Translate this page into:
Clinicopathological study of annexin A5 & apelin in pre-eclamptic placentae with emphasis on foetal outcome
For correspondence: Dr Senjuti Dasgupta, Department of Pathology, Medical College Kolkata, Kolkata 700 073, West Bengal, India e-mail: dasguptasenjuti@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Pre-eclampsia has remained an elusive disease with serious impacts on both maternal and foetal health. Two novel markers, annexin A5 (ANXA5) and apelin are currently of considerable interest. The present study aimed to determine the placental expression of ANXA5 and apelin in pre-eclamptic placentae and also to elucidate if there is any correlation between the expression of these markers and the clinical features of both, mother and neonate. The comparison between gross and histopathological features of pre-eclamptic placentae and controls was another objective.
Methods:
A prospective, observational study was undertaken for one year. Placentae of pre-eclamptic patients and matched controls (matched for age, ethnic and socio-economic background) were collected along with the clinical data. Gross and histopathological analyses were done and immunohistochemical study of placental sections with ANXA5 and apelin was also undertaken.
Results:
79 pre-eclamptic patients and equal numbers of matched controls were included in the study. The difference in weight and dimensions of placentae between the pre-eclampsia group and matched controls was significant. Histopathological features noted in the pre-eclamptic placentae included decidual vasculopathy, infarction, perivillous fibrin deposition, increased syncytial knots and distal villous hypoplasia. There was a significant reduction in the expression of both ANXA5 and apelin in pre-eclamptic placentae compared to controls. Among pre-eclamptic patients, the intensity of ANXA5 and apelin expression showed a significant association with respect to neonatal resuscitation. Furthermore, the intensity of apelin showed expression a significant correlation with the requirement of sick neonatal care unit treatment.
Interpretation & conclusions:
The results of the present study suggest that both ANXA5 and apelin levels are reduced in pre-eclamptic placentae. Hence, it is recommended to further explore the impact of these markers on pregnancy outcomes by undertaking randomized controlled trials.
Keywords
Annexin A5
apelin
clinicopathological analysis
immunohistochemical study
placenta
pre-eclampsia
Pre-eclampsia is a multisystem disorder, characterized by new-onset hypertension and proteinuria occurring after 20 wk of pregnancy1. It is encountered in 5-7 per cent of all pregnancies worldwide and has serious adverse effects both on maternal and foetal health. The pre-eclamptic pregnancies need to be monitored closely and are associated with both maternal and foetal morbidity and mortality. The associated foetal complications include pre-maturity and growth restriction1.
Pre-eclampsia imposes a serious burden on the social, health and financial aspects of pregnancy. Till date, no cure has been found for this disorder. The only definitive management available is the termination of pregnancy, which is associated with the risk of premature birth2.
Annexin A5 (ANXA5) and apelin are the novel markers of placenta which play an important role in maintaining placental homoeostasis. ANXA5 is a part of annexin family of proteins, which consists of 12 highly conserved and structurally related proteins in both humans and mice3. ANXA5 plays an important role in various cellular processes including mineralization of cartilage, intracellular signalling and inhibition of protein kinase C and phospholipase A24. It has been proposed that the binding of ANXA5 to phosphatidylserine on vascular endothelial cells in the placenta is vital for the suppression of inappropriate blood coagulation during pregnancy4.
The placental trophoblasts express ANXA5 and the protein is immunolocalized to the apical surfaces of syncytiotrophoblasts that line the chorionic villi. ANXA5 has a thrombomodulatory function in maintaining placental circulation and placental integrity5.
Apelin is the product of APLN gene. It is an endogenous ligand of APJ, which is a G protein-coupled receptor. APJ is closely associated with angiotensin receptor (AT1)6. The apelin/APJ complex plays a significant role in cardiovascular pathophysiology7. It acts as a potent inotropic agent and also causes endothelium-mediated vasodilation8. The principal site of synthesis of apelin is the lung, but it is also produced in the adipose tissue, placenta and lactating breast9. A paracrine role of apelin has been suggested in human chorionic villi as it has been identified in the cytotrophoblasts, syncytiotrophoblasts and foetal endothelial cells1011.
Pre-eclampsia has continued to allure the medical fraternity with its mysterious pathophysiology (of which only some aspects are now known) and also by the continued search for its cure. A healthy placenta is vital for a healthy foetus. This study was hence aimed at analyzing the gross and histopathological features of pre-eclamptic placentae and compare these findings with those in matched control placentae. It further aimed to shed new light on the understanding of the cumulative roles of ANXA5 and apelin in the placental health in cases of pre-eclampsia. The objective was also to find out if there was any correlation between placental expression of these two markers in pre-eclampsia and clinical features pertaining both to the mother and neonate.
