Translate this page into:
Performance of second-trimester maternal biochemistry screening (quadruple test vs. triple test) for trisomy 21: An Indian experience
For correspondence: Dr Anita Kaul, Department of Fetal Medicine, Apollo Centre for Fetal Medicine, Indraprastha Apollo Hospital, New Delhi 110 076, India e-mail: anitagkaul@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Down syndrome (DS) is one of the most common causes of developmental delay. In India, there is no protocol for prenatal screening of DS. Second-trimester biochemical screening is still being done by triple test. Quadruple test is with better sensitivity and specificity but is not advised routinely. So, the objective of this study was to evaluate the sensitivity and accuracy of the second-trimester screening (quadruple test with genetic sonogram) for trisomy 21 as compared to biochemical testing.
Methods:
This retrospective observational study was carried out in a Fetal Medicine Centre to analyze the odds of being affected with DS, given a positive risk (OAPR) upon screening in the quadruple test; triple test and quadruple test plus a genetic sonogram for high-risk singleton pregnancies (in view of advanced maternal age; an anomaly scan showing some abnormality, etc).
Results:
3175 high-risk singleton pregnancies were screened for trisomy 21. 394 women underwent amniocentesis on the basis of triple test, quadruple test or quadruple plus genetic sonogram positive. 17 foetuses were diagnosed to have DS. The quadruple test was found to have a higher OAPR as compared to the triple test (1:30.1 as compared to 1: 40.2). Quadruple test plus the genetic sonogram was found to have the highest OAPR of 1:6.
Interpretation & conclusions:
Best screening for trisomy 21 is provided with quadruple test with genetic sonogram which can lower the rates of unnecessary amniocentesis in high-risk population.
Keywords
Down syndrome
genetic
quadruple test
second trimester screening
triple test
ultrasound
Down syndrome (DS) is the most common cause of developmental delay and accounts for 15-30 per cent of individuals with intellectual disabilities1. Published data suggest that, in India, 21,400 children with DS are born every year2 and the birth prevalence is reported to vary from one in 1361 to one in 692 in various studies34. Although the knowledge about screening for DS is increasing both in the public and private sector, a definitive protocol from professional bodies or the Government is still not available.
In India, in many of the low resource settings a genetic sonogram done at 18-20 wk gestation is the only screening tool available for the detection of aneuploidies. This genetic sonogram, if done in a regular low volume clinic has a detection rate (DR) of 56 per cent for a three per cent false positive rate [International Society of Prenatal Diagnosis (ISPD) 2015], whereas if done by dedicated foetal medicine clinics, has a DR of 80 per cent5.
In many parts of the country even today, second-trimester maternal serum screening in the form of the triple test [serum alpha foetoprotein, serum total beta-human chorionic gonadotrophin (HCG) and serum unconjugated estriol) is generally being used to screen for foetal aneuploidies. Quadruple test (additional biochemical marker, i.e. serum inhibin A in addition to the triple test) though known to have a higher sensitivity as compared to the triple test is still not being used for screening of aneuploidies in India universally because of a lack of awareness, cost constraints and laboratory availability.
First trimester combined screening [free serum beta-HCG with serum pregnancy-associated plasma protein (PAPP-A) with nuchal translucency/nasal bone (NT/NB) sonogram] is the main DS screening test with a DR of 93.8 per cent with a 1.9 per cent false positive rate6. This is the standard investigation in the Western world but is yet to be established in India.
The present study was undertaken to compare the odds of being affected with DS (i.e. diagnosing trisomy 21) given a positive risk [odds of being affected given a positive risk (OAPR)] of qadruple test compared to the triple test, and to further evaluate the improvement in DRs of quadruple test when it is combined with a genetic sonogram at 18-20 wk of gestation in a tertiary care referral centre in New Delhi.
Material & Methods
This was a retrospective observational study conducted at Apollo Centre for Fetal Medicine, Indraprastha Apollo Hospital, New Delhi, India between December, 2009 to December, 2017 after attaining the required clearance from the Institutional Ethics and Review board and in accordance with the Helsinki Declaration of 1975, as revised in 2000.
