
| 6 | 0 | 0 | 0 | 1 (0.1) | 0 | 1 (0.1) |
| 7 | 2 (0.2) | 1 (0.1) | 3 (0.3) | 0 | 2 (0.2) | 0 |
| Any of the seven days | 584 (59.8) | 139 (14.2) | 570 (58.4) | 113 (11.6) | 680 (69.7) | 173 (17.7) |
| McNemar-Bowker Test | 403.4 | 411.6 | 466.6 | |||
| P | <0.001 | <0.001 | <0.001 | |||
| Adverse events | After first dose of vaccination | After second dose of vaccination | ||||||
|---|---|---|---|---|---|---|---|---|
| Incidence n (%) | Severity | Incidence n (%) | Severity | |||||
| Mild n (%) | Moderate n (%) | Severe n (%) | Mild n (%) | Moderate n (%) | Severe n (%) | |||
| Local adverse events | ||||||||
| Pain at injection site | 546 (55.9) | 362 (37.1) | 142 (14.5) | 42 (4.3) | 125 (12.8) | 97 (9.9) | 22 (2.3) | 4 (0.4) |
| Tenderness soreness | 206 (21.1) | 88 (9) | 46 (4.7) | 14 (1.4) | 36 (3.7) | 27 (2.8) | 3 (0.3) | 3 (0.3) |
| Swelling induration | 99 (10.1) | 47 (4.8) | 22 (2.3) | 4 (0.4) | 16 (1.6) | 14 (1.4) | 2 (0.2) | 0 |
| Redness erythema | 65 (6.7) | 29 (3) | 14 (1.4) | 2 (0.2) | 13 (1.3) | 8 (0.8) | 2 (0.2) | 0 |
| Pruritus associated with injection | 35 (3.6) | 14 (1.4) | 3 (0.3) | 1 (0.1) | 3 (0.3) | 0 | 0 | 0 |
| Systemic adverse events | ||||||||
| Fever | 388 (39.8) | 94 (9.6) | 106 (10.8) | 59 (6) | 47 (4.8) | 41 (4.2) | 5 (0.5) | 0 |
| Headache | 359 (36.8) | 90 (9.2) | 97 (9.9) | 49 (5) | 50 (5.1) | 33 (3.4) | 10 (1) | 5 (0.5) |
| Muscle pain/myalgia | 313 (32.1) | 78 (8) | 98 (10) | 45 (4.6) | 48 (4.9) | 39 (4) | 7 (0.7) | 2 (0.2) |
| Fatigue | 280 (28.7) | 80 (8.2) | 71 (7.3) | 41 (4.2) | 39 (4) | 31 (3.2) | 7 (0.7) | 1 (0.1) |
| Joint pain | 213 (21.8) | 54 (5.5) | 57 (5.8) | 24 (2.5) | 23 (2.4) | 16 (1.6) | 6 (0.6) | 1 (0.1) |
| Nausea | 128 (13.1) | 46 (4.7) | 23 (2.4) | 16 (1.6) | 14 (1.4) | 12 (1.2) | 1 (0.1) | 0 |
| Warmth | 125 (12.8) | 87 (8.9) | 27 (2.8) | 11 (1.1) | 19 (1.9) | 14 (1.4) | 4 (0.4) | 1 (0.1) |
| Generalized pain | 116 (11.9) | 42 (4.3) | 32 (3.3) | 14 (1.4) | 19 (1.9) | 12 (1.2) | 4 (0.4) | 0 |
| Vomiting | 66 (6.8) | 17 (1.7) | 11 (1.1) | 13 (1.3) | 5 (0.5) | 4 (0.4) | 0 | 0 |
| Acute allergic reaction | 20 (2) | 1 (0.1) | 11 (1.1) | 2 (0.2) | 2 (0.2) | 2 (0.2) | 0 | 0 |
| Rash | 14 (1.4) | 6 (0.6) | 3 (0.3) | 2 (0.2) | 2 (0.2) | 1 (0.1) | 1 (0.1) | 0 |
Effectiveness: Overall, COVID-19 infection among HCWs occurred in 300 of the 2766 fully vaccinated, 269 of the 2111 partially vaccinated and 371 of the 1673 unvaccinated during the follow up period of three and half months. The long rank test (121.1, df=2) indicated significant difference in cumulative probability of COVID-19 infections among these three groups (P=0.001). Cumulative COVID-19 incidence over time is shown in Fig. 2. The incidence of COVID-19 was estimated as 11.7 per cent among fully vaccinated, 12.7 per cent among partially vaccinated and 22.2 per cent among unvaccinated HCWs. The incidence of COVID-19 was significantly less among vaccinated (11.7%) as compared to not vaccinated (22.2%) (P=0.001). The difference was also significant between the fully vaccinated (10.8%) and partially vaccinated (12.7%) (P=0.040). As the profile of HCWs among fully, partially and unvaccinated was different, cox-proportional hazard model was applied to calculate the hazard rate for COVID-19 infection among unvaccinated versus fully vaccinated after adjusting for covariates. The model indicated that the hazard was higher among males (HR=1.42, 95% CI: 1.20-1.68, P<0.0001), age >50 yr (HR=1.52, 95% CI: 1.07-2.15, P=0.019), and clinical role in the hospital (HR=1.42, 95% CI: 1.20-1.68, P<0.001). After adjustment for age, gender, and role in hospital, hazard of COVID-19 infection was more than two times among unvaccinated as compared to fully vaccinated. This indicated hazard/risk ratio as 0.478. Thus, the effectiveness of vaccine was 52.2 per cent (Table IV).