Material & Methods
This prospective, observational study was carried out for a period of one year at the department of Pathology, Medical College, Kolkata, West Bengal, India, a tertiary care centre between November 2019 and October 2020. The cases of pre-eclampsia admitted in the department of Gynaecology and Obstetrics of the institute during this time span were consecutively included in the study after procuring approval the Institutional Ethics Committee.
Inclusion & exclusion criteria: The inclusion criteria were systolic blood pressure (SBP) greater than 130 mmHg, diastolic blood pressure (DBP) greater than 90 mmHg on at least two measurements and taken least 6 h apart, in association with proteinuria of more than 300 mg in 24 h urine. Patients with a history of miscarriage and accompanying diseases, pregnant women who had placental hematoma or multiple gestations were excluded from the study. Initially, 87 patients were included in the study. However, eight patients were later excluded as five of these did not give consent and the other three had one or more complications of pregnancy, in addition to pre-eclampsia. Frequency matching was done to delineate the control group, which included healthy pregnant females with no maternal complications. Their age group, ethnic and socioeconomic background were similar to those of the study subjects. Healthy females with multiple gestations were excluded from the control group. Foetal growth restrictions (FGRs) without pre-eclampsia were also excluded from this group.
Sample size and study participants: The sample size was determined using the formula z2pq/d2 where z is the value of standard normal distribution corresponding to a significance level of 0.05 (z=1.96), p is prevalence of pre-eclampsia in India (P=25.3%), q is 1−p and d is permissible error (10%)1213. By using the above formula, the minimum sample size calculated was approximately 73. The power of the study with this sample size when calculated using Epi Info website (www.cdc.gov/epiinfo/EpiInfo) was 80 per cent.
All the patients included in the study were divided into two groups – the patients with DBP <110 mmHg and SBP <160 mmHg were designated as blood pressure (BP) group 1 and those with DBP ≥110 mmHg and SBP ≥160 mmHg, or only DBP ≥110 mmHg, or only SBP ≥160 mmHg were designated as BP group 214. Only BP was considered to categorize patients as mild and severe pre-eclampsia as there were patients both in BP group 1 as well as 2 who complained of headache, visual disturbance and epigastric pain. The patients in BP group 2 did not have oliguria, convulsion, thrombocytopaenia and elevated serum transaminase. Therefore, these features were not considered to categorize into mild and severe pre-eclampsia groups.
Study parameters and protocol: Maternal and foetal outcomes were studied in all cases. The placentae of these patients were collected in 10 per cent neutral-buffered formalin. Placentae from 79 matched controls (who underwent delivery in the same institute) were also similarly collected. Gross examination of all specimens was done in detail. Relevant sections were taken from the specimens and submitted for processing. Sections from the placenta were taken from the following sites: umbilical cord cut margin, umbilical cord at a distance of 2 cm from insertion, placental membranes, area of insertion of umbilical cord in placental parenchyma, area of infarct and placental parenchyma. In case of velamentous insertion of the umbilical cord, sections were also taken from the cord along with the surrounding membrane.
Sections were cut from the paraffin blocks and stained with haematoxylin and eosin (H and E) stain for the histopathological examination. Immunohistochemistry (IHC) was performed on the placental tissue sections using ANXA5 and apelin antibodies. The ANXA5 clone orb36728 and apelin clone orb247041 (both manufactured by Biorbyt, UK) were used. ANXA5 primary antibody dilution was done at 1:100 and that of apelin at 1:200. Negative controls were obtained by omitting the primary antibody. For ANXA5 antibodies, the positive control used was sections of hepatocellular carcinoma. Human glial tissue was used as positive control for apelin antibodies (provided in the kit).
Scoring: All slides were reported by two pathologists independently. The histopathological findings were recorded after careful examination of H and E stained slides. The ANXA5 and apelin-stained IHC slides were reported both in terms of extent and intensity of staining. The slides were first observed under low power to delineate the ‘hot spots’. Then, ×400 was used to count at least 300-500 cells in 10 high-power fields within these ‘hot spots’. The percentage of positive cells were scored as follows – no immunoreactive cells (score 0), upto 10 per cent (score 1), 10-50 per cent (score 2), 51-80 per cent (score 3) and above 80 per cent immuoreactive cells (score 4). The intensity of staining of the cells was also scored – very weak (score 0.5), weak (score 1), mild (score 2) and strong (score 3)15. In case of discrepancies between the findings of the two pathologists, a consensus was reached after re-examining the slides together.
Statistical analysis: Normally distributed continuous variables were expressed as mean and range. These variables were compared between pre-eclampsia and control groups and between BP group 1 and 2 of pre-eclampsia using unpaired t test - two-tailed. The categorical variables were expressed as percentages and compared between the groups using Fischer’s exact test or Chi-square test. All statistical analyses were done using GraphPad Prism 6™ (San Diago, CA, USA). P<0.05 was considered statistically significant.