OAPR is the likelihood of a woman having a DS pregnancy confirmed by chorionic villous sampling (CVS) or amniocentesis if her screen risk is high7. If a screening test has a high OAPR, more affected pregnancies will be successfully diagnosed for every miscarriage caused by invasive testing8. So, it is important that the false-positive rate (FPR)/screen positive rate is kept as low as possible so to minimize the number of women offered invasive procedures which will, in turn, reduce the number of miscarriages of healthy foetuses. In this study OAPR was calculated as the ratio of true screen positive to false screen positive89.
Sample size: Taking the prevalence of DS in the general population as approximately 0.1 per cent and the prevalence of DS in high-risk population based on literature10, and our data of prevalence data of trisomy 21 in patients referred to our centre (the study cohort) as 1:250 with five per cent margin of error and 80 per cent power of the study based on the width of the 95 per cent confidence interval (CI) around an expected OAPR for the triple test, the required sample size for assessment of triple test OAPR was calculated as 1471. As this was a retrospective study with no issues related to attrition or loss to follow up a total of 1490 patients who had undergone the triple test were included. This encompassed a period of eight years. At the same time, all patients who had undergone the quadruple test (n=1685) during the same period were included. 5 ml blood was collected in plain vial from each patient for testing and the triple and quadruple tests were done on the serum of the patients.
High-risk singleton pregnancies (in view of advanced maternal age; an anomaly scan showing some abnormality, etc.) referred to our centre between December, 2009 to December, 2017 were included in the study. Most of the patients with positive 2nd-trimester biochemical screening, i.e. who had a risk >1:250 on triple or on quadruple test were referred cases from all over India making the whole of the study population a high-risk population. The cut-off risk for a biochemical test to be positive was taken as a risk > 1: 250 at the time of screening.
The study hospital had a universal first trimester combined screening protocol (serum beta-HCG with serum PAPP-A with NT/NB sonogram) in place done at 11-13+6 wk of gestation, meaning only a few of the patients required a second-trimester quadruple test (this was only offered to women with a risk between 1:250 and 1:1000).
All women with a screen positive triple test or quadruple test were either given the choice of a risk re-assessment scan (i.e. new risks being generated after performing a genetic sonogram in conjunction with quadruple test) or directly proceeding with diagnostic testing using amniocentesis, fluorescent in situ hybridization and karyotyping. The amniocentesis and further testing were done on 30 ml amniotic fluid aspirated using 18F needle under ultrasound guidance. The ultrasound examinations were performed using real-time, high-resolution scanning with a Voluson E8 System (GE Healthcare, Milwaukee, WI, USA) with a 3.5-MHz convex probe with 5-90° volume angle, 398 HZ frame rate and 3-26 cm depth.
Genetic sonogram meant checking for all the soft markers i.e. ventriculomegaly, absence of NB, nuchal thickness >6 mm at 18-22 wk, presence of intracardiac echogenic focus in the heart, presence of aberrant right subclavian artery (ARSA), echogenic bowel, short humerus and short femur, renal pelvis dilation >4 mm at 18-20 wk and then re-calculating risks by entering the data on our Obstetric software, ASTRAIA software GmbH® (Bayern, Germany) based on the Agathokleous risk calculation meta analysis11. All these soft markers for DS were seen using the Fetal Medicine Foundation (FMF) criteria. Before the Agathokleous publication, risks were given without the addition of ventriculomegaly and ARSA. If a patient had a low risk on the new re-calculated risk they were given the option of no further testing after detailed counselling regarding the risks and benefits of observation and follow up.
After the introduction of cell-free foetal deoxy ribonucleic acid, non-invasive pre-natal screening (NIPS) in 201512 in the screening of DS, this non-invasive test with a sensitivity of 99.9 per cent was also offered in addition to amniocentesis which is the 100 per cent diagnostic test for detection of chromosomal abnormalities. The NIP-test was conducted by CENTOGENE Lab (Rostock, Germany) on 10 ml blood sample collected in CentoNIPT Streck tube. The CentoNIPS® is based on the in vitro diagnostic test Illumina VeriSeq™ NIPT solution.
