Translate this page into:
Smoking & risk of advanced liver fibrosis among patients with primary biliary cholangitis: A systematic review & meta-analysis
For correspondence: Dr Patompong Ungprasert, Department of Rheumatic and Immunologic Diseases, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH, 44195, USA e-mail: p.ungprasert@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Studies have suggested that smoking may accelerate the progression of fibrosis among patients with primary biliary cholangitis (PBC), although the data are limited. The current review was undertaken with the aim to comprehensively analyze this possible association by identifying all relevant studies and summarizing their results.
Methods:
A comprehensive literature review on MEDLINE and EMBASE databases was performed from inception through February 2019 to identify all relevant studies. Eligible studies included cross-sectional studies that recruited patients with PBC and collected data on the smoking status and presence or absence of advanced liver fibrosis for each participant. Odds ratios (OR) with 95 per cent confidence intervals (CI) was desirable for inclusion or sufficient raw data to calculate the same for this association. Adjusted point estimates from each study were extracted and combined together using the generic inverse variance method of DerSimonian and Laird. I2 statistic, which quantifies the proportion of total variation across studies was used to determine the between-study heterogeneity.
Results:
Three cross-sectional studies with 544 participants were included. The pooled analysis found a significantly increased risk of advanced liver fibrosis among patients with PBC who were ever-smokers compared to those who were nonsmokers with the pooled OR of 3.00 (95% CI, 1.18-7.65). Statistical heterogeneity was high with I2 of 89 per cent.
Interpretation & conclusions:
This meta-analysis found that smoking is associated with a significantly higher risk of advanced liver fibrosis among patients with PBC. Further prospective studies are still required to determine whether this association is causal.
Keywords
Cigarettes
liver fibrosis
meta-analysis
primary biliary cholangitis
smoking
Primary biliary cholangitis (PBC) is a chronic autoimmune disease of the liver characterized by inflammation and intrahepatic bile duct destruction, resulting in intrahepatic cholestasis1. PBC is a relatively uncommon disease with the reported prevalence of only 20-400 cases per million persons in Northern Europe and North America23. The precise aetiology of PBC is unknown but is believed to be an interplay between genetic and environmental factors45. Patients with PBC may present with abnormal liver chemistry tests without any symptoms, symptoms of cholestasis (pruritus, yellow eyes and fatigue) or signs and symptoms of cirrhosis67.
Cigarette smoking is a known cause of several preventable non-communicable diseases such as coronary artery disease (CAD), cerebrovascular disease, chronic obstructive pulmonary disease (COPD) and malignancy89. The impact of smoking cessation on the prevention of those diseases is substantial. For instance, a study of postmenopausal women found that smoking cessation can decrease the risk of stroke by almost 40 per cent10. Recent studies found that smoking may also have deleterious effects on the liver because of the increased oxidative stress burden and lipid peroxidation, which may lead to hepatic injury and fibrosis11. The effect may be more pronounced among patients who already have chronic inflammation in the liver, including patients with PBC, although clinical data from epidemiologic studies are still limited121314. The current systematic review and meta-analysis was conducted with the aim to comprehensively analyze the association between risk of liver fibrosis and history of smoking among patients with PBC.
Material & Methods
Information sources and search strategy: A systematic literature search was carried out using the MEDLINE and Embase databases from inception to February 2019 to identify original studies reporting the relationship between history of smoking and risk of advanced liver fibrosis in patients with PBC. The systematic literature review was independently conducted by three investigators using the search strategy that included the terms such as, ‘primary biliary cholangitis’, ‘primary biliary cirrhosis’, ‘smoking’, and ‘cigarettes’ (Supplementary Table I). A manual search for additional potentially relevant studies was also carried out using the references of the included studies as well as some selected review articles. This study was conducted in accordance with the Preferred reporting items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (checklist available as Supplementary Table II). EndNote X7 (Clarivate Analytics, Pennsylvania, United States) was used for study retrieval.
