Translate this page into:
The species distribution of ticks & the prevalence of Kyasanur forest disease virus in questing nymphal ticks from Western Ghats of Kerala, South India
For correspondence: Dr R. Balasubramanian, ICMR-National Institute of Virology, Kerala Unit, T D Medical College & Hospital, Alappuzha 688 005, Kerala, India e-mail: balasniv@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Kyasanur forest disease (KFD) is a zoonotic tick-borne disease across the Western Ghats of India. With the discovery of a cluster of human KFD cases in the Wayanad district of Kerala, the present study was focused on detecting KFD virus (KFDV) in tick populations. To manage this disease, it is necessary to understand the diversity of the tick species and factors influencing the distribution, abundance and prevalence of infected ticks in Wayanad district.
Methods:
Surveys were conducted from November 2016 to May 2018 in four forest ranges of Wayanad district. Ticks were collected by the dragging method and were identified to species level and assayed for virus detection using real-time polymerase chain reaction.
Results:
A total of 25,169 ticks were collected from 64 sites. Of the identified species, Haemaphysalis spinigera was the most abundant (56.64%), followed by H. turturis 9047 (35.94%), H. bispinosa 999 (3.96%), Amblyomma integrum 691 (2.74%), H. kyasanurensis (0.55%), Rhipicephalus sanguineus (0.08%), Hyalomma marginatum (0.02%), H. cuspidata (0.01%), R.microplus (0.01%) and Dermacentor auratus (0.003%). The nymphal stage was predominant from December to February having peak activity in January. A total of 572 pools were screened for the presence of KFDV, of which 21 pools were positive. The infection rates in H. spinigera and H. turturis tick were 2.62 and 1.04 per cent, respectively.
Interpretation & conclusions:
The circulation of KFDV was detected and its correlation with the prevalence in ticks near the fragmented forest and teak plantation areas of Wayanad district. Residents and visitors of these regions may become vulnerable to tick bites and to an increased risk of KFD as the distribution of established, infected tick populations continues to expand.
Keywords
Climate
forest
Haemaphysalis spinigera
Kyasanur forest disease
pool
ticks
virus
Wayanad
Ixodid ticks (Acarina, Ixodidae) are the major vectors for infectious bacterial, viral and protozoan pathogens affecting humans and animals. Amongst the many diseases transmitted by ticks, Kyasanur forest disease (KFD) is a viral zoonotic disease which has become endemic in many areas of the Western Ghats of India12. The KFD virus (KFDV) is mainly transmitted by Haemaphysalis spinigera species of tick, and it has also been isolated from seven other species of the genus Haemaphysalis spp., as well as from Dermacentor, Rhipicephalus and Ixodes genera. Monkeys (black-faced langurs and red-faced bonnet monkeys) become infected with KFDV through the bite of infected ticks; the virus is then transmitted to other ticks feeding on infected monkeys3. Large mammals such as deer and domestic ruminants mainly serve as reproductive hosts for ticks, and KFDV is maintained in nature through small mammals, shrews, bats, monkeys and in ticks4. Exposure to tick bites occurs when people visit forests for farming or grazing livestock animals, collecting dry wood, trekking, etc. Human beings are the dead-end host, and person-to-person transmission of KFD has not been demonstrated so far.
The disease has been primarily reported in Karnataka, but for the past five years, cases have been reported from the adjacent States of Karnataka such as Tamil Nadu, Kerala, Goa and Maharashtra. Chakraborty et al5 reported that from 1957 to 2017, there were an estimated 9594 cases of KFD within 16 districts of India. The most significant human outbreaks of KFD were between 1957 to 1981 mostly in Shimoga, with some cases in Uttar Kannada6. Since 1982, new foci of disease have been recognized, with 1984 new cases reported from Dakshina Kannada. In the following years, the disease spread geographically to Chikmagalur and Udupi districts of Karnataka State. The growth in number of cases continued in 20035. In Kerala, the first reported human case was in 2013 from Wayanad district; furthermore, the first large outbreak occurred in 2015. In 2014, a new focus of virus activity was reported from the tribal area of Nilambur in the Malappuram district of Kerala. The most recent focus occurred during 2015 to 2017, when the disease spread to other States of Goa and Maharashtra578. The KFDV is spreading to other parts of India that were previously free of the virus and has become endemic. To improve understanding about KFDV epidemiology and manage the risk of the disease, knowledge on species diversity, biotic and abiotic conditions that promote the distribution of vector tick populations (both infected and non-infected with KFD) is necessary.
