Translate this page into:
Electroencephalography-based cortical sources of working memory in the subjects with opioid addiction: A pilot study
For correspondence: Dr Simran Kaur, Department of Physiology, All India Institute of Medical Sciences, New Delhi 110 029, India e-mail: simranaiims@outlook.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Working memory impairments in the subjects of opioid addiction may stem from an aberrant cortical activity in the executive areas, and may help in early identification of individuals with addictive tendencies and may also be used as a neurofeedback mechanism in adjunct to the existing therapeutics.
Methods:
Electrical neuroimaging via 128-channel electroencephalography (EEG) recording was done in 15 male subjects with opioid addiction (29.45±5.6 yr) during the performance of Sternberg Working Memory Task. EEG data were acquired and analyzed for cortical sources during task as compared to resting (baseline) condition.
Results:
Working memory deficits were manifested as decrease in accuracy percentage in the subjects with opioid addiction, while no significant difference was seen in reaction time, on comparison with laboratory-acquired matched controls. Standardized low-resolution brain electromagnetic tomography (sLORETA)-based EEG source analysis revealed higher cortical activity in the anterior cingulate cortex, inferior, middle and superior temporal gyri, inferior frontal gyrus, superior parietal lobule, inferior parietal lobule and precuneus, whereas significant lower activity was seen in superior and middle frontal gyri, parietal lobule, cingulate cortex and pre- and postcentral gyri when the task was compared to baseline in the subjects with opioid addiction. Further, a negative correlation was seen between the accuracy of task performance and activation ratio for the significant gyri in the subjects with opioid addiction.
Interpretation & conclusions:
EEG cortical sources revealed the failure of deactivation of default-mode network (DMN) during the task amongst the subjects with opioid addiction. In addition, there was a decrease in the executive function areas in the subjects with opioid addiction. This lack of sufficiently active executive network and persistence of DMN during the task (as compared to baseline) may potentially form the basis of functional impairments in the subjects with opioid addiction.
Keywords
Cortical sources
opioid addiction
quantitative electroencephalography
sLORETA
working memory deficits
Addiction is defined as the compulsive use of a substance or engagement in behaviour despite its negative consequences1. There has always remained a social reservation in recognizing and accordingly accrediting addiction, per se, as a disease necessitating treatment. Therapeutic objectives of medical personnel have always aimed at setting right the derangements in the normal physiology of an individual, inflicted by the substance of abuse. However, recognition of the underlying aetiology of addiction and realization that addiction could stem from a disorder in volition could create a shift in the way we look at them2.
The prevalence of opioid addiction, as reported by a Global study conducted in 2010, was 0.22 per cent (with a 95% uncertainty interval=0.20-0.25%) based on multiple search strategies in online databases and feedback from experts across 187 countries and 21 region3. In Rapid Situation and Response Assessment conducted amongst the subjects with opioid addiction from five South Asian countries, reported that 76 per cent injected buprenorphine, 76 per cent injected heroin, 70 per cent used heroin by inhalation route and 64 per cent using propoxyphene4. The prevalence of comorbidities has been reported to be around 56.6 per cent in the subjects with opioid addiction ranging from mood to other psychiatric disorders as well as, other substance abuse disorders, which can be as severe as delirium and convulsions5.
Literature has reported that an inherent defect in an individual’s working memory can lead to impairment in volition and irrational heights to attain the substance of abuse despite being well aware of the ominous consequences it brings with it6. Even during the abstinence, neurocognitive deficits were seen in the domain of working memory in the subjects with opioid addiction, which had a significant correlation with the days of withdrawal7. The authors of this study reported that although the correlations do not imply causation, it does provide an impetus for further research.
Working memory is a term referring to a brain system that provides temporary storage and active manipulation of the information necessary for such complex cognitive tasks as language comprehension, learning and reasoning8. Working memory is known to have three subsystems: the central executive, the visuospatial sketch pad and the phonological loop9. The central executive is an attentional-controlling system, and the two subsystems, the visuospatial sketch pad and the phonological loop, play a role in the manipulation of visuospatial and verbal information, respectively10. It has not only been implicated in the causation of addiction but also in the subsequent prognosis and in the effectiveness of interventions aimed at curbing the menace of addiction. In a study conducted amongst adolescents, it was found that the effectiveness of educational interventions aimed at curbing alcohol and marijuana addiction was less effective amongst those who fared worse in tests aimed at assessing their working memory. This relationship between impairment of working memory and effectiveness of interventions also highlighted the fact that motivational interviewing was found to bring desirable results regardless11. Further, studies conducted amongst the subjects with opioid addiction also confirm the association between opioid addiction and deficits in working memory12. Similarly, studies conducted by Arias et al13 found that one-third of the subjects with opioid addiction were associated with greater neurocognitive impairment including in the domain of working memory.