| Database: Ovid MEDLINE |
| 1. Primary biliary cholangitis.mp. or exp Liver Cirrhosis, Biliary/ |
| 2. Primary biliary cirhosis.mp. |
| 3. Or/1-2 |
| 4. Smoking.mp or exp smoking/ |
| 5. Cigarette smoking.mp |
| 6. or/4-5 |
| 7. 3 and 6 |
| Database: EMBASE |
| 1. ‘Primary biliary cirrhosis’ or ‘primary biliary cirrhosis’/exp |
| 2. Primary AND biliary AND (‘cholangitis’/exp or cholangitis) |
| 3. or/1-2 |
| 4. ‘Smoking and smoking related phenomena’ or ‘smoking and smoking related phenomena’/exp |
| 5. ‘Cigarette smoking’ or ‘cigarette smoking’/exp |
| 6. ‘Tobacco use’ or ‘tobacco use’/exp |
| 7. Or/4-6 |
| 8. 3 and 7 |
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 3 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | 4 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | 4-5 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 4-5 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 4-5 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 4-5 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 5-6 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | 5-6 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | Table 1 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | 5-6 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | 5-6 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | 5-6 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | Not applicable |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 6-7 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | Table 1 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | Table 1 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot | Figure 2 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | 6-7 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | 6-7 |
| Additional analysis | 23 | Give results of additional analyses, if done [e.g., sensitivity or subgroup analyses, meta-regression (see Item 16)] | Not applicable |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers) | 7-8 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | 8-9 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 9 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | 9 |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6 (6): e1000097. doi: 10.1371/journal.pmed1000097. For more information, visit: www.prisma-statement.org
Selection criteria: Only cross-sectional studies that recruited patients with PBC with data on (i) smoking status, and (ii) presence or absence of advanced liver fibrosis for each participant were selected. Odds ratios (OR) with 95 per cent confidence intervals (CI) or sufficient raw data to calculate the same for this association should have been reported. Inclusion was not restricted by study size. When more than one study using the same database/cohort was available, only the study with the most comprehensive data/analyses was included. Retrieved articles were independently reviewed to determine their eligibility by the same three investigators. Any discrepancy was resolved by discussion. The modified Newcastle-Ottawa scale was used for quality assessment of the included studies as described previously15.
Data abstraction: A structured data asbtraction form was used to extract details such as title of the study, publication year, name of the first author, calendar year(s) when and in which country the study was conducted, number and demographic data of participants, definiton of advanced liver fibrosis, method(s) used to evalaute liver fibrosis, definition of positive history of smoking (i.e., definition of ever-smokers), method(s) used to determine smoking status, adjusted effect estimates with 95 per cent CI as well as covariates that were adjusted for in the multivariable analysis.
To ensure the accuracy, this data extraction process was independently performed by two investigators. The data abstraction forms were cross-checked by the senior investigator. Any data discrepancy was resolved by referring back to the original articles.
Statistical analysis: Data analysis was performed using the RevMan 5.3 software (Cochrane, London, UK). Adjusted point estimates for the association between ever-smoker status and advanced liver fibrosis were extracted from each study and combined together using the generic inverse variance method as described earlier16, to assign the weight of each study in the pooled analysis inversely.
Random-effects, rather than fixed-effects model, was utitlized for the meta-anlayses as the assumption of the latter that all studies, regardless of study design and participants, should produce the same result is almost always not true for clinical research. Cochran’s Q test and I2 statistic were used to determine the between-study heterogeneity. This I2 statistic quantified the proportion of total variation across studies due to true heterogeneity rather than chance. A value of I2 of 0-25 per cent represents insignificant, 26-50 per cent represents low, 51-75 per cent represents moderate heterogeneity and more than 75 per cent represents high heterogeneity, respectively17. If enough number of eligible studies were identified, visualization of funnel plot was used to assess for the presence of publication bias.
Results
Two-hundred and ninety-six potentially eligible articles were identified as per the described search strategy (107 from MEDLINE and 189 from Embase). After the exclusion of 105 duplicated articles, 191 articles underwent title and abstract review. A total of 170 articles were excluded at this stage since these did not fulfill the eligibility criteria based on type of article, study design, population or measured outcomes, leaving 21 articles for full-text review. Eighteen of these were excluded after the full-length review as these did not report the outcome of interest. Finally, three cross-sectional studies121314 with 544 participants were included in the meta-analysis. It should be noted that the study by Zein et al14 consisted of two cohorts that were recruited from different centers. The effect estimates for each cohort were reported separately and, therefore, were both included in the meta-analysis. The literature review and selection process are depicted in Fig. 1. The characteristics and quality assessment of these studies are detailed in the Table. In brief, all included studies diagnosed PBC based on clinical presentation, serology, and histopathology. The definition of ever-smokers was consistent across the studies (defined as current or history of smoking of ≥5 packs at any time during the patient’s lifetime up to the time of PBC diagnosis)121314.