Results
Among the 79 patients of pre-eclampsia included in the study, 54 (68.4%) were assigned to BP group 1 and the rest (25, 31.6%) to BP group 2. Sixteen (20.3%) of pre-eclamptic patients delivered pre-term babies, whereas eight (10%) were delivered post-term and 55 (69.62%) delivered at term. The minimum gestational age at which a pre-term baby was delivered was 26 wk. None of the babies was previable. While there was neither maternal nor foetal loss in the study population, 30 (38%) babies were born with low birth weight, 12 (15.2%) required resuscitation at birth and 40 (50.6%) needed treatment in the sick neonatal care unit (SNCU). These features showed a significant association with pre-eclampsia, when the patients were compared with their matched controls. It was found that 32 per cent of babies born to mothers of BP group 2 required resuscitation while seven per cent of babies born to mothers of BP group 1 required resuscitation (P=0.0105). However, the rest of the aforementioned features were not significantly associated with increasing severity of BP (Table I).
| Clinical features | Comparison between PE and control groups | Comparison between BP Groups 1 and 2 | ||
|---|---|---|---|---|
| PE, n (%) | Controls, n (%) | BP Group 1, n (%) | BP Group 2, n (%) | |
| Term versus pre-term | n=71 | n=71 | n=54 | n=17 |
| Term | 55 (77)* | 71 (100)* | 39 (72) | 16 (94) |
| Pre-term | 16 (23)* | 0* | 15 (28) | 1 (6) |
| Birth weight | n=79 | n=79 | n=60 | n=19 |
| Normal birth weight | 49 (62)* | 79 (100)* | 38 (63) | 12 (63) |
| Low birth weight | 30 (38)* | 0* | 22 (37) | 7 (37) |
| Resuscitation | n=79 | n=79 | n=60 | n=19 |
| Spontaneous cry at birth | 67 (84.8)* | 79 (100)* | 56 (93)* | 13 (68)* |
| Resuscitation required at birth | 12 (15.2)* | 0* | 4 (7)* | 6 (32)* |
| Treatment in SNCU | n=79 | n=79 | n=60 | n=19 |
| Treatment in SNCU not required | 39 (49.4)* | 79 (100)* | 29 (48) | 12 (63) |
| Treatment in SNCU required | 40 (50.6)* | 0* | 31 (52) | 7 (37) |
P *<0.05. SNCU, sick neonatal care unit; BP, blood pressure; PE, pre-eclampsia
The mean birth weight of babies of pre-eclampsia group of patients was found to be 2.6±0.57 kg (range 0.8-3.6 kg) and that of BP groups 1 and 2 was 2.66±0.53 kg (range 0.85-3.5 kg) and 2.69±0.70 kg (range 1.09-3.6 kg), respectively. The mean placental weight in pre-eclampsia patients was found to be 415.23±110 g (range 200-700 g), mean placental diameter was 17.88±2.49 cm (range 13-26 cm) and mean placental thickness was 2.42±0.84 cm (range 1-5.7 cm). There was a significant difference in weight, diameter and thickness of placentae between pre-eclampsia group and their matched controls. However, the same was not true for the two BP groups of pre-eclampsia (Table II). The umbilical cords of pre-eclamptic placentae were noted to have eccentric insertion in 36 cases (45.6%), central in 24 (30.4%), marginal in 15 (18.9%) and velamentous in four (5.1%) (Fig. 1A and B). Infarcts were found in 48 (60.8%) cases, with a size range of 1-2 cm. The number of cotyledons varied from 18 to 24.