The aim of the present study was to determine the diversity of tick species, their distribution and relative abundance at selected forest sites in Wayanad district and to ascertain the level of infection in tick populations. In addition, the possible effect of climatic condition on the distribution of different tick species in this district was given special consideration.
Material & Methods
Study area: Wayanad, a hilly district in northeastern Kerala, lies between latitudes 11° 27’ and 15° 58’ North, 75° 47’ and 70° 27’ East, has a total area of 2131 sq km in the Western Ghats. The population of this district is 780,619 (391,273 males and 389,346 females), of this and the tribal population comprises 17 per cent, which is the largest in the State of Kerala9. The altitude varies from 700 to 3100 m above sea level. This region experiences a moderate, semi-tropical climate with an average temperature of 18°C-35°C in winter and summer, respectively, with a mean rainfall of 2786 mm. The annual average relative humidity is above 60 per cent. The area receives most of its rainfall under the influence of the southwest monsoon and less rainfall under the influence of the northeast monsoon. This district has the largest forest cover of 83.33 per cent, and is fairly deciduous, interspersed with evergreen forests10.
Site selection: In Wayanad district, the forest area is administratively divided into four divisions – Sulthan Bathery, Muthanga, Kurichiyat and Tholpetty. These four forest divisions were assigned as four forest zones, and the study was conducted between November 2016 to May 2018 during every month along these zones. Suitable 4-6 locations in each site (all zones) were selected based on the history of KFDV being positively reported and suitable ecological and geographical features which favour the activity of questing ticks. Amongst the four forest divisions, Muthanga was selected as zone 1 with 13 sites, Sulthan Bathery as zone 2 with 13 sites, Kurichiyat as zone 3 with 22 sites and Tholpetty as zone 4 with 16 sites (Fig. 1). A total of 121 surveys were carried out with 60 sampling events in Kurichiyat, 21 in Muthanga, 19 in Sulthan Bathery and 21 in Tholpetty. There were two parallel 100 m transects which were dragged in from each of the five locations, for a total sample area of 1000 m2 per site per visit in all zones. In each zone, the same time period was selected for tick collection and the difference in survey events and number of survey sites in each zone was mainly due to the difference in forest ecology, habitat type and prevalence of KFDV reported from those study sites.
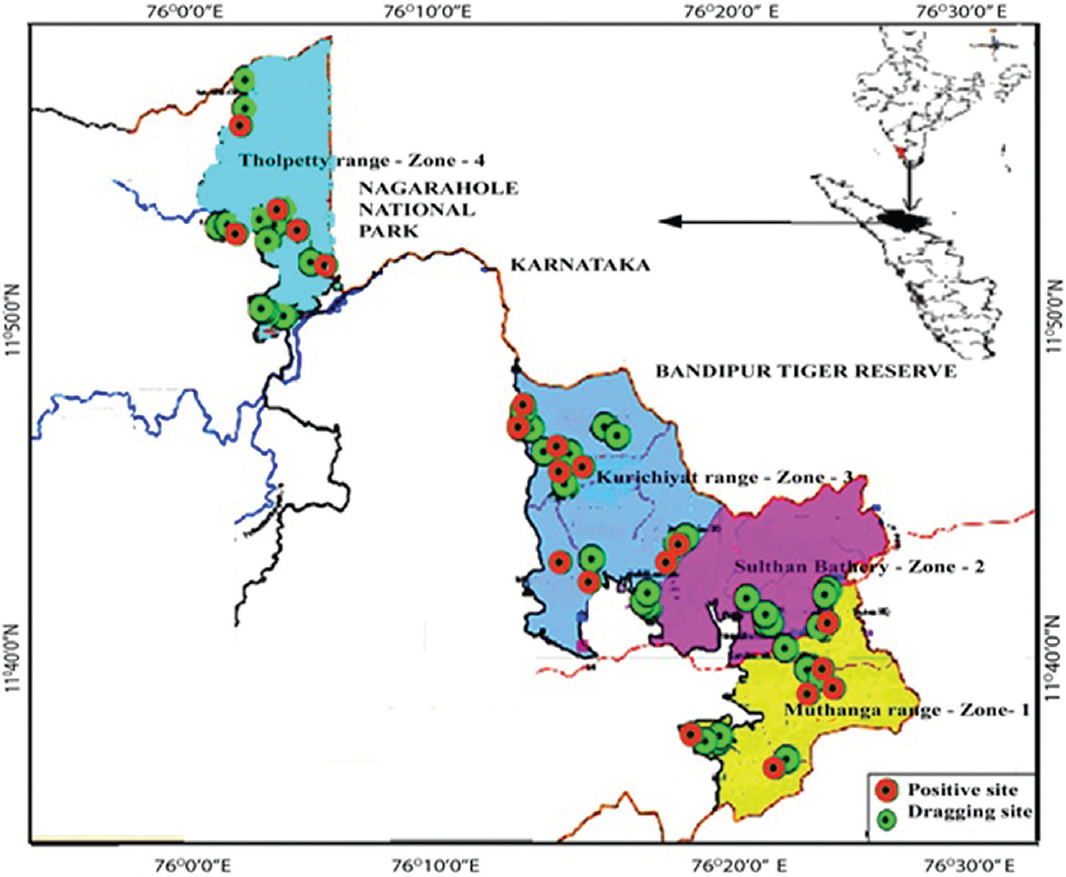