- Literature review process.
| Study | Zein et al14 | Corpechot et al12 | Mantaka et al13 |
|---|---|---|---|
| Country | USA | France | Greece |
| Study design | Cross-sectional | Cross-sectional | Cross-sectional |
| Year | 2006 | 2010 | 2018 |
| Total participants | First cohort: 77 patients with PBC | 164 patients with PBC | 148 patients with PBC |
| Second cohort: 155 patients with PBC | |||
| Participants | First cohort: Patients with PBC who were seen at one of the three teaching hospitals of Case Western Reserve University (University Hospital, Veteran Affair Medical left and MetroHealth Medical Center) in Cleveland, Ohio, from 1 January 1998 to 31 October 2005 were identified from the databases of the hospitals. Only patients who had liver histopathology available in the system were included | Participants were patients with PBC who previously participated in a prospective epidemiological study. Most of them were recruited from Saint-Antoine hospital. Only patients who had liver histopathology available in the system were included. They were re-interviewed for this study in 2008 | Participants were patients with PBC who were seen at the University of Crete Medical School hospital, Greece. Only patients who had liver histopathology available in the system were included |
| Second cohort: Patients with PBC who were seen at Cleveland Clinic in Cleveland, Ohio, from 1 January 1998 to 30 March 2006 were identified from the database of the hospital. Only patients who had liver histopathology available in the system were included | |||
| Diagnosis of PBC | First cohort: Presence of ICD-9 code for PBC in the database plus 1. Detectable AMA, 2. Cholestatic biochemical profile ≥six months and 3. Compatible liver histology | Presence of at least two of the following criteria; 1. Cholestatic biochemical profile ≥six months, 2. presence of AMA or anti-gp210 ANA (titer ≥1:40 and detectable on ELISA) and 3. compatible liver histology | Based on standard biochemical, immunological, and histological criteria |
| Second cohort: Same as the first cohort | |||
| Determination of smoking status | First cohort: Data on smoking status were retrieved from health questionnaire filled by the patients during visits with healthcare providers of those hospitals. Second cohort: Same as the first cohort | Smoking status was determined through health questionnaires answered by the patients for this study. | Smoking status was determined through health questionnaires answered by the patients for this study. |
| Definition of advanced fibrosis | First cohort: Stage of liver fibrosis was defined based on liver biopsy using Ludwig’s classification. Stage 3 and 4 were considered advanced fibrosis | Stage of liver fibrosis was defined based on liver biopsy using Ludwig’s classification. Stage 3 and 4 were considered advanced fibrosis | Stage of liver fibrosis was defined based on liver biopsy using Metavir-based classification system. F3 and F4 were considered advanced fibrosis |
| Second cohort: Same as the first cohort | |||
| Females (%) | First cohort: 87.6 | 90.2 | 86.5 |
| Second cohort: 91.3 | |||
| Average age (yr) | First cohort: 53.0 | 50.0 | 65.6 |
| Second cohort: 52.0 | |||
| Race (%) | First cohort: Caucasian (91.8) | NA | NA |
| Second cohort: Caucasian (95.7) | |||
| History of smoking (%) | First cohort: 51.0 | 26.0 | 32.7 |
| Second cohort: 50.3 | |||
| Patients with advanced liver fibrosis (%) | First cohort: 49.4 | 20.5 | 17.5 |
| Second cohort: 51.5 | |||
| Confounder adjusted in multivariate analysis | None | Sex and significant alcohol consumption | Sex and significant alcohol consumption |
| Newcastle-Ottawa score | Selection: 3 | Selection: 3 | Selection: 3 |
| Comparability: 1 | Comparability: 2 | Comparability: 2 | |
| Exposure: 3 | Exposure: 3 | Exposure: 3 |
PBC, primary biliary cirrhosis; AMA, antimitochondrial antibody; ICD-9, international classification of diseases-9; ANA, antinuclear antibody; ELISA, enzyme-linked immunosorbent assay; BMI, body mass index; NA, not available
Risk of advanced liver fibrosis among patients with primary biliary cholangitis (PBC) who were ever-smokers versus patients who were non-smokers: The pooled analysis found a significantly increased risk of advanced liver fibrosis among patients with PBC who were ever-smokers compared to patients who were non-smokers with the pooled OR of 3.00 (95% CI, 1.18-7.65) as shown in Fig. 2. Statistical heterogeneity was high with I2 of 89 per cent.