Twin pregnancies and women who had undergone amniocentesis based on a positive combined first-trimester screening were excluded from the study. DS pregnancies, including those missed by screening, were ascertained from hospital records and cytogenetic laboratories. The screening performance (in terms of the OAPR) of the quadruple test was compared with triple test and with quadruple test and genetic sonogram.
Statistical analysis: Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp. Armonk, NY, USA). Descriptive analysis of DR, false positive rate, PPV, NPV and OAPR were calculated for the triple test; quadruple test and the quadruple test with genetic sonogram for data analysis.
Results
During the eight years of the study period, a total of 3175 high-risk singleton pregnancies were included for analysis. The demographic characters of the study population are shown in Table I. 1490 high-risk women with singleton pregnancies were screened by the triple test, which had been done outside, as at the study site triple test was not done. These were patients who had had triple test done from elsewhere and had come/referred to us in view of either screen positive triple test or for an anomaly scan in view of any suspicious finding or had missed their first trimester combined screening.
| Demographic character | Triple test (n=1490) | Quadruple test (n=1685) |
|---|---|---|
| Mean age (yr) | 36.6 | 35.2 |
| Mean weight (kg) | 64.3 | 67.4 |
| Mean BMI (kg/m2) | 22.8 | 23.1 |
| Mean gestational age at sampling (months of gestation) | 18.1 | 17.9 |
BMI, body mass index