- Spatial distribution map of host-seeking nymphal ticks in four forest ranges of Wayanad district. 1. Muthanga (Zone 1), 2. Sulthan Bathery (Zone 2), 3. Kurichiyat (Zone 3) and 4. Tholpetty (Zone 4), forest ranges from which obtained tick samples has been highlighted in different colours. The location of the positive sample for Kyasanur forest disease is marked with a red colour. Source: Kerala forest department, Sulthan Bathery, Wayanad district, Kerala.
Tick collection: Tick collection was carried out by three trained technicians, and the same trained team was involved throughout the study. Questing ticks were collected by dragging a 1.5 m long ×1.0 m wide flannel cloth attached to a wooden stick and a 5 m long nylon rope attached to each end of the stick. Collection was made by dragging the flannel cloth over vegetation stopping at 1 and 2 min intervals, and all nymphs and adults from each transect were collected and kept in separate vials labelled with the date, locality and area of collection. These samples were transported to the laboratory of the department of Medical Entomology and Zoology, ICMR-National Institute of Virology (NIV), Kerala Unit, Alappuzha. Sampling was performed on rain-free days, avoiding early morning and mid day hours to minimize the potentially confounding effects of heavy dew and extreme heat on sampling efficiency. The collected ticks were identified to species using stereo zoom microscope (Olympus SZ61, Japan) using morphological identification keys1112. The mean tick density of collected ticks from each zone was calculated using methods described previously13. Specimens were assigned to pool (not exceeding 50 ticks per pool) based on species, sex, location and date of collection and kept at −80°C until processing for virus detection at ICMR-NIV, Pune.
Specimen processing: Processing of specimens was performed at the biosafety level-4 (BSL-4) laboratory, ICMR-NIV, Pune. Homogenization of tick pools was done by the addition of minimum essential medium. A pool of approximately 50 ticks was added to a 2 ml plastic tube containing 1 ml minimum essential medium along with a 7 mm stainless steel bead. The tube was transferred to the TissueLyser II (Qiagen, Germany) adapter which was adjusted at 30 Hz/sec for 5 min. Tick pool homogenate of 140 μl was further used for RNA extraction. The tick samples were tested for KFDV viral RNA by real-time PCR14.
Measurement of environmental parameters: Air temperature and relative humidity were measured 5 cm above the soil surface using a temperature–humidity probe in each collection (IBS HTC-1, India). Detailed climate data were obtained from a meteorological station of Regional Agricultural Research Station, Ambalavayal, Wayanad district, and these data were used for climatic factor analysis.