As mentioned before, working memory is crucial to the cognition of an individual, and deficits in it may lead to a flaw in one’s volition, the first step towards addiction, and hence, it warrants a sensitive quantitative estimation and a detailed description. This has been attempted through various diagnostic modalities such as functional magnetic resonance imaging (fMRI), positron-emission tomography (PET) and electroencephalography (EEG). While EEG may have conventionally fallen short due to its limited spatial resolution in the past, with the 128 Electrode HydrocCel Geodesic Sensor Net (HCGSN), spatial resolution has improved to 5 mm, leading to it being an effective tool for conducting electrical neuroimaging studies. Further, EEG resolves the temporal drawbacks of PET and fMRI and is reported to resolve the events milliseconds apart, whereas fMRI is limited to 2-6 sec14. Moreover, the literature pertaining to working memory deficits in opioid addiction using 128-channel EEG is limited. Based on this, the present study was designed to investigate the effect of working memory in patients of opioid addiction and elucidate the EEG cortical sources of working memory using sLORETA (standardized low-resolution brain electromagnetic tomography) software. We hypothesized that the subjects with opioid addiction would demonstrate deficits in the performance of working memory task, which may be reflected by the cortical sources as deciphered by EEG source analysis.
Material & Methods
This is a cross-sectional observational study conducted between April and July 2019, performed on 15 subjects of opioid addiction at Stress and Cognitive Electroimaging Laboratory, department of Physiology, AIIMS, New Delhi, after obtaining the ethical clearance from the Institute Ethics Committee. The opioid dependent patients were recruited from the department of Psychiatry, National Drug Dependence and Treatment Centre (NDDTC) Ghaziabad, All India Institute of Medical Sciences. The sample size was calculated after consultation with the department of Statistics, AIIMS, New Delhi, as a pilot exploratory study.
Inclusion criteria: Patients were diagnosed by the clinician based on diagnostic and statistical manual of mental disorders-5 (DSM-5) criteria15 and had a frequency of heroin intake of 3-4 times per week for at least six months prior to the test and were in the detoxification phase at the time of the said study.
After obtaining written informed consent, right-handed males were recruited based the pre-defined inclusion criteria (age: 18-45 yr, with normal or corrected visual acuity of 6/6), while subjects with any other comorbid psychiatric/neurological/sleep disorders were excluded from the study.
The data of the subjects with opioid addiction were compared to healthy control data previously acquired in the laboratory. Both the groups were matched with respect to age, gender (males) and socio-economic background (lower-middle class). Based on a semi-structured questionnaire, it was assessed that the subjects of both groups had minimum education of 8th standard in an English medium and were well versed with the English alphabets, as required for the adequate performance in the simple English alphabet-based Sternberg task. The behavioural data had been acquired on the same task at the Stress and Cognitive Electroimaging Laboratory under similar conditions in both the groups.
Stimulus presentation and cognitive task: The Sternberg Working Memory Task was designed using E-prime v2.0, which facilitated the logging of the events of the trial to the EEG data through an experiment control interface based on a local area network. The stimulus presentation was done on a 17-inch LCD display placed 70 cm from the subject. Two blocks of the task, each consisting of 20 trials, were presented to each subject. The trial structure of the task is given in Fig. 1.