Out of these 1490 patients, 329 (22.1%) were screened as positive on triple test. All of these patients (329 screened positive) were then counselled in detail and were given either the option for risk re-assessment (quadruple test plus genetic sonogram for soft markers) or NIPS (from year 2015) or amniocentesis. 208 (63.2%) patients opted for amniocentesis directly of which six patients had trisomy 21. 121 women opted for risk reassessment, of which 11 patients were screened positive on risk reassessment and went ahead for amniocentesis. Of these 11 patients, two patients had trisomy 21. Of the remaining 110 patients who were screened negative on risk-reassessment, none had trisomy 21 on follow up. Of the 1161 patients who had screen negative triple test, two patients had trisomy 21 on subsequent follow up (Fig. 1).
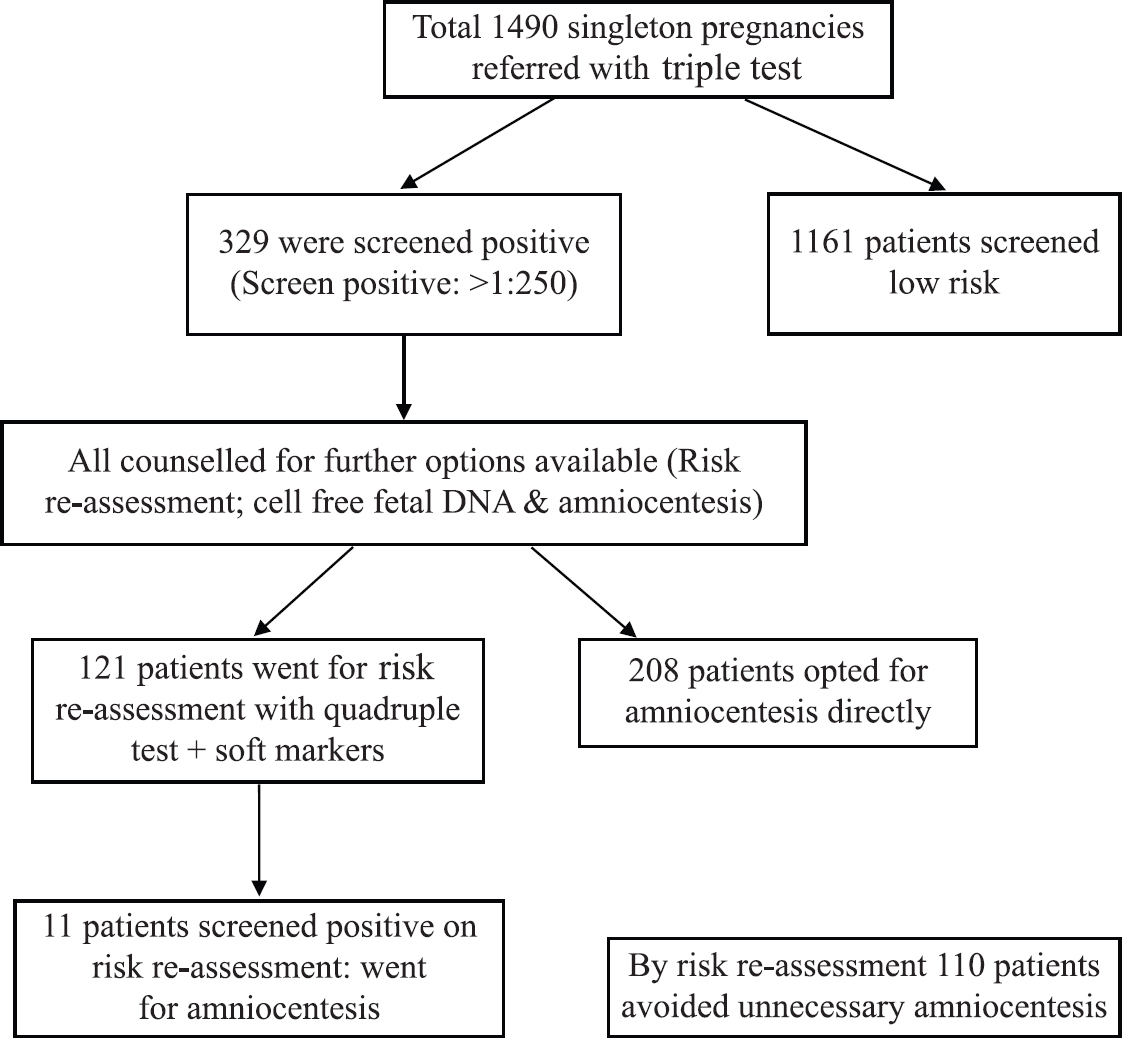
- Management of singleton pregnancies with positive triple test.
1685 women with high-risk singleton pregnancies were screened by quadruple test during the study period. Out of these 1685 patients, 311 were screened positive on quadruple test. Women with screened positive quadruple test were also counselled in detail and were given the option of risk re-assessment (quadruple test plus genetic sonogram for soft markers) or NIPS (from year 2015) or amniocentesis. 155 (49.8%) women opted for amniocentesis directly of which six patients had trisomy 21. 156 women opted for risk re-assessment using quadruple test with genetic sonogram for soft markers. Out of these 156 women, 20 were screened positive for trisomy 21 and went ahead for amniocentesis with three patients confirmed with trisomy 21. Of the remaining 136 patients who were screened negative using risk re-assessment, one patient had trisomy 21 detected on follow up. Of the 1374 patients who were quadruple negative on initial screening, one patient had trisomy 21 on follow up (Fig. 2).