Mapping of tick population: Geographic coordinates of areas containing ticks were marked using a handheld Geographic Positioning System device (Garmin eTrex H, Taiwan) and processed with MapSource software. Coordinates of habitat containing ticks were integrated into a GIS database using the software ArcGIS (ESRI India Version – 10.4, Esri India Technologies Pvt. Ltd., Gurugram, Haryana, India) to map the distribution of ticks in the associated area. All digital data in the GIS were displayed in the coordinate system.
Results
A total of 25169 ticks were collected from 64 sites. Amongst the identified species, Haemaphysalis spinigera was the most abundant (56.64%), followed by H. turturis 9047 (35.94%), H. bispinosa 999 (3.96%), Amblyomma integrum 691 (2.74%), H. kyasanurensis (0.55%), Rhipicephalus sanguineus (0.08%), Hyalomma marginatum (0.02%), H. cuspidata (0.01%), R. microplus (0.01%) and Dermacentor auratus (0.003%). The present study revealed that H. spinigera and H. turturis were the most common vector species. The mean tick density of the four zones ranged from 1.8 to 4.92 per minute of drag sampling. The maximum tick density was obtained during the daytime temperature range between 28°C and 30°C.
Virus detection: A total of 572 pools consisted of 24,606 ticks belonging to five species such as A. integrum (9 pools/485), H. bispinosa (24 pools/995), H. kyasanurensis (2 pools/96), H. spinigera (320 pools/14160) and H. turturis (217 pools/8870) were tested. The KFD viral RNA was detected in 21 (3.67%) pools out of the 572 pools tested. The infection rate in H. spinigera (15 pools) and H. turturis (6 pools) ticks was 2.62 and 1.04 per cent, respectively. None of the ticks, belonging to A. integrum, H. bispinosa and H. kyasanurensis, were infected (Table). All of these pools were positive for KFDV from December to February.
| Tick species | Zone 1 | Zone 2 | Zone 3 | Zone 4 | Total (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of ticks | +ve/pool | Number of ticks | +ve/pool | Number of ticks | +ve/pool | Number of ticks | +ve/pool | ||
| Amblyomma integrum | 182 | 0/3 | 5 | - | 469 | 0/6 | 35 | - | 691 (2.74) |
| Dermacentor auratus | 1 | - | 0 | - | 0 | - | 0 | - | 1 (0.003) |
| Haemaphysalis bispinosa | 105 | 0/3 | 493 | 0/20 | 377 | 0/1 | 24 | - | 999 (3.96) |
| Haemaphysalis cuspidata | 3 | - | 0 | - | 0 | - | 0 | - | 3 (0.01) |
| Haemaphysalis spinigera | 3477 | 4/98 | 472 | 0/30 | 7252 | 7/135 | 3055 | 4/57 | 14,256 (56.64) |
| Haemaphysalis turturis | 3420 | 2/61 | 839 | 0/34 | 2766 | 3/82 | 2016 | 1/40 | 9047 (35.94) |
| Haemaphysalis kyasanurensis | 40 | 0/2 | 13 | - | 9 | - | 77 | - | 139 (0.55) |
| Hyalomma marginatum | 1 | - | 0 | - | 6 | - | 0 | - | 7 (0.02) |
| Rhipicephalus microplus | 1 | - | 0 | - | 3 | - | 1 | - | 5 (0.01) |
| Rhipicephalus sanguineus | 7 | - | 3 | - | 5 | - | 6 | - | 21 (0.08) |
| Total | 7243 | 6/167 | 1825 | 0/84 | 10,887 | 10/224 | 5214 | 5/97 | 25,169 |
Distribution of tick species: The distribution of the ticks and virus prevalence in each zone is shown in the Table. Zone 1 shows the highest (4.92) mean tick density per min sampling with a mean range from 0.8 to 8.48 per min dragging, in which four pools of H. spinigera and two pools of H. turturis were positive for KFDV. In zone 2, the mean tick density per min was 1.84 and the mean ranged from 0.4 to 3.21 per min dragging and none of the pools were positive for KFDV.