| Placental weight (g) | Placental thickness (cm) | Placental diameter (cm) | Birth weight to placental weight ratio | |
|---|---|---|---|---|
| Comparison between PE and control groups | ||||
| PE | 415±110* | 2.4±0.84* | 17.8±2.49* | 6.64±1.73* |
| Control | 478±46.81* | 2.9±0.29* | 19.2±1.92* | 5.8±0.48* |
| Comparison between BP Groups 1 and 2 | ||||
| BP group 1 | 423.5±8.36 | 2.42±0.72 | 17.85±2.21 | 6.48±1.63 |
| BP group 2 | 389.2±41.5 | 2.41±1.17 | 17.98±3.35 | 7.11±1.98 |
All values depicted as mean±SD. P *<0.05
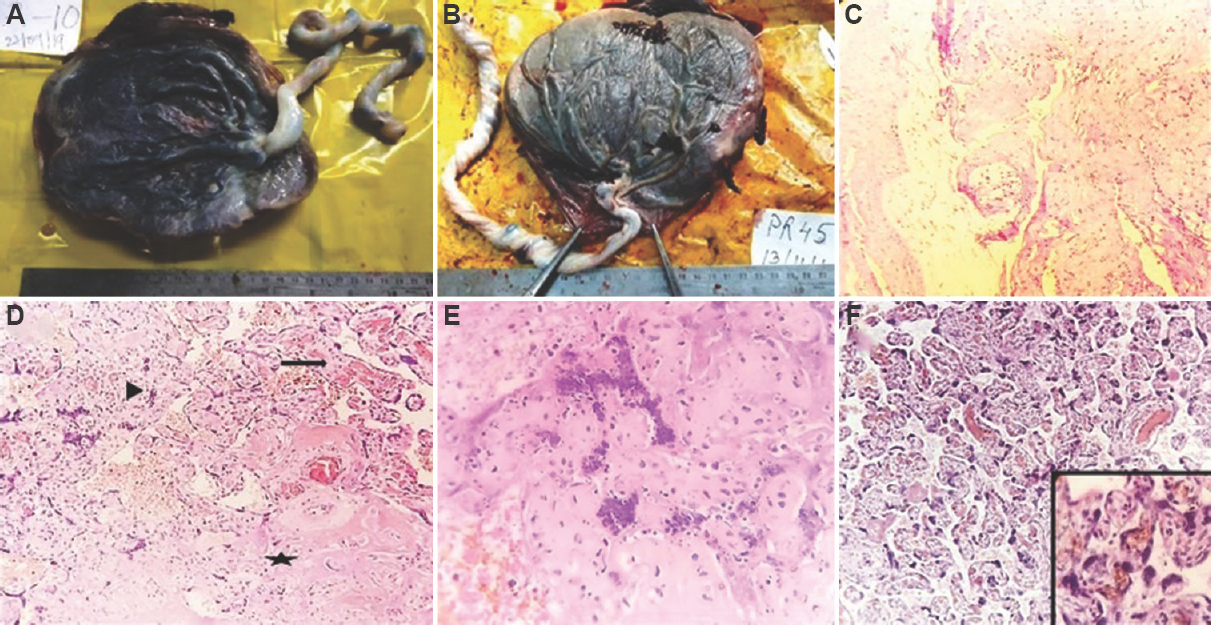
- (A) Placenta with eccentric insertion of the umbilical cord. (B) Placenta with velamentous insertion of the umbilical cord. (C) Section from the placental membrane showing atheroma formation in a vessel (H and E, ×100). Inset showing high-power view of the same (H and E, × 400). (D) Microscopic appearance of infarct showing degenerative changes in villi (star). There was gradient of ischaemic injury at the periphery with collapse of intervillous space (arrowhead). Distal villous hypoplasia is noted in adjacent parenchyma (arrow). (E) Microscopic appearance of infarct showing collapse of intervillous space, villous agglutination, smudging of nuclei, pyknosis and karyorrhexis (H and E, ×400). (F) Section showing increased syncytial knots in terminal villi (H and E, ×100). Inset showing high-power view of the same.
Microscopic examination of H and E stained slides of the placental sections showed the presence of the following features – decidual vasculopathy, infarction, perivillous fibrin deposition, increased syncytial knots and distal villous hypoplasia (Fig. 1C-F). Decidual vasculopathy was noted in 28 (35%) of pre-eclampsia cases. These cases had one or more of the following findings in the vessels present in the microscopic sections of the placental membranes – lack of physiologic conversion (diagnosed when neither transformation of arteries to enlarged tortuous rigid vessels nor evidence of replacement of arterioles by fibrinoid was noted), mural hypertrophy of arterioles, atherosis (recognized by noting fatty infiltration in endothelial cells and presence of macrophages containing phagocytosed fat), thrombosis (presence of laminations composed of alternating layers of red blood cells and fibrin) and fibrinoid necrosis of media16. Infarction and perivillous fibrin deposition were found in 62 (78%) cases. Infarction was characterized by the presence of one or more of the following features - congestion of villous capillaries, intravillous haemorrhage, villous agglutination, collapse of intervillous spaces, loss of nuclear basophilia of syncytium, smudging of nuclei, pyknosis, karyorrhexis and ghost villi16. Increased presence of syncytial knots (>30% terminal villi possessing syncytial knots)14 was noted in 14 (18%) cases. Distal villous hypoplasia was found in six (7%) cases. These microscopic features showed statistically significant association with pre-eclampsia when comparison was made with their matched controls (Table III).