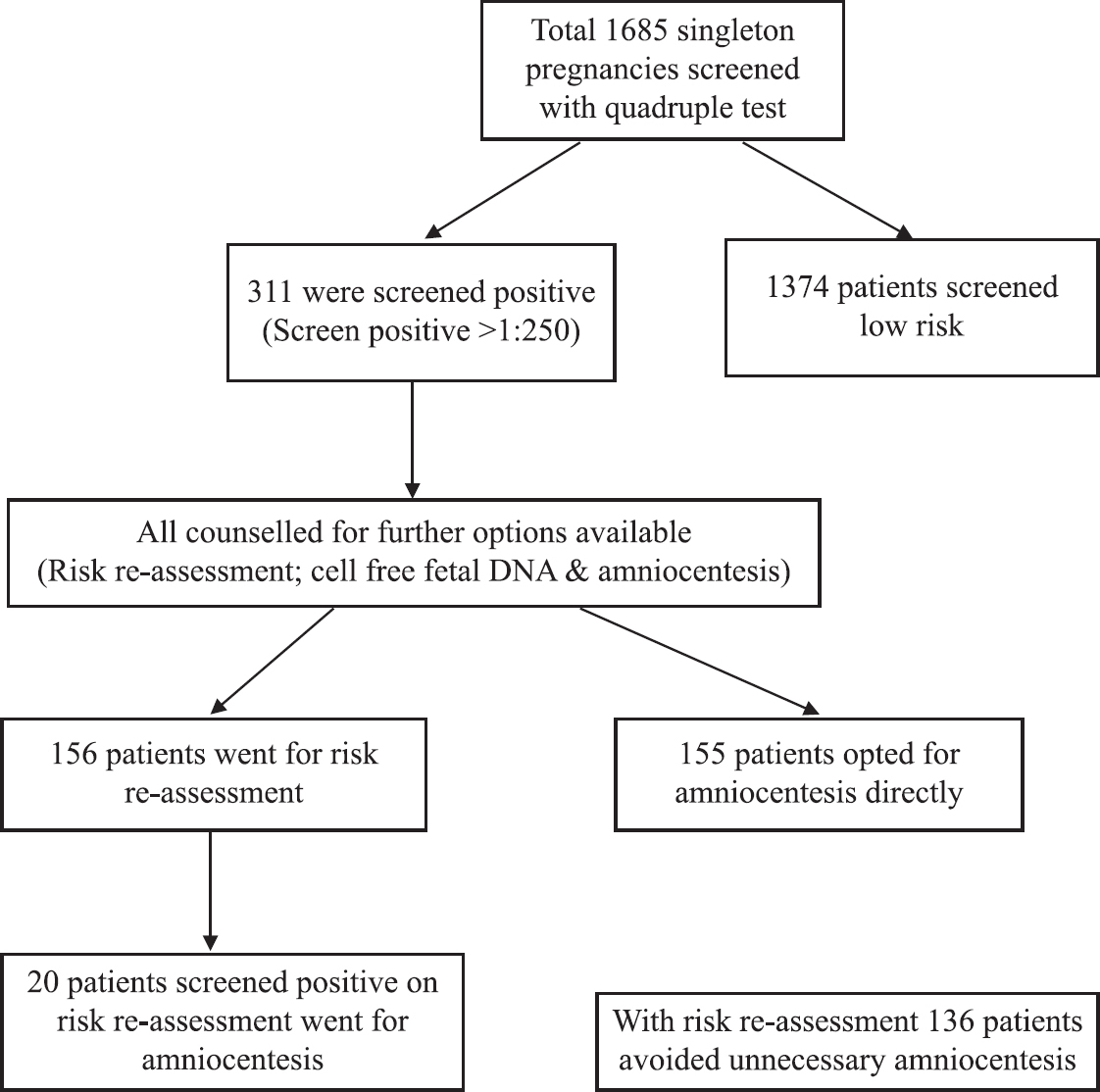
- Management of singleton pregnancies with positive quadruple test.
With the introduction of NIPS in the year 2015, this test was introduced in the study centre at the end of the year 2015 and subsequently offered to all patients who screened positive on either triple or quadruple test. However due to high cost, limited data on the sensitivity of NIPS initially, added apprehension of patients due to screen positive biochemical tests along with the fear of getting invasive testing done if NIPS comes positive, women who were screened positive either on triple test or quadruple test or quadruple test plus soft markers directly opted for amniocentesis in our study during that period (2015-2017) with none of the patients undergoing NIPS.
Out of the total 3175 high-risk pregnancies screened, 21 foetuses were diagnosed to have trisomy 21, giving a prevalence of trisomy 21 of 1:151 at our centre. The OAPR was found to be 1:40.2 for triple test, 1:30.1 for quadruple test and 1:6 for quadruple test plus genetic sonogram (Table II).
| Test | Total positive | True positive | OAPR |
|---|---|---|---|
| Triple test | 329 | 8 | 1:40.2 |
| Quadruple test | 311 | 10 | 1:30.1 |
| Quadruple plus soft markers | 31 | 5 | 1:6 |
OAPR, odd’s of being affected given a positive risk
In our study, quadruple test had a higher DR of 90 per cent, 95 per cent CI (58.8-99.7) with 17.8 per cent false positive rate as compared to a DR of 80 per cent, 95 per cent CI (44.4-97.5) with false positive rate of 21.6 per cent of triple test.