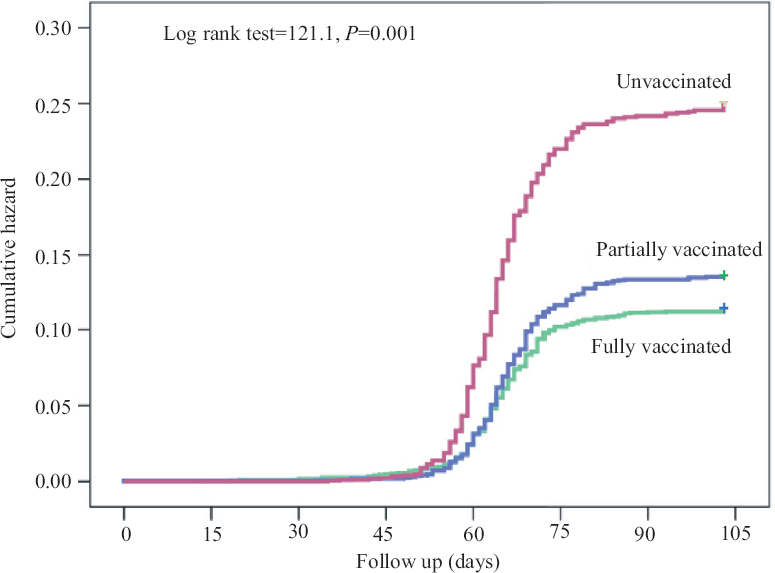
- Kaplan-Meier curve - Cumulative probability of COVID-19 infection.
| Vaccination status | Number of HCWs | COVID-19 positive | COVID-19 negative | COVID-19 infection incidence rate, per cent (95% CI) | |
|---|---|---|---|---|---|
| Fully vaccinated (two doses) | 2766 | 300 | 2466 | 10.8 (9.7-12.0) | |
| Partially vaccinated (one dose) | 2111 | 269 | 1842 | 12.7 (11.3-14.2) | |
| Unvaccinated | 1673 | 371 | 1302 | 22.2 (20.2-24.2) | |
| Comparison of incidence rates of COVID-19 infection | |||||
| Fully versus partial vaccinated (%) | 10.8 versus 12.7; P=0.040 | ||||
| Vaccinated versus unvaccinated (%) | 11.7 versus 22.2; P=0.001 | ||||
| Cox proportional hazard model | |||||
| Title | Beta coefficient | HR | 95% CI for HR | P | |
| Lower | Upper | ||||
| Male | 0.352 | 1.42 | 1.20 | 1.68 | <0.001 |
| Age >50 yr | 0.418 | 1.52 | 1.07 | 2.15 | 0.019 |
| Clinical HCW | 0.352 | 1.42 | 1.20 | 1.68 | <0.001 |
| Unvaccinated | 0.736 | 2.09 | 1.78 | 2.44 | <0.001 |
| Hazard of COVID-19 infection among fully vaccinated versus unvaccinated after adjusting for age and role in hospital | 47.8 (1/2.09×100) | ||||
| Effectiveness of the vaccine=1-Risk/HR | 52.2 | ||||
HR, hazard ratio; CI, confidence interval; HCWs, healthcare workers
Discussion
The vaccine appeared immunogenic, with seropositivity increasing to 98 per cent after two doses. Singh et al4 reported seropositivity of 98.1 per cent in their serological evaluation after two doses of ChAdOx1 nCoV in HCWs. Bradley et al5 reported that ‘‘the response to the first dose of SARS-CoV-2 vaccine among previously infected individuals could help guide the allocation of the limited supply of mRNA-based vaccines”. Ibarrondo et al6 reported that “in persons with prior COVID-19 one dose boosted levels to the high end of severe natural infection even in those who never had robust responses from infections increasing no further after the second dose”.