In zone 3, the mean tick density per min was 3.04 and the mean ranged from 0.1 to 5.75 per min dragging, in which seven pools of H. spinigera and three pools of H. turturis were positive for KFDV. This zone shows the highest prevalence of KFDV where both monkey deaths and human cases of KFD occurred. In zone 4, the mean tick density was 4.23 per min sampling and the mean ranged from 0.2 to 7.72 per min dragging, where four pools of H. spinigera and one pool of H. turturis were positive. The results revealed that H. spinigera and H. turturis were predominant species in all zones except zone 2 where H. bispinosa was the second predominant species.
Seasonal activity of Ixodid ticks: At all of the study sites, the number of collected ticks was increased from December to February and then decreased until May. Temperature ranges from 28°C to 30°C and relative humidity of 60-80 per cent was more favourable to support high questing nymph tick density (8.48 ticks/m sampling) during December to February. In addition to temperature, rainfall was also one of the most important climatic factors which affected the tick’s persistence. From the onset of summer rain in April (18 mm) and May (25 mm), the tick density was reduced to <1 ticks/min sampling. H. spinigera and H. turturis were predominant in all months and reached a peak in January. However, after the onset of summer rain, H. turturis dominated over H. spinigera. As summarized in Fig 2, more than 34.12 and 29.74 per cent of the total ticks were collected in January and February, whereas only 3.16 and 0.34 per cent of the total ticks were collected in April and May, respectively (Fig. 2), indicating higher tick activity in winter months and lower activity in summer months. The activity of Haemaphysalis nymph ticks began around November, increased between January and February and declined in May. Haemaphysalis species, especially H. spinigera, H. turturis and H. bispinosa were found from all zones, especially in winter and early summer. The relative abundance of Haemaphysalis ticks gradually declined from 33.21 to 0.32 per cent from January to May.
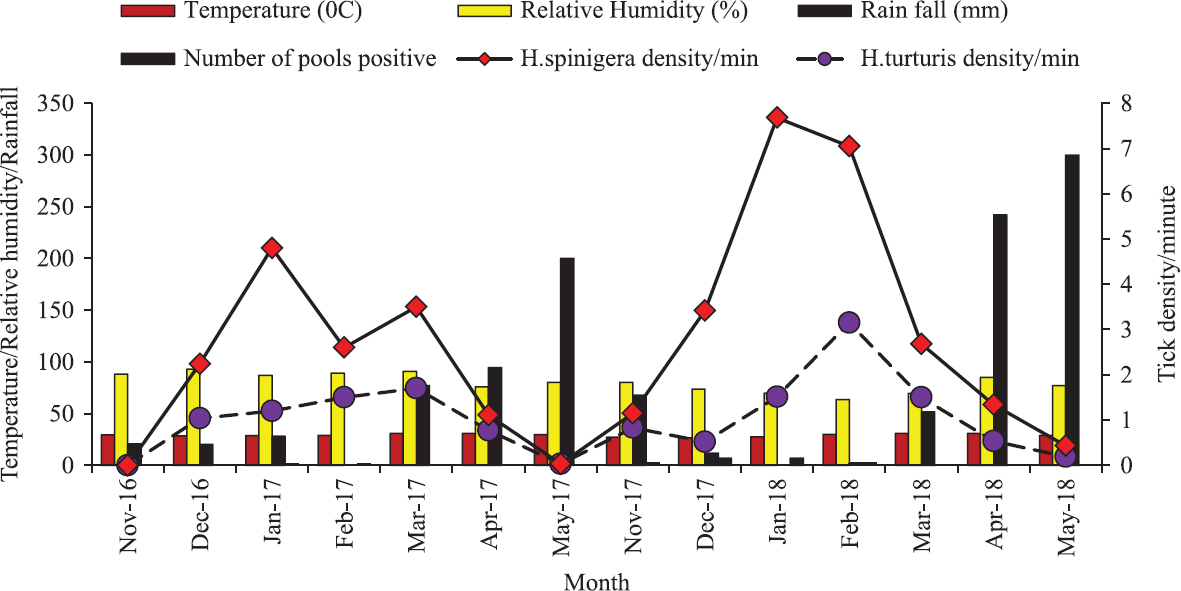
- Seasonal density of Haemaphysalis turturis, Haemaphysalis spinigera and total ticks with respect to climatic factors from Wayanad district.