| Histopathological features | Comparison between PE and control groups | Comparison between BP Groups 1 and 2 | ||
|---|---|---|---|---|
| PE, n (%) | Controls, n (%) | BP Group 1, n (%) | BP Group 2, n (%) | |
| Decidual vasculopathy | n=79 | n=79 | n=60 | n=19 |
| Present | 28 (35)* | 0* | 21 (35) | 6 (31) |
| Absent | 51 (65)* | 79 (100)* | 39 (65) | 13 (69) |
| Infarction/perivillous fibrin deposition | n=79 | n=79 | n=60 | n=19 |
| Present | 62 (78)* | 18 (23)* | 46 (77) | 16 (84) |
| Absent | 17 (22)* | 61 (77)* | 14 (23) | 3 (16) |
| Syncytial knots | n=79 | n=79 | n=60 | n=19 |
| ≤30 | 14 (18)* | 64 (81)* | 10 | 1 |
| >30 | 65 (82)* | 15 (19)* | 50 | 18 |
| Distal villous hypoplasia | n=79 | n=79 | n=60 | n=19 |
| Present | 6 (7)* | 0* | 5 | 1 |
| Absent | 73 (93)* | 79 (100)* | 55 | 18 |
P *<0.05. BP, blood pressure; PE, pre-eclampsia
IHC analysis showed maximum expression of ANXA5 in the syncytiotrophoblasts of chorionic villi whereas that of apelin was strongest in the villous stroma (Fig. 2A-F). There was reduced expression of both these markers in pre-eclamptic placentae compared to the matched controls and the reduction was statistically significant. The expression of these markers in pre-eclamptic placentae was also compared with respect to the various features reflective of foetal outcome (pre-term vs. term babies, low vs. normal birth weight, those requiring resuscitation vs. those who did not and those requiring treatment in SNCU vs. those that did not). The intensity of expression of ANXA5 showed a significant association with respect to requirement of resuscitation among pre-eclamptic patients. Furthermore, apelin intensity showed significant correlation with regard to requirement of resuscitation and SNCU treatment among the pre-eclamptic subjects (Tables IV and V). However, the grade and intensity of ANXA5 and grade of apelin (grade) did not significantly differ among the two BP groups. Of note, the intensity of apelin showed a significant decrease with the increasing severity of BP when the two BP groups were compared (P=0.01).
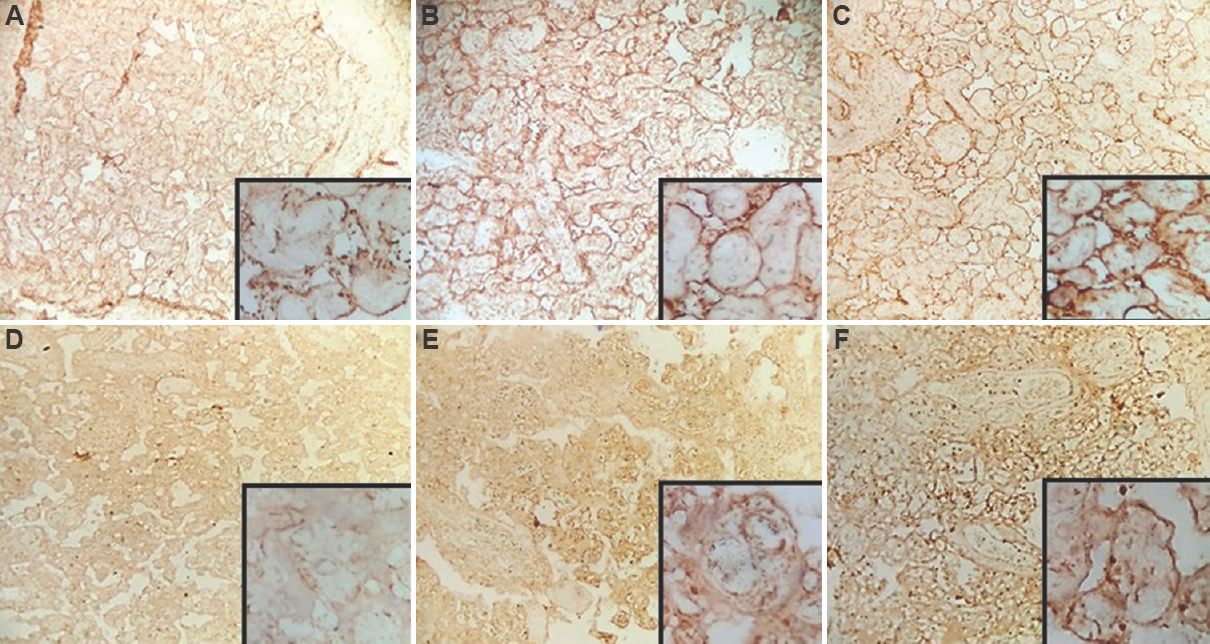
- (A-C) Annexin A5 expression in the placenta of pre-eclampsia: Expression intensity (A) 1; (B) 2; (C) 3 (H and E, ×100). (D-F) Apelin expression in the placenta of pre-eclampsia: Expression intensity (D) 1; (E) 2; (F) 3 (H and E, ×100). The insets show high-power view of the respective images.