The accuracy of quadruple test was found to be 82.1 per cent, 95 per cent CI (80.16-83.88) against the 78.32 per cent, 95 per cent CI (76.14-80.4) accuracy of triple test. Quadruple test plus the genetic sonogram for soft markers was found to have the highest accuracy of 90.6 per cent, 95 per cent CI (86.6-93.7) for screening of trisomy 21.
Discussion
The overall incidence of DS is reportedly one in 13613 to one in 6924 live births and is the most common genetic cause of developmental delay. The risk of DS increases gradually up to the age of 33 yr and subsequently increases exponentially till the age of 45 yr after which it plateaus12. In the United Kingdom (UK), the National Down’s Syndrome Cytogenetic Register indicated that without improved screening tools between 1989 and 2008, the continuous rise in maternal age would have caused a 48 per cent increase in live births with Down’s syndrome10.
Apart from the maternal age, biochemical and ultrasonographic markers introduced since the early 1980s have markedly increased the sensitivity of screening programmes13. Next-generation sequencing is the latest non-invasive screening tool for the detection of aneuploidies14.
For a pregnancy diagnosed as screen positive for DS on biochemical screening whether on dual test (free beta-HCG + PAPP-A) or triple/quadruple test, the options available include combining the dual test with the NT/NB scan done by a foetal medicine expert. This increases the DR of the combined first-trimester screening to 93-95 per cent with a 3 per cent FPR. Similarly, a pregnancy screened positive for DS on quadruple test, risk re-assessment (in which quadruple test is combined with the genetic sonogram) gives a DR of 80 per cent with a three per cent FPR (ISPD 2015)5. Nevertheless, in both situations, CVS/amniocentesis remains the gold standard diagnostic test for the detection of DS. The risk of iatrogenic foetal loss with these invasive tests is approximately 0.7-1 per cent8.
In many of the developing countries including India, a proper screening modality is yet to be incorporated in national antenatal screening programmes. In the West, on the other hand, screening begins as early as in the first trimester in which first trimester screening is done combining the dual test with the NT/NB scan. Second trimester screening begins at 16 wk in which quadruple test is done and at 18 wk a genetic sonogram is done to assess the risk of having a DS child13. The results of the genetic sonogram and the quadruple test are combined again with the first-trimester screening results. This is called as sequential screening for DS and has a DR of 95 per cent13, a method that needs to be adopted universally. In India, in many of the government and private settings, screening generally begins in the second trimester and triple test which was introduced in 198815, is still being increasingly used as a second-trimester biochemical screening tool for DS. The first trimester extended combined test which has a DR of 93.8 per cent with 1.9 per cent FPR in the Indian setting has not yet been uniformly adopted6.
The reliability of the quadruple test as compared to the triple test in the screening of DS is well established. SURUSS study16, 2003 found similar results with a 77 per cent DR for triple test against an 84 per cent DR for quadruple test keeping the false positive rate (FPR) of five per cent. The study concluded that quadruple test is a better screening tool but because the only commercially available assay for inhibin A was not suitable for use in a routine laboratory (insufficiently stable and intra batch assay variation was excessive, 17%), it could not be used in national screening protocols16. However, by 2007, the UK National Screening Committee had incorporated the quadruple test in its screening programme.
A study conducted in Taiwan assessed quadruple test in 21,481 women and found a DR of 81.1 per cent with 4.4 per cent FPR17. The FPR of triple test in the present study was 21.6 per cent whereas the FPR for quadruple test was 17.8 per cent and for quadruple test + genetic sonogram it was 8.9 per cent. Our rates of FPR were higher than the Taiwan study as that was based on the general population, whereas the present study was based on high-risk population with a higher incidence of trisomy 21 compared to the general population. As the incidence risk increases in the study population, the FPR of screening tests also increases18. In a study from western India, 2111 women were investigated by triple-marker screening between 14 and 20 wk of gestation, of whom 224 women were found to be screen positive for trisomy 21 and further on karyotyping of 105 of the screen-positive cases, eight had trisomy 21 and one had mosaic trisomy 21 quoting the DR and FPR of that study19. In another study which reported the two-year data of a referral institute from northern India, in four out of 68 women (4.4%) with triple-test positivity for DS, amniotic fluid karyotyping was found to show trisomy 2120.