Discussion
The present study evidently showed the species distribution of ticks and the prevalence of KFDV in Wayanad district, in accordance with suitable microclimate provided by the landscape topography and climate. A total of seven species of ticks were recorded in Wayanad area earlier15, but in the present survey, ten species were identified. The difference could probably imply the necessity of right method of collection, i.e. dragging of targeting questing ticks during the survey. The present study has re-confirmed the advantages of the dragging methods161718 than the flagging, visual search, leaf litter examinations and carbon dioxide traps19. Amongst the ten questing tick species, the population of H. spinigera and H. turturis was dominant, particularly during the winter season, as the density of infected H. spinigera nymphs has already been suggested as a strong predictor for KFD risk of infection20. Amongst the four surveyed forest zones, zone 3 was deemed a high risk for infection because of the comparatively high KFDV positivity in H. spinigera (5.29%) and H. turturis (4.05%). This could probably be explained by the availability of host animals due to adequate food, water and the shelter preferred by host animals. This is similar to earlier reports of human KFD cases, monkey deaths and the detection of virus in the locality212223.
The study areas of four different zones come under the moist deciduous forest with different landscape features such as fragmented natural forest, teak and eucalyptus plantation, bamboo and plain grasslands. In zones 1 and 3, the highest tick density was associated with its fragmented forest topography with suitable habitat coverage provided by secondary plant growth such as invasive weeds such as Lantana camara, Eupatorium and Chromolaena odorata. In these zones, forest fragmented with teak plantation was identified with an abundance of larval ticks because teak leaves are preferred for tick egg hatching due to large surface area and ability to retain water that provides a suitable microclimate for larval sustainability. The other two zones, Sulthan Bathery (zone 2) and Tholpetty (zone 4), are mainly landscaped and vegetated by open grassland habitats and open bamboo forest. Most of these landscapes are plain without any shadow, and this may not provide good habitat coverage for host animals and ticks by causing fast desiccation, which leads to low tick density.
Changes of climatic factors such as temperature, relative humidity and rainfall alter tick biology2425. From the evidence of previous outbreak history, KFD cases began in the last week of December, peaked in January as well as February and then declined. In this study, tick infection was also reported during December to February. This period was characterized by moderate temperatures (28°C-30°C) and relative humidity (60-80%) which favours the high questing nymphal activity. However, temperatures exceeding 30°C and the onset of summer rainfall during the month of March to May were associated with fewer numbers of ticks.
The limitations of this study were that the ticks were collected only from the areas where the tribes and forest people were able to access; so data of ticks located in highly elevated areas and inaccessible forest areas was not collected. This area may also contain positive ticks. Our data did not include ticks that fed on animals (e.g. small mammals and birds), which may contribute to a large proportion of positive ticks. Therefore, our findings may not provide an accurate representation of all locations with endemic tick populations within this district.
The present study provides baseline information to build public awareness about the potential exposure to KFD. It also provides an evidence for developing KFD prevention efforts and implementing environmental management strategies to control further spread of vector ticks and their associated pathogens. The broad geographic distribution and high abundance of H. spinigera and H. turturis, the recovery of KFDV from field specimens and the host-seeking phenology of nymphs coincides with the seasonal occurrence of human KFD cases supports the role of H. spinigera and H. turturis in KFD transmission in this region. The human cases of KFD between 2013 and 201520 showed the KFD transmission from December to May in Wayanad district and in this study also KFDV detected in tick pools from the month of December to February which supports a correlation between relative abundance of ticks and numbers of KFD cases in the district.
Overall, in the present study, the level of circulation of KFDV was evaluated and its correlation with the prevalence in tick species. The results suggest that there is a need for increasing public awareness regarding the higher density and infection prevalence of ticks near the fragmented forest and teak plantation areas of Wayanad district. Residents and visitors of these regions are vulnerable may bites of principal vectors and to have an increased risk of KFD.
Acknowledgment:
Authors acknowledge the support of the Kerala forest department, Wayanad district forest department (File No:WL10-26277/2016), to facilitate our work in forest area and also thank Director, Regional Agricultural Research Station, Ambalavayal, Wayanad, for providing the climate data.