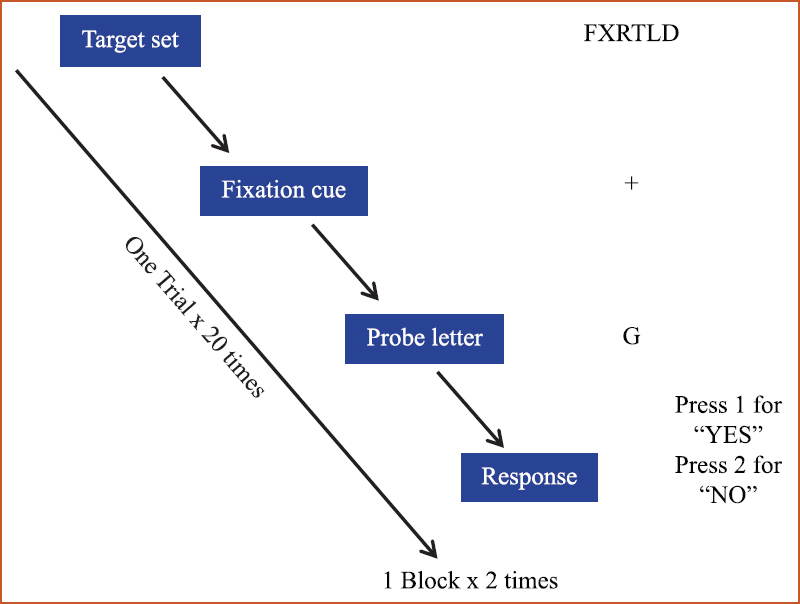
- Trial structure for the Sternberg task paradigm.
The Sternberg Working Memory Task was divided into: (i) encoding phase, wherein an array of six alphabets was presented on the screen for 2 sec and the subject was asked to memorize for subsequent recall; (ii) retention phase, wherein a ‘+’ sign was presented on the screen as a fixation cue for 2 sec, during which the subject was required to retain the array of alphabets that were presented in the previous phase; (iii) recall phase, wherein a probe letter was presented on the screen for 4 sec, to which the subject was required to press on the response pad provided, key ‘1’ if the letter was present in the original array of alphabets presented in the 1st phase or key ‘2’ if the letter was not present in the original array of alphabets presented; and (iv) response, response elicited was recorded through keyboard/response pad.
The responses were generated as reaction time and accuracy, in the data aid sheet. The Sternberg trials were randomized with respect to positive trial (when the target letter was present in the set) and negative trial (when the target letter was not present in the remembered set).
Electroencephalography (EEG) acquisition and pre-processing: EEG acquisition was done at a sampling rate of 1000 Hz using a 128-channel HCGSN system (Electrical Geodesic Inc.). Subsequently, raw EEG data were processed offline using NetStation v5.4.1.2. version and EEGLAB v14 toolbox in MATLAB r2018b (Mathworks Inc.) after data exportation. The data were first band pass filtered at 1-00 Hz with a notch filter of 50 Hz. The segmentation strategy involved 500 msec artefact-free epochs, just before the presentation of the probe letter, from all the forty trials of Sternberg task (http://www.sccn.ucsd.edu/eeglab/). The last 500 msec of data was taken from the retention period to ensure that the cortical changes were due to optimum retention and not due to familiarity of the stimuli. Furthermore, it ensured sufficient time for the maintenance rehearsal for better recall16. Forty segments of 500 msec were taken from the resting (eyes open) condition to elucidate the cortical activity during the task as compared to the baseline. Fig. 2 depicts the segmentation strategy taken for each of the Sternberg trials. Subsequently, artefacts such as eye movement, eye blinks and bad channels were removed using NetStation’s semi-automated artefact-detection algorithm and bad channels were interpolated with spherical spline interpolation. Thereafter, data were exported to EEGLAB toolbox operating in MATLAB software (R2017a; http://eeglab.org) for further analysis and average referencing was done to remove the reference site bias. Independent component analysis was performed using the RUNICA algorithm in EEGLAB toolbox to remove the component characteristics of eye movements, muscle, cardiac artefacts and power line noise. These steps of pre-processing were performed in the subjects of opioid addiction, across the segmented data obtained from the eyes open condition and the epochs before probe letter presented during the task.