| Parameter | ANXA5 grade | ANXA5 intensity | |||||
|---|---|---|---|---|---|---|---|
| Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) | Intensity 1, n (%) | Intensity 2, n (%) | Intensity 3, n (%) | |
| Maternal blood pressure | |||||||
| Pre-eclampsia (n=79) | 13 (16.5)* | 34 (43)* | 30 (38)* | 2 (2.5)* | 16 (20.3)* | 38 (48.1)* | 25 (31.6)* |
| Matched controls (n=79) | 3 (3.8)* | 3 (3.8)* | 15 (19)* | 58 (73.4)* | 3 (3.8)* | 3 (3.8)* | 73 (92.4)* |
| Pre-term babies (n=71) | |||||||
| Pre-term babies in PE (n=16) | 4 (25) | 6 (37.5) | 6 (37.5) | 0 | 2 (12.5) | 10 (62.5) | 4 (25) |
| Term babies in PE (n=55) | 10 (18.1) | 23 (41.8) | 20 (36.3) | 2 (3.8) | 13 (23.6) | 23 (41.8) | 19 (34.6) |
| Neonatal birth weight (n=79) | |||||||
| Low birth weight in PE patients (n=30) | 5 (16.6) | 17 (56.6) | 7 (23.8) | 1 (3) | 5 (16.7) | 17 (56.7) | 8 (26.6) |
| Normal birth weight in PE patients (n=49) | 7 (14.2) | 18 (36.7) | 22 (44.8) | 2 (4.3) | 10 (20.4) | 19 (38.7) | 20 (40.9) |
| Resuscitation (n=79) | |||||||
| Neonates requiring resuscitation among PE patients (n=12) | 1 (8.3) | 3 (25) | 7 (58.4) | 1 (8.3) | 2 (16.7)* | 2 (16.7)* | 8 (66.6)* |
| Resuscitation not required among PE patients (n=67) | 13 (19.4) | 31 (46.2) | 21 (31.5) | 2 (2.9) | 13 (19.4)* | 35 (52.2)* | 19 (28.4)* |
| Treatment in SNCU (n=79) | |||||||
| Neonates sent to SNCU among PE patients (n=40) | 8 (20) | 21 (52.5) | 10 (25) | 1 (2.5) | 9 (22.5) | 20 (50) | 11 (27.5) |
| Neonates not requiring SNCU treatment among PE patients (n=39) | 6 (15.3) | 13 (33.3) | 19 (48.7) | 1 (2.7) | 6 (15.3) | 18 (46.1) | 15 (38.6) |
P *<0.05. ANXA5, annexin A5
| Parameter | Apelin grade | Apelin intensity | |||||
|---|---|---|---|---|---|---|---|
| Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) | Intensity 1, n (%) | Intensity 2, n (%) | Intensity 3, n (%) | |
| Maternal blood pressure | |||||||
| Pre-eclampsia (n=79) | 55 (69.6)* | 20 (25.4)* | 2 (2.5)* | 2 (2.5)* | 61 (77.2)* | 16 (20.3)* | 2 (2.5)* |
| Matched controls (n=79) | 3 (3.8)* | 3 (3.8)* | 24 (30.4)* | 49 (62)* | 3 (3.8)* | 3 (3.8)* | 73 (92.4)* |
| Pre-term babies (n=71) | |||||||
| Pre-term babies in PE (n=16) | 8 (50) | 6 (37.5) | 2 (12.5) | 0 | 10 (62.5) | 6 (37.5) | 0 |
| Term babies in PE (n=55) | 41 (76.3) | 12 (20.1) | 2 (3.6) | 0 | 42 (77.3) | 11 (19.1) | 2 (3.6) |
| Neonatal birth weight (n=79) | |||||||
| Low birth weight in PE patients (n=30) | 16 (53.4) | 12 (40) | 1 (3.3) | 1 (3.3) | 23 (76.7) | 6 (20) | 1 (3.3) |
| Normal birth weight in PE patients (n=49) | 37 (75.5) | 9 (18.3) | 3 (6.2) | 0 | 37 (75.5) | 10 (19.5) | 2 (5) |
| Resuscitation (n=79) | |||||||
| Neonates requiring resuscitation among PE patients (n=12) | 8 (66.7) | 2 (16.7) | 1 (8.3) | 1 (8.3) | 10 (83.4)* | 1 (8.3)* | 1 (8.3)* |
| Resuscitation not required among PE patients (n=67) | 47 (70.1) | 18 (26.8) | 2 (3.1) | 0 | 50 (74.6)* | 17 (25.4)* | 0* |
| Treatment in SNCU (n=79) | |||||||
| Neonates sent to SNCU among PE patients (n=40) | 23 (57.5) | 15 (37.5) | 1 (2.5) | 1 (2.5) | 24 (60)* | 15 (37.5)* | 1 (2.5)* |
| Neonates not requiring SNCU treatment among PE patients (n=39) | 33 (84.5) | 6 (15.5) | 0 | 0 | 36 (92.3)* | 3 (7.7)* | 0* |
P *<0.05.
Discussion
The exact pathogenesis of pre-eclampsia is still a matter of intensive research. The principal pathophysiologic processes include abnormal placental vasculature, endothelial and coagulation abnormalities. This leads to uteroplacental ischaemia. There is also reduced endothelial production of prostacyclin and increased synthesis of pro-coagulant factors. These changes lead to a hyper-coagulable state in pre-eclampsia17.