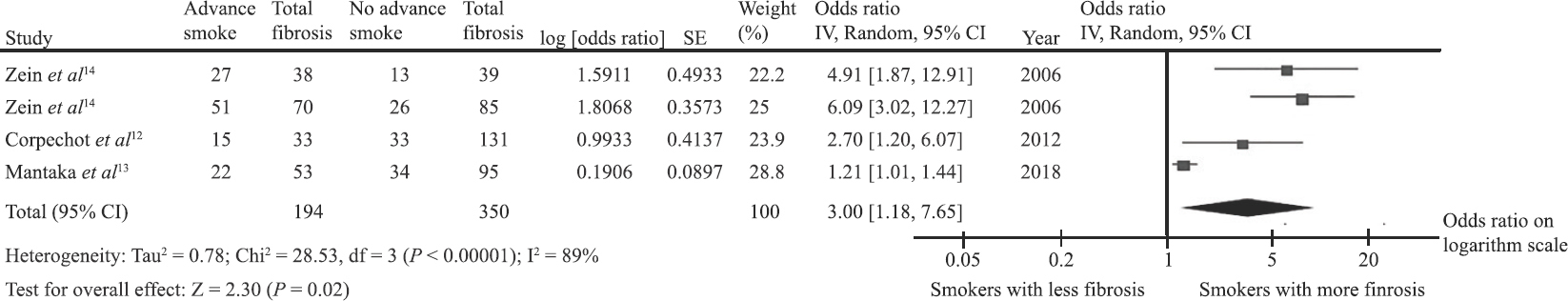
- Forest plot of the meta-analysis.
Discussion
As per our acknowledge, this study is the first systematic review and meta-analysis that summarizes all available data on the association between smoking status and risk of advanced liver fibrosis among patients with PBC. The pooled analysis found a three-fold increased risk of advanced liver fibrosis among patients with PBC who were ever-smokers compared to patients without history of tobacco exposure. The mechanism behind the increased risk is not known with certainty. Possible explanations are discussed below.
First, smoking has been shown to alter the balance of T helper cells, Th1 and Th2, and several cytokine levels, including IL-5 and IL-131819. IL-13 has been implicated in progression of fibrosis in animal studies and smoking can increase the production of IL-1311202122. It has been demonstrated that IL-5 can augment the progression of liver fibrosis by up-regulating activity of IL-1323. Th1 cells that are inducible by smoking24 have been shown to accelerate the progression of fibrosis by activating hepatic stellate cells to secrete more profibrogenic markers through the IFN-γ/STAT pathway25.
The second possible explanation involves pro-angiogenic factors. A study in patients with chronic hepatitis C virus infection found that the level of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF) and VEGF-D, are higher among smokers compared to non-smokers and the higher level was independently associated with advanced fibrosis26. Tissue hypoxia induced by smoking is the likely cause of the higher level of these factors.
Third, smoking can lead to insulin resistance as demonstrated by Houston et al27 in the CARDIA study. Furthermore, studies have also demonstrated that insulin resistance is associated with higher prevalence of severe hepatic fibrosis in patients with NAFLD2728. Some other studies have suggested that hyperinsulinemia can stimulate influx of fatty acid to the liver, leading to hepatic triglyceride accumulation293031. This excessive fatty deposition will cause cellular injury through oxidative stress and hepatocyte apoptosis, which will eventually lead to hepatic fibrosis32.
The present study has some limitations that may affect the validity of the results. First, statistical heterogeneity was high in this meta-analysis. We believe that the difference in background populations and methods used to evaluate liver fibrosis were the main source of the between-study variation. In addition, there was variation in adjustment of the effect estimates as two studies1213 adjusted their effect estimates for sex and alcohol consumption while one study14 did not. Second, a formal assessment for the presence of publication bias could not be performed due to the limited number of included studies. Therefore, it is possible that publication bias in favour of studies that showed positive association may have been present and may have skewed the pooled result. Third, all of the studies were conducted in Western countries and the results may not be generalizable to other populations. Fourth, subgroup analysis comparing heavy, regular, occasional, and ex-smokers could not be performed compared to non-smokers as the included studies did no provide such data. Similarly, there was no subgroup data to perform subgroup analysis based on sex and age. Lastly, this was a systematic review and meta-analysis of observational studies. Thus, it is still possible that the observed association was not causal but was a function of a confounding effect. Other factors related to the smoking habit, but not smoking itself, could still be the actual etiology of the increased risk. This limitation is true for all observation studies but is of particular concern for the current study because only minimal to none adjustment for potential confounders was performed by the primary studies.