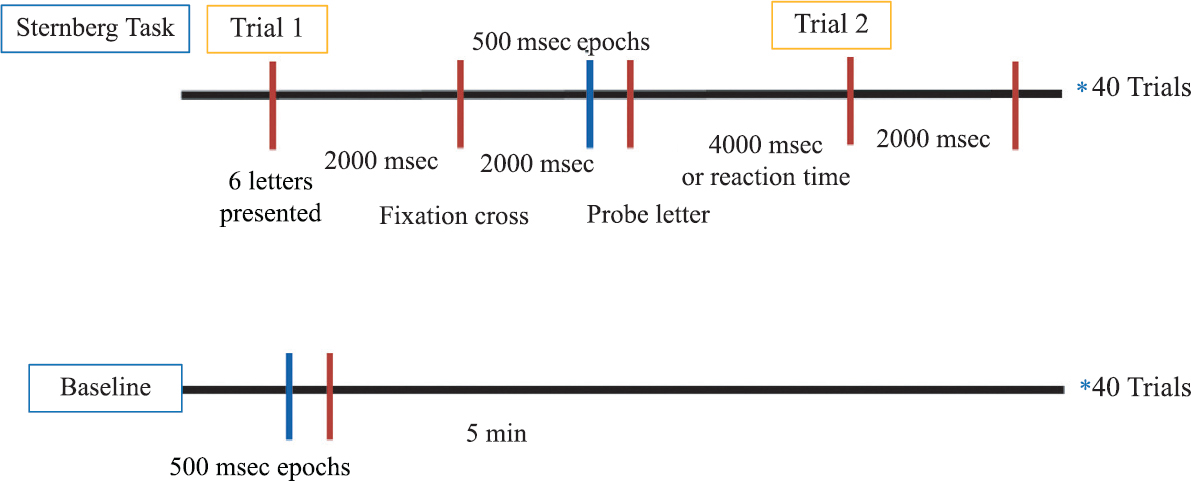
- Segmentation strategy: 500 msec artefact-free epochs before the presentation of the probe letter, from forty trials of Sternberg task was compared to forty segments of 500 msec from the resting (eyes open) condition.
Cortical sources analysis: Standardized low-resolution brain electromagnetic tomography was used to calculate the intracortical electrical sources17. It is based on solving the inverse problem without assuming a priori number of underlying sources and computes electric neural activity as standardized current density (unit: amperes per square meter, A/m2). The probabilistic location of grey matter in the average MRI atlas of the Montreal Neurological Institute (MNI) Atlas was used to constrain the location of 6239 source voxels. Three orthogonal dipole moments (x, y and z) were defined and solved for each of the source voxels. The source space was restricted to 6239 cortical voxels (5 mm3) that were each assigned to a gyrus, representing 66 gyri and corresponding Brodmann areas (BAs) using MNI 152 template. Five orthogonal views were plotted for each condition using viewer and explorer tools of sLORETA by giving statistically compared output files. Gyri and BAs pertaining to each lobe that showed significantly different activity during each event condition and coordinates of the voxels activated in the MNI-brain were also extracted.
Statistical analysis: The statistical analysis was done using GraphPad Prism Version 9. (GraphPad Software San Diego, CA, USA). For the behavioural parameters, normality tests (D’Agostino and Pearson test and Shapiro–Wilk test) were applied, and the data were found to be normally distributed. Thus, parametric tests were applied for the behavioural analysis.
To find out the difference of cortical source localization between conditions voxel-by-voxel in each group, independent t test was used based on sLORETA current source density power. sLORETA applies a statistical nonparametric mapping method (SnPM)18 for analysis of current source density based on Fisher’s permutation method (with the threshold set at the 5% probability level). It thus compares the mean current source power in each voxel and its distribution in the permuted values. By evaluating the empirical probability distribution of the “maximal-statistics” in the null hypothesis, permutation and randomization tests have demonstrated effectiveness in controlling Type I error in neuroimaging studies19. For the present study, sLORETA used 5000 data randomizations to determine the critical probability t threshold values for the actually observed test values with correction for multiple comparisons across all voxels, without the need to rely on the Gaussian distribution of data. The levels of significance were corrected for multiple comparisons, for which significance was determined at <0.0520.
The current source density values of cortical gyri with statistically significant were extracted and averaged for the 500 msec of the stimulus and baseline EEG epochs. Then, the ratio of mean current source density in stimulus/baseline was computed to calculate an activation ratio. Correlation of the activation ratios with the behavioural data (reaction times and accuracy) was conducted. Since the data were normally distributed (Shapiro–Wilk test), analysis was done using Pearson’s correlation and significant correlation is reported in Table I.