Financial support & sponsorship: This study received financial support from the Department of Health Research, New Delhi (Concept Proposal Number: 2014-0495).
Conflicts of Interest: None.
References
- Prevalence and spatial distribution of Ixodid tick populations in the forest fringes of Western Ghats reported with human cases of Kyasanur forest disease and monkey deaths in South India. Exp Appl Acarol. 2018;75:135-42.
- [Google Scholar]
- Clinical &epidemiological significance of Kyasanur forest disease. Indian J Med Res. 2018;148:145-50.
- [Google Scholar]
- New focus of Kyasanur forest disease virus activity in a tribal area in Kerala, India, 2014. Infect Dis Poverty. 2015;4:12.
- [Google Scholar]
- Historical expansion of Kyasanur forest disease in India from 1957 to 2017:A retrospective analysis. Geohealth. 2019;3:44-55.
- [Google Scholar]
- Kyasanur Forest disease. In: Monath TP, ed. The Arboviruses:Epidemiology and Ecology. Colorado: CRC Press; 1988. p. :93-116. Taylor and Francis Group
- [Google Scholar]
- Recent scenario of emergence of Kyasanur forest disease in India and public health importance. Curr Trop Med Rep. 2016;3:7-13.
- [Google Scholar]
- On the transmission pattern of Kyasanur forest disease (KFD) in India. Infect Dis Poverty. 2015;4:37.
- [Google Scholar]
- An investigation on first outbreak of Kyasanur forest disease in Wayanad district of Kerala. J Entomol Zool Stud. 2015;3:239-40.
- [Google Scholar]
- Kyasanur forest disease:A new virus disease in India:Summary of preliminary report of investigations of the VRC on the epidemic disease affecting forest villagers and wild monkeys of Shimoga district, Mysore state 1957. Indian J Med Sci. 1957;11:41-342.
- [Google Scholar]
- Ticks of domestic animals in Africa, a guide to identification of species (2nded). Edinburgh: Scotland; p. :2013.
- Haemaphysalis ticks of India (1sted). London: Elsevier; p. :2011.
- Diagnosis of Kyasanur forest disease by nested RT-PCR, real-time RT-PCR and IgM capture ELISA. J Virol Methods. 2012;186:49-54.
- [Google Scholar]
- Distribution and prevalence of ticks on livestock population in endemic area of Kyasanur forest disease in Western Ghats of Kerala, South India. J Parasit Dis. 2019;43:256-62.
- [Google Scholar]
- Comparison of two methods for collecting free-living ticks in the Amazonian forest. Ticks Tick Borne Dis. 2010;1:194-6.
- [Google Scholar]
- Efficiency of flagging and dragging for tick collection. Exp Appl Acarol. 2013;61:119-27.
- [Google Scholar]
- Flagging versus dragging as sampling methods for nymphal Ixodes scapularis (Acari:Ixodidae) J Vector Ecol. 2013;38:163-7.
- [Google Scholar]
- Complementary data on four methods for sampling free-living ticks in the Brazilian Pantanal. Rev Bras Parasitol Vet. 2014;23:516-21.
- [Google Scholar]
- Difference in vector ticks dropping rhythm governs the epidemiology of Crimean-Congo haemorrhagic fever &Kyasanur forest disease in India. Indian J Med Res. 2016;144:633-5.
- [Google Scholar]
- An outbreak of Kyasanur forest disease in Kerala:A clinic epidemiological study. IJFCM. 2016;3:272-5.
- [Google Scholar]
- Spread of Kyasanur forest disease, Bandipur tiger reserve India, 2012-2013. Emerg Infect Dis. 2013;19:1540-1.
- [Google Scholar]
- Kyasanur forest disease - First reported case in Kerala. J Assoc Physcians India. 2016;64:90-1.
- [Google Scholar]
- Seasonal dynamics of tick species in an urban park of Rome. Ticks Tick Borne Dis. 2013;4:513-7.
- [Google Scholar]
- Lyme disease risk in Southern California:Abiotic and environmental drivers of Ixodes pacificus (Acari:Ixodidae) density and infection prevalence with Borrelia burgdorferi. Parasit Vectors. 2017;5:7.
- [Google Scholar]