In summary, the current study demonstrated that smoking is associated with a significantly higher risk of advanced liver fibrosis among patients with PBC. Further prospective studies are required to determine whether this association is indeed causal.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- European Association for the Study of the Liver. EASL clinical practice guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-72.
- [Google Scholar]
- Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology. 2000;119:1631-6.
- [Google Scholar]
- Epidemiology of primary biliary cirrhosis in Victoria, Australia: High prevalence in migrant populations. Gastroenterology. 2004;127:470-5.
- [Google Scholar]
- Risk factors and comorbidities in primary biliary cirrhosis: A controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194-202.
- [Google Scholar]
- Primary biliary cholangitis: 2018 practice guidance from the American Association for the study of liver diseases. Hepatology. 2019;69:394-419.
- [Google Scholar]
- Primary biliary cirrhosis: Survival of a large cohort of symptomatic and asymptomatic patients followed for 24 years. J Hepatol. 1994;20:707-13.
- [Google Scholar]
- Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses –United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-8.
- [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults and trends in smoking cessation –United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1227-32.
- [Google Scholar]
- Smoking cessation, weight gain, and risk of stroke among postmenopausal women. Prev Med. 2019;118:184-90.
- [Google Scholar]
- Demographic, lifestyle, medical and familial factors associated with primary biliary cirrhosis. J Hepatol. 2010;53:162-9.
- [Google Scholar]
- Association of smoking with liver fibrosis and mortality in primary biliary cholangitis. Eur J Gastroenterol Hepatol. 2018;30:1461-9.
- [Google Scholar]
- Smoking and increased severity of hepatic fibrosis in primary biliary cirrhosis: A cross validated retrospective assessment. Hepatology. 2006;44:1564-71.
- [Google Scholar]
- Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154.
- [Google Scholar]
- Th1 and Th2 cytokines and IgE levels in identical twins with varying levels of cigarette consumption. J Clin Immunol. 2004;24:617-22.
- [Google Scholar]
- Disruption in Th1/Th2 immune response in young adult smokers. Addict Behav. 2007;32:1-8.
- [Google Scholar]
- Involvement of IL-13 in tobacco smoke-induced changes in the structure and function of rat intrapulmonary airways. Am J Respir Cell Mol Biol. 2010;43:220-6.
- [Google Scholar]
- IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol. 2004;173:4020-9.
- [Google Scholar]
- IL13 gene polymorphisms modify the effect of exposure to tobacco smoke on persistent wheeze and asthma in childhood, a longitudinal study. Respir Res. 2008;9:2.
- [Google Scholar]
- Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect Immun. 2006;74:1471-9.
- [Google Scholar]
- The effects of cigarette smoking on T cell subsets. A population-based survey of healthy caucasians. Am Rev Respir Dis. 1989;139:1446-51.
- [Google Scholar]
- Interactions between Th1 cells and Tregs affect regulation of hepatic fibrosis in biliary atresia through the IFN-γ/STAT1 pathway. Cell Death Differ. 2017;24:997-1006.
- [Google Scholar]
- Relationship of smoking and fibrosis in patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2006;4:797-801.
- [Google Scholar]
- Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. BMJ. 2006;332:1064-9.
- [Google Scholar]
- Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356-62.
- [Google Scholar]
- Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology. 2005;42:987-1000.
- [Google Scholar]
- Mechanism of insulin resistance in human liver cirrhosis. Evidence of a combined receptor and postreceptor defect. J Clin Invest. 1985;75:1659-65.
- [Google Scholar]
- Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799-806.
- [Google Scholar]
- The progression of liver fibrosis in non-alcoholic fatty liver disease. Korean J Gastroenterol. 2017;69:341-7.
- [Google Scholar]