| Parameter/gyrus with significant activation | Pearson’s correlation |
|---|---|
| Reaction time | −0.46689 |
| Accuracy | 1 |
| Anterior cingulate | −0.653* |
| Cingulate gyrus | −0.649* |
| Inferior frontal gyrus | −0.664* |
| Inferior parietal lobule | −0.647* |
| Inferior temporal gyrus | −0.653* |
| Medial frontal gyrus | −0.672* |
| Middle frontal gyrus | −0.674* |
| Middle temporal gyrus | −0.656* |
| Orbital gyrus | −0.670* |
| Paracentral lobule | −0.678* |
| Postcentral gyrus | −0.659* |
| Precentral gyrus | −0.675* |
| Precuneus gyrus | −0.667* |
| Subgyral gyrus | −0.684* |
| Superior frontal gyrus | −0.678* |
| Superior parietal lobule | −0.674* |
| Superior temporal gyrus | −0.653* |
| Supramarginal gyrus | −0.618* |
| Transverse temporal gyrus | −0.637* |
P *<0.05
Results
Fifteen males of mean age 29.45±5.6 yr with opioid addiction performed the Sternberg task. The results are provided under the following two headings:
Behavioural scores of Sternberg Working Memory Task: The reaction time and accuracy of the subjects with opioid addiction and controls were normally distributed as per the normality tests applied. Thus, parametric tests (unpaired t test) were used to find out the significant difference between both the groups for both the parameters. Table II highlights the results of the behavioural parameters while performing the Sternberg task, wherein there was no significant difference in the reaction time, but the accuracy percentage among the subjects with opioid addiction was significantly lower as compared to laboratory-matched control data (Fig. 3A and B).
| Parameters | Subjects with Opioid addiction | Controls |
|---|---|---|
| Reaction time (msec) | 942.8±203.0 | 999.3±219.1 |
| Accuracy (%)*** | 68.07±6.63 | 84.53±7.558 |
P ***<0.0001 (for comparisons between opioid addiction and controls)
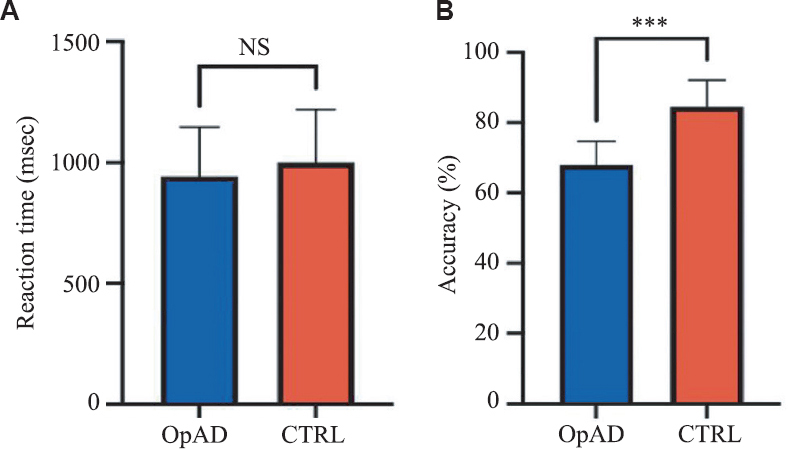
- Behavioural results of Sternberg task in the subjects with opioid addiction as compared to controls. (A) Reaction time (B) Accuracy. OpAD: Subjects with opioid addiction, CTRL, controls.
EEG sources during task as compared to baseline: The orthogonal plots, given below, depict the differential cortical activity during Sternberg task as compared to the baseline in the subjects with opioid addiction using sLORETA (Fig. 4). The scale provided in the figure is a measure of current source density in (nAm/m2). Blue-coloured areas indicate lower activity, while red–yellow colour highlights higher cortical activity.