The present study conducted in a tertiary care referral hospital found the OAPR of quadruple test to be 1:30.1 against an OAPR of 1:40.2 when using the triple test as a second-trimester biochemical screening tool. Also the OAPR of quadruple test plus genetic sonogram detectable at second trimester was as high as 1:6. This meant that for every six amniocentesis done based on quadruple test plus the soft markers, one foetus was be affected by DS.
The present study showed a higher DR/sensitivity of the quadruple test against the triple test as compared to the results obtained from the previous study19, again because the whole of the study population was a high-risk group as aforementioned resulting even in a high OAPR of quadruple test when compared to the triple test. When entropolated to the general population, the sensitivity/DR would be less. This was the only draw back of the present study other than the fact that the laboratories which reported a screen positive were heterogeneous.
Overall the quadruple test is a better second-trimester biochemical screening tool for DS and should be used for screening of aneuploidies in case the first trimester combined test has been missed. The false-positive rate of the test can be further lowered by combining it with the genetic sonogram done by an FMF Anomaly Certified sonologist. This test should be incorporated as a part of the national prenatal screening programme. The quadruple test along with a genetic sonogram forms an accurate screening test for trisomy 21 and can avoid unnecessary invasive testing (amniocentesis) in women with just a positive biochemical screening. This would additionally reduce unnecessary financial and psychological burden on the families and prevent chances of iatrogenic miscarriages associated with amniocentesis.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Aetiologic spectrum of mental retardation &developmental delay in India. Indian J Med Res. 2012;136:436-44.
- [Google Scholar]
- Past, present &future scenario of thalassaemic care &control in India. Indian J Med Res. 2011;134:507-21.
- [Google Scholar]
- Study of still birth and major congenital anomaly among newborns in the high-level natural radiation areas of Kerala, India. J Community Genet. 2013;4:21-31.
- [Google Scholar]
- Down syndrome in tribal population in India:a field observation. J Neurosci Rural Pract. 2016;7:40-43.
- [Google Scholar]
- Position statement from the Chromosome Abnormality Screening Committee on behalf of the Board of the International Society for Prenatal Diagnosis. Prenat Diagn. 2015;35:725-34.
- [Google Scholar]
- Observational study comparing the performance of first-trimester screening protocols for detecting trisomy 21 in a North Indian population. Int J Gynaecol Obstet. 2017;137:14-9.
- [Google Scholar]
- An update on antenatal screening for Down's syndrome and specific implications for assisted reproduction pregnancies. Human Reprod Update. 2006;12:513-8.
- [Google Scholar]
- Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling:A systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;45:16-26.
- [Google Scholar]
- Wrongful deaths and rightful lives –Screening for Down syndrome. Downs Syndr Res Pract. 2008;12:79-86.
- [Google Scholar]
- Revised estimates of the maternal age specific live birth prevalence of Down's syndrome. J Med Screen. 2002;9:2-6.
- [Google Scholar]
- Meta-analysis of second trimester markers of trisomy 21. Ultrasound Obstet Gynecol. 2013;41:247-61.
- [Google Scholar]
- Screening for Down syndrome using first-trimester combined screening followed by second trimester ultrasound examination in an unselected population. Gynecol Obstet Fertil. 2007;35:303-11.
- [Google Scholar]
- Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood:A study in a clinical setting. Am J Obstet Gynecol. 2011;204(205):e1-11.
- [Google Scholar]
- The triple test as a screening technique for Down syndrome:Reliability and relevance. Int J Womens Health. 2010;2:83-8.
- [Google Scholar]
- First and second trimester antenatal screening for Down's syndrome:The results of the Serum, Urine and Ultrasound Screening Study (SURUSS) Health Technol Assess. 2003;7:1-77.
- [Google Scholar]
- Second-trimester maternal serum quadruple test for Down syndrome screening:A Taiwanese population-based study. Taiwan J Obstet Gynecol. 2010;49:30-4.
- [Google Scholar]
- Second trimester screening for fetal aneuploidy through triple marker test:The two-year experience of the genetics unit of a referral institute. Perinatology. 2008;10:149-54.
- [Google Scholar]