In our study, 69.7 per cent of HCWs reported at least one adverse events after first dose of vaccination. This finding was similar to that reported by Jayadevan and Shenoy7. Kaur et al8 reported that “around one half of the vaccine recipients developed adverse events at any time post vaccination. The adverse events were reported more by those in the age group ≤50 yr (70.8%) as compared to those in the age group >50 (59.4%). Further, adverse events were seen more among those with past history of COVID-19 (76.8%) as compared to those with no past history (68.5%)”. This findings was similar to that of Ramasamy et al9 who noted that “ChAdOx1 nCoV-19 appears to be better tolerated in older adults than in younger adults”. Kaur et al8 reported that ‘individually fever, injection site pain and headache were commonly observed any adverse event following immunization’. As to the severity of symptoms after the first dose of vaccination, these were reported mainly mild and moderate, the severe were only in about four per cent. Kaur et al8 also reported that majority of events were mild to moderate in severity. The vaccine was observed to be providing protection at the level of 52.2 per cent. Voysey et al10 after analyzing data from four trials (Brazil, and South Africa and the UK) reported that ‘in participants who received two standard doses, vaccine efficacy was 62.1 per cent’.
This study had certain limitations. The data on antibody levels could not be collected for unvaccinated HCWs and for those who completed only single vaccination at comparable time points with those who received both the vaccination. Such data would have given important insights on change in antibody levels with different vaccination status over time.
The ChAdOx1 nCoV-19 vaccine (Recombinant) appeared to be safe, immunogenic and showed effectiveness against COVID-19 disease.
Acknowledgment:
The authors acknowledge the support and guidance of Shri Pankaj Sahni (CEO), Dr Arvinder Singh Soin, Chairperson (Liver Transplant Surgery), Shri Rajiv Sikka (Head IT) and the IT team at Medanta. The authors also thank to Shri Manbir Singh, Senior Lab Technician, Microbiology, Neha Jakhar, Trainee Microbiology; the Clinical Research team consisting of Shri Dheeraj Sharma, Ms Rita Maity, Servshree Rehan Khan, Bajarang, Ms Arunima, Ms Ruchika, Shri Iqbal, Ms Rhea Aggarwal (Medical student), Mahatma Gandhi Institute of Medical Sciences, Sewagram, Clinical research and Pharmacy interns from Apeejay Stya University, GD Goenka University, Jamia Hamdard University, and all staff of the Medanta-The Medicity Hospital, Gurugram and all the participants of this research project.
Financial support & sponsorship: This study received funding support from an intramural grant of the Medanta Institute of Education and Research.
Conflicts of Interest: None.
References
- A real world evaluation of the safety and immunogenicity of the covishield vaccine, ChAdOx1 nCoV-19 corona virus vaccine (recombinant) in health care workers (HCW) in national capital region (NCR) of India:A preliminary report. medRxiv 2021 Doi:10.1101/2021.04.14.21255452
- [Google Scholar]
- DiaSorin. Liaison®SARS-CoV-2 S1/S2 IgG. The fully automated serology test for the detection of SARS-CoV-2 IgG antibody. Available from: https://www.diasorin.com/sites/default/files/allegati/liaisonr_sars-cov2_s1s2_igg_brochure. pdf.pdf
- Centers for Disease Control and Prevention. Lesson 3:Measures of risk. Section 6:Measures of public health impact. Available from: https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section6.html
- Antibody response after first and second-dose of ChAdOx1-nCoV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India:The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine. 2021;39:6492-6509.
- [Google Scholar]
- Antibody responses boosted in seropositive healthcare workers after single dose of SARS-CoV-2 mRNA vaccine. medRxiv 2021 Doi:10.1101/2021.02.03.21251078
- [Google Scholar]
- Primary, recall, and decay kinetics of SARS-CoV-2 vaccine antibody responses. ACS Nano. 2021;15:11180-91.
- [Google Scholar]
- Survey of symptoms following COVID-19 vaccination in India. medRxiv 2021 Doi: https://doi.org/10.1101/2021.02.08.21251366
- [Google Scholar]
- A prospective observational safety study on ChAdOx1 nCoV-19 corona virus vaccine (recombinant) use in healthcare workers-first results from India. EClinicalMedicine. 2021;38:101038.
- [Google Scholar]
- Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002):A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979-93.
- [Google Scholar]
- Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2:An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111.
- [Google Scholar]