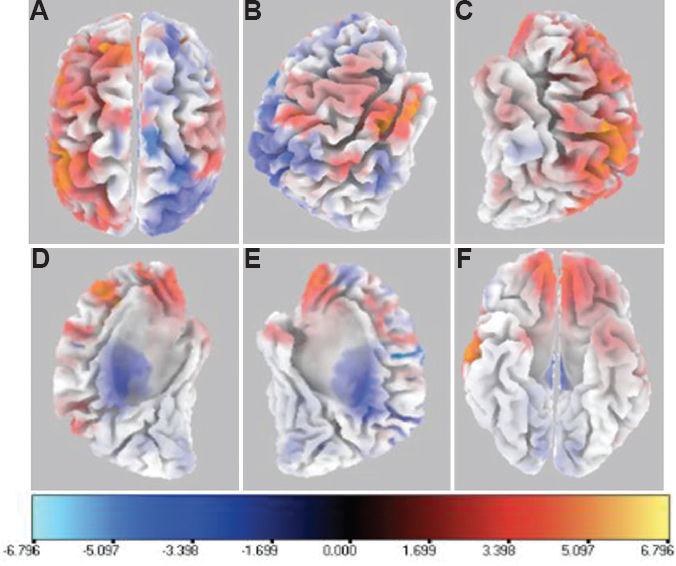
- Six orthogonal views for 500 msec epoch segment showing mean difference in current source density for gyri with significantly different cortical activity during Sternberg Working Memory Task as compared to baseline. (A) top view; (B) right view; (C) left view; (D) left hemisphere right view; (E) right hemisphere left view; and (F) bottom view. The scale provided measures the t statistics based on SnPM analysis comparing the current source density in (nA/mm2) of task versus baseline conditions. Blue-coloured areas indicate lower activity, while red–yellow colour highlights higher cortical activity.
The cortical sources were localized based on the significant current source density (csd) values of cortical gyri, averaged for the 500 msec of the stimulus and baseline EEG epochs. Then, the ratio of mean current source density in stimulus/baseline was computed to calculate an activation ratio. These activation ratios were correlated through Pearson correlation with the behavioural data of reaction times and accuracy from the Sternberg task. We found a negative correlation between the activation ratios and accuracy. However, no correlation was seen with the reaction time of the subjects of opioid addiction while performing a working memory task (Table I).
Discussion
From a psychological perspective, addiction is a disorder of altered cognition. Anatomically, the brain regions and processes that underlie addiction overlap with those essential for cognitive functions, including learning, memory, attention, reasoning and impulse control21. The impairment in working memory is one of the cognitive deficits reported in the subjects with opioid addiction2223. Our study findings have corroborated the cognitive decline in the subjects of opioid addiction with lower accuracy but comparable reaction time when compared to already acquired laboratory data. This implied that there is no slowing of the mental faculties in these patients but a derailment in the conclusions drawn. This could possibly bear long-term detrimental consequences and implications on their functioning in the society as well. While executing tasks within the provided time frame, there seemed to be no particular dysfunction in the volition, but significant impairment may occur in decision making while abstaining from the use of substance. However, lack of evoking a significant difference in reaction time amongst the subjects with opioid addiction could have also stemmed from the small sample size.
On exploring the EEG cortical sources, a higher activity was seen in anterior cingulate cortex, inferior and, middle temporal gyrus, superior temporal and, inferior frontal gyrus, superior parietal lobule, inferior parietal lobule (IPL) and the precuneus. Interestingly, most of these areas form part of the default-mode network (DMN). DMN comprises of network of activated areas when the resting brain is idle and not actively engaged in attention-demanding tasks. These areas get deactivated in focussed attention and are anti-correlated with the executive/attention a networks that are activated during performance of cognitive tasks24, like the one used in this study – the Sternberg Working Memory Task. The DMN may be divided into anterior (aDMN) and posterior (pDMN). The aDMN contains the mPFC, dorsal medial prefrontal cortex, anterior cingulate cortex, posterior cingulate cortex, anterior temporal lobe, inferior frontal gyrus and lateral parietal cortex, whereas the pDMN contains the posterior cingulate cortex, precuneus, posterior IPL, angular, hippocampal and temporal areas2425. The precuneus demonstrates decreased activation during most externally driven tasks. Studies claim a pivotal role of the precuneus in the DMN on account of its strong connections with the rest of the acclaimed DMN areas. Utevsky et al26 reported that during the performance of a task, increased connectivity (compared with rest) was seen between the precuneus and the right frontoparietal network, whereas during rest, increased connectivity was seen between the precuneus and the DMN. Thus, in the current study, surprisingly, the subjects with opioid addiction demonstrated activation of DMN areas during the task contrary to what is expected. Zhang and Volkow27 also reported that drug abuse alters the DMN connectivity affecting both cognitive and emotional processing. DMN reorganization was seen in the patients with opioid addiction, and strong associations were seen between abstinence duration and coupling strength between the areas of the network. Sullivan et al28 also documented that alcoholism can disrupt neural synchrony between nodes of intrinsic functional networks that are maximally active when resting relative to engaging in a task, the DMN pattern. Yuan et al29 reported that abnormal functional organization in the heroin-dependent subjects may provide evidence for functional impairments in decision making.