The effects of pre-eclampsia are profound both on the placenta as well as the foetus. Most studies show a significant negative correlation between birth weight, placental weight and pre-eclampsia when compared to control placentae181920. Similar findings were obtained even in the present study. Few studies also found the decline in weights to be directly related to the severity of the disease1820. One of the studies reported the feto-placental weight ratio in normal pregnancy to be approximately 5.72±0.93 and that in pre-eclampsia to be 6.35±2.0519. The present study found comparable results and the difference was also noted to be significant (P=0.02).
Majority (36, 45.6%) of the umbilical cords of pre-eclamptic placentae in the present study were found to have eccentric insertion. Predoi et al21 stated that eccentric cord insertion results in a disturbed vascular distribution in the chorionic villi, defective transport gradient and a decline in birth weight for a particular placental weight.
Salmani et al19 found prominent histopathological features in pre-eclamptic placentae such as areas of calcification, hyalinization, increased syncytial knots and proliferation of medial coat of blood vessels. They opined that these modifications reduce the uteroplacental circulation and thereby lower the birth weight of neonates. Kambale et al20 also did not report any significant differences in the frequencies of microscopic features of placenta with increasing severity of pre-eclampsia.
In recent times, two novel markers have been studied by some researchers to better understand the complex pathogenesis of pre-eclampsia – ANXA5 and apelin. ANXA5 can be found in platelets, endothelial cells and placental villi. It is an anionic phospholipid binding protein with anticoagulant activity. By inhibiting prothrombin activation, it prevents thrombus formation under normal venous and arterial flow conditions. It has been suggested that ANXA5 may protect the syncytial surface against clot formation. Lower amount of ANXA5 expression on trophoblasts has been noted in pre-eclamptic patients compared to healthy pregnant women15.
In the present study, the expression of ANXA5 was found to be diminished in pre-eclamptic placentae, when compared with matched controls. Colcimen et al15 corroborated the reduced levels of ANXA5 immunoreactivity in pre-eclamptic placentae with progress of diffuse arterial microthrombi in placental beds of pre-eclampsia patients. Shu et al22 stated that the degree of decrease in expression of ANXA5 in pre-eclamptic trophoblasts correlated with the rise of markers for blood coagulation activation.
However, when the two BP groups were compared, the difference in expression of ANXA5 was not found to be significant with the increasing BP. Similarly, Ornaghi et al5 did not find any significant association between severity of pre-eclampsia and reduction of ANXA5 expression. However, they found significantly low ANXA5 expression in pre-eclamptic women with FGR compared with matched controls. They concluded that ANXA5 expression is related only to FGR and not to pre-eclampsia.
ANXA5 expression was not significantly lower with increasing severity of pre-eclampsia in the present study. However, the authors would beg to express their reservations regarding this conclusion offered by Ornaghi et al5. Their study sample consisted of 103 patients of pre-eclampsia, among which only 34 cases were complicated by FGR. Even though the controls were matched for gestational age, it is perhaps too early to completely rule out the role of ANXA5 in pre-eclampsia. Ueki et al4 have offered their ANXA5-deficient mouse model to understand the pathogenesis of pre-eclampsia. They found direct evidence that maternal ANXA5 serves as an anti-thrombotic agent in pregnancy.
Apelin is a bioactive multifunctional peptide that participates in the regulation of systemic circulation through its cardiovascular actions and also functions as an adipocytokine23. It has been hypothesized that apelin functions in placental angiogenesis and that its altered expression impairs the angiogenesis, leading to the onset of pre-eclampsia. However, studies on the role of apelin in pre-eclampsia have found conflicting results. In different studies, apelin expression in the pre-eclamptic placenta has been reported to increase or decrease, compared with normotensive pregnancies23. Even though studies that explored the role of apelin in pre-eclampsia yielded conflicting results, the consistent finding in all the studies is that there is an association between apelin and pre-eclampsia, which justifies the analysis of this protein in the present study.
Van Mieghem et al9 reported slightly lower placental apelin expression in pre-eclamptic placentae compared to controls9. Lower apelin content in pre-eclamptic placentae compared to normal ones has also been reported by Yamaleyeva et al24. Apelin levels have been found to be reduced both in serum and placentae of pre-eclamptic patients in studies conducted by Inuzuka et al23 and Bortoff et al25. However, increased placental expression of apelin has been reported in pre-eclampsia by Colcimen et al15 and Cobellis et al1013.
Wang et al1 developed a rat model of pre-eclampsia and the findings of their study yielded several interesting and potentially remarkable results. They found apelin to be effective in ameliorating hypertension and proteinuria, improving pregnancy outcomes, restoring endothelial nitric oxide synthase/nitric oxide signalling and relieving oxidative stress. They concluded that it has the potential of being a novel drug for the prevention and treatment of pre-eclampsia.