The importance of the prefrontal cortex in executive tasks like working memory is a well-established fact30. Experiments lesioning the prefrontal cortex have shown a significant impairment in the performance of individuals in working memory tasks. Analysis of the EEG data also brought forth a deactivation in certain voxels of the superior and middle frontal gyrus while the task was being performed. The superior, middle and inferior frontal gyri, which are part of the dorsolateral prefrontal cortex, are involved in executive functions, such as working memory, cognitive flexibility, planning, inhibition and abstract reasoning31. The deactivation of these frontal executive areas during the performance of an executive task involving working memory signifies the presence of hypofrontality, the cognitive signatures of which may be found in their poor performance on the parameters of the Sternberg task, as well as the inability to make strategic decisions. Goldstein and Volkow32 documented that there is dysfunction of prefrontal cortex in the patients with addiction during both cognitive and emotional challenges. Hester and Garavan33 reported that prefrontal hypoactivation correlates with compromised control over prepotent urges in the subjects with cocaine addiction using GO-NO-GO task, wherein working memory was varied. Thus, this could be reflective of an inability of the subjects with opioid addiction in activating the frontal areas, part of the executive network, sufficient enough to meet the requirements of the task. The activation during working memory tasks is not just limited to the frontal cortex; studies also report the participation of the parietal cortex in this frontoparietal network34. Our study revealed decreased activation of both superior and IPL during the performance of a task. Superior parietal lobe has a crucial role in the visual guidance of action, whereas IPL is responsible for maintaining attention on current task goals as well as encoding salient events in the environment. IPL forms a crucial node in a frontoparietal system and has been associated with sustaining attention, detecting salient or novel events, phasic alerting and switching between task-sets35. In addition, some voxels of IPL were found to be activated during the performance of a task. Since IPL also forms the part of DMN, the activation of these voxels may be due to failure to suppress DMN-related areas in the subjects with opioid addiction36.
To envisage the basis of the functional impairments in subjects with opioid addiction, the behavioural parameters were correlated with the activation ratios of the significant gyri, wherein only accuracy was seen to be negatively correlated. Since most of the areas were DMN areas, in order to get higher accuracy, there might be a relative deactivation of the DMN areas in order to perform well. Thus, responsiveness to increasing cognitive demand might have led to the necessary reallocation of finite neural resources needed for cognitive performance37. However, since the performance was suboptimal, a negative correlation was also seen with the areas implicated for executive function as well.
Hence, a clear failure to disengage the DMN in performing the working memory task was noted. As performing a task requires engagement of the executive network and deactivation of the DMN, the lack of achieving the same would perhaps reason out for their impaired performance in the working memory task in terms of their accuracy.
Our study addresses pertinent aspects in the subjects with opioid addiction with respect to working memory deficit (lower accuracy) and the underlying cortical sources while the task is being performed, where there is limited literature present with respect to neuroimaging. However, this study had some limitations including, time constraints, which may be addressed by undertaking a longitudinal study with the same research question. The study could have been done on a larger sample size with simultaneous acquisition of neuroimaging modalities such as EEG in control data in order to be a representative of the population in question. In addition, the Sternberg task paradigm had an inherent confounding factor, wherein presence of the same probe letter in a former trial, could affect the reaction time and accuracy of the subsequent trial, though in our study since same paradigm was followed in subjects of opioid addiction and healthy controls, which negated the effect of this confounder.
Overall, the present study opens avenues for further research. The authors have looked into the cortical sources of the subjects with opioid addiction using source analysis. Other EEG analyses such as ERP, power spectral analysis, event-related spectral perturbations or microstate analysis can further help to understand the neural underpinnings in the patients with opioid addiction. Furthermore, several other addictions such as alcohol, internet or gambling disorders can be studied using the same paradigm in order to decipher working memory deficits in these patients as compared to healthy controls.
Financial support & sponsorship: The first author (SS) received funding as part of ICMR-short term studentship (STS) Scheme (Ref No: 2019-01723).
Conflicts of Interest: None.
References
- The discovery of addiction. Changing conceptions of habitual drunkenness in America. J Stud Alcohol. 1978;39:143-74.
- [Google Scholar]
- The place of volition in addiction:Differing approaches and their implications for policy and service provision. Drug Alcohol Rev. 2013;32:195-204.
- [Google Scholar]
- The global epidemiology and burden of opioid dependence:Results from the global burden of disease 2010 study. Addiction. 2014;109:1320-33.
- [Google Scholar]
- Rapid situation and response assessment (RSRA) on drugs and HIV in Bangladesh, Bhutan, India, Nepal and Sri Lanka. Available from:https://www.unodc.org/pdf/india/Presentation_RSRA_June_25_2008.pdf
- [Google Scholar]
- A study of comorbidity in psychoactive substance dependence patients. Indian J Psychiatry. 1994;36:133-7.
- [Google Scholar]
- Cognitive function during early abstinence from opioid dependence:A comparison to age, gender, and verbal intelligence matched controls. BMC Psychiatry. 2006;6:9.
- [Google Scholar]
- Working memory.Psychology of learning and motivation. 1974. New York Academic Press; :47-89. Available from:https://www.sciencedirect.com/science/article/pii/S0079742108604521
- [Google Scholar]
- Working memory capacity and addiction treatment outcomes in adolescents. Am J Drug Alcohol Abuse. 2018;44:185-92.
- [Google Scholar]
- Working memory impairment in cannabis- and opioid-dependent adolescents. Subst Abuse. 2014;35:387-90.
- [Google Scholar]
- Neurocognitive, psychiatric, and substance use characteristics in opioid dependent adults. Addict Behav. 2016;60:137-43.
- [Google Scholar]
- Functional magnetic resonance imaging for imaging neural activity in the human brain:The annual progress. Comput Math Methods Med. 2012;2012:613465.
- [Google Scholar]
- DSM-5 as a Framework for psychiatric diagnosis. In: Holes RE, Yudofsky SC, Roberts LW, eds. Textbook of psychiatry. Washington (DC): American Psychiatric Publishing; 2014.
- [Google Scholar]
- Consolidating working memory:Distinguishing the effects of consolidation, rehearsal and attentional refreshing in a working memory span task. J Mem Lang. 2015;81:34-50.
- [Google Scholar]
- Functional imaging with low-resolution brain electromagnetic tomography (LORETA):A review. Methods Find Exp Clin Pharmacol. 2002;24(Suppl C):91-5.
- [Google Scholar]
- Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab. 1996;16:7-22.
- [Google Scholar]
- Nonparametric permutation tests for functional neuroimaging:A primer with examples. Hum Brain Mapp. 2001;15:1-25.
- [Google Scholar]
- Deactivation of default-mode network and early suppression of decision-making areas during retrieval period by high-arousing emotions improves performance in verbal working memory task. Cogn Affect Behav Neurosci. 2019;19:231-8.
- [Google Scholar]
- Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacol. 2006;31:1036-47.
- [Google Scholar]
- Investigation of brain electrophysiological properties among heroin addicts:Quantitative EEG and event-related potentials. J Neurosci Res. 2017;95:1633-46.
- [Google Scholar]
- Neuronal oscillations and functional interactions between resting state networks. Hum Brain Mapp. 2013;35:3517-28.
- [Google Scholar]
- Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34:932-40.
- [Google Scholar]
- A selective insular perfusion deficit contributes to compromised salience network connectivity in recovering alcoholic men. Biol Psychiatry. 2013;74:547-55.
- [Google Scholar]
- Combining spatial and temporal information to explore resting-state networks changes in abstinent heroin-dependent individuals. Neurosci Lett. 2010;475:20-4.
- [Google Scholar]
- The human frontal lobe:An introduction. In: The human frontal lobes:Functions and disorders (2 nd ed). New York, USA: The Guilford Press; 2007. p. :3-11.
- [Google Scholar]
- Dysfunction of the prefrontal cortex in addiction:Neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652-69.
- [Google Scholar]
- Executive dysfunction in cocaine addiction:Evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017-22.
- [Google Scholar]
- The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47:1434-48.
- [Google Scholar]
- On the relationship between the “default mode network“and the “social brain. “Front Hum Neurosci. 2012;6:189.
- [Google Scholar]
- Is a responsive default mode network required for successful working memory task performance? J Neurosci. 2015;35:11595-605.
- [Google Scholar]