In the present study, one of the limitations was that the number of subjects in BP group 2 were only 20 (25.4%). This may be the reason why the expression of ANXA5 (grade and intensity) and apelin (grade) did not significantly differ among the two BP groups. The authors feel that the fact that the intensity of apelin showed significant decrease with the increasing severity of BP when the two BP groups were compared (P=0.01) is an incidental finding. Appropriate statistical analysis for testing the associations of some parameters was also not possible due to the small sample size. A larger study sample with more subjects with severe pre-eclampsia is required for drawing conclusions regarding these aspects.
Overall, in the present study, a significant decrease in the expression of ANXA5 and apelin was noted in pre-eclamptic placentae as compared to the matched controls. The results of this study, highlight the need for randomized controlled trials in the future for further exploration of the impact of expression of these markers on maternal and foetal outcomes.
Acknowledgment
The authors acknowledge Dr Sanchari Dutta for her valuable conceptual inputs.
Financial support & sponsorship: The study was funded by the Department of Science and Technology and Department of Biotechnology, Government of West Bengal (G.O. No.: 147(Sanc)/ST/P/S&T/9G-4/2018).
Conflicts of Interest: None.
References
- Potential targets for the treatment of preeclampsia. Expert Opin Ther Targets. 2015;19:1517-30.
- [Google Scholar]
- Loss of maternal annexin A5 increases the likelihood of placental platelet thrombosis and foetal loss. Sci Rep. 2012;2:827.
- [Google Scholar]
- Immunohistochemical expression of annexin A5 in preeclamptic placentas. Placenta. 2011;32:264-8.
- [Google Scholar]
- The apelin receptor APJ: Journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol. 2013;219:R13-35.
- [Google Scholar]
- Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34-41.
- [Google Scholar]
- Apelin in normal pregnancy and pregnancies complicated by placental insufficiency. Reprod Sci. 2016;23:1037-43.
- [Google Scholar]
- Modulation of apelin and APJ receptor in normal and preeclampsia-complicated placentas. Histol Histopathol. 2007;22:1-8.
- [Google Scholar]
- Expression of angiotensin II receptor-like 1 in the placentas of pregnancy-induced hypertension. Int J Gynecol Pathol. 2012;31:227-35.
- [Google Scholar]
- The incidence of pregnancy hypertension in India, Pakistan, Mozambique, and Nigeria: A prospective population-level analysis. PLoS Med. 2019;16:e1002783.
- [Google Scholar]
- Hypertensive disorders of pregnancy and risk of diabetes in Indian women: A cross-sectional study. BMJ Open. 2016;6:e011000.
- [Google Scholar]
- Hypertensive disorders. In: Cunningham FG, Leveno KJ, Bloom SL, Dashe JS, Hoffmann BL, Casey BM, eds. Williams obstetrics (25th ed). New York: McGraw-Hill Education; 2018. p. :710-54.
- [Google Scholar]
- Investigation of role of vascular endothelial growth factor, Annexin A5 and Apelin by immunohistochemistry method in placenta of preeclampsia patients. Cell Mol Biol (Noisy-le-grand). 2017;63:42-5.
- [Google Scholar]
- Placental malperfusion. In: Baergen RN, ed. Manual of Benirschke and Kaufmann’s pathology of the human placenta (1st ed). China: Springer; 2005. p. :332-50.
- [Google Scholar]
- The female genital tract. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran pathologic basis of disease (9th ed). New Delhi: Elsevier; 2014. p. :991-1042.
- [Google Scholar]
- Study of placental changes in pregnancy induced hypertension. Int J Reprod Contracept Obstet Gynecol. 2013;2:524-7.
- [Google Scholar]
- Study of structural changes in placenta in pregnancy-induced hypertension. J Nat Sci Biol Med. 2014;5:352-5.
- [Google Scholar]
- Placental morphology and fetal implications in pregnancies complicated by pregnancy-induced hypertension. Med J DY Patil Univ. 2016;9:341-7.
- [Google Scholar]
- Placental damages in preeclampsia – From ultrasound images to histopathological findings. J Med Life. 2015;8:62-5.
- [Google Scholar]
- Immunohistochemical study of annexin V expression in placentae of preeclampsia. Gynecol Obstet Invest. 2000;49:17-23.
- [Google Scholar]
- Decreased expression of apelin in placentas from severe pre-eclampsia patients. Hypertens Pregnancy. 2013;32:410-21.
- [Google Scholar]
- Downregulation of apelin in the human placental chorionic villi from preeclamptic pregnancies. Am J Physiol Endocrinol Metab. 2015;309:E852-60.
- [Google Scholar]
- Decreased maternal plasma apelin concentrations in preeclampsia. Hypertens Pregnancy. 2012;31:398-404.
- [Google Scholar]






