Translate this page into:
Potential of neutrophil to lymphocyte ratio in predicting sustained remission in rheumatoid arthritis compared to other immune activation markers
For correspondence: Dr S. Chandrashekara, Department of Rheumatology & Clinical Immunology, ChanRe Rheumatology & Immunology Center & Research, No. 65 (414), 20th Main, West of Chord Road, 1st Block, Rajajinagar, Bengaluru 560 010, Karnataka, India e-mail: chandrashekara_s@yahoo.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Cells and cell proportions may indicate the equilibrium status of the immune system. The present study was conducted to evaluate the role of cytokines and the immunocompetent cells as biomarkers of remission in rheumatoid arthritis (RA) patients intended to withdraw or reduce disease-modifying anti-rheumatic drug (DMARD) treatment.
Methods:
This prospective observational study involved newly diagnosed and treated RA patients who fulfilled 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) criteria. The patients were classified based on disease activity score (DAS)28-C-reactive protein (CRP)(3) score into remission (≤2.6) and treatment-naïve/active (>2.6) groups. Remission patients were followed up for six months and were reclassified into those in sustained remission (≤2.6) and relapse (>2.6) based on the DAS28-CRP(3) score. Various cytokines and cell surface markers were quantitated using whole blood samples, and the CD3+/CD19+ and FOXP3+/CD4+ ratios were calculated. The clinical, demographic, cytokine and cellular phenotype characteristics were compared between remission and treatment-naïve groups. The factors associated with sustained remission were verified.
Results:
Of the 72 patients, 52 were in remission and 20 were DMARD naïve and had active disease. Duration of illness, interleukin-6 (IL-6) and IL-10 were significantly different between remission and treatment naïve/active disease patients. Increased likelihood for achieving sustained remission was noted in RA patients with baseline NLR ≤2. Other demographic/clinical variables and cell phenotypes, namely age, gender, duration of illness, CD3+, CD4+, FOXP3+, CD19+, CD3+/CD19+, FOXP3+/CD4+ and cytokines - IL-6 and IL-10 were not associated with sustained remission.
Interpretation & conclusions:
The present preliminary study highlighted the potential of NLR in predicting sustained remission in RA patients with a cut-off <2. Further study with a large sample size should be done to confirm this finding.
Keywords
Cytokines
disease-modifying anti-rheumatic drugs
neutrophil to lymphocyte ratio
rheumatoid arthritis
sustained remission
The currently followed management approach of early and aggressive management for rheumatoid arthritis (RA) has increased the number of patients achieving the state of remission. The five-year data, from the BeSt study on disease activity score (DAS)-steered treatment, showed that nearly 25 per cent of the patients with RA had drug-free remission, while 46 per cent had restarted disease-modifying anti-rheumatic drugs (DMARDs) monotherapy due to relapse1. These observations substantiate the possibility of withdrawing the drug in a good proportion of patients with RA. The European League Against Rheumatism (EULAR) and other international guidelines have incorporated drug withdrawal as one of the recommendations2. However, one of the major drawbacks in implementing the guidelines is to identify the ideal patients for drug withdrawal.
The immunocompetent cells like T and B cells, macrophages and neutrophils play a major role in RA pathogenesis. The altered balance between anti- and pro-inflammatory factors has been suggested to be responsible for progression and maintenance of the disease3. Ratios of cell sub-populations or the cytokines may be useful in identifying the imbalance involved in the autoimmune disease. Based on this hypothesis, an exploratory study was planned to identify cytokines and the immunocompetent cells, which might serve as biomarkers of remission in patients with RA. These biomarkers may assist in identifying the patients in whom the DMARD therapy can be stopped or dosage can be reduced. The current study was carried out in two phases. The first phase involved the determination of cytokine levels and cellular phenotypes in the peripheral blood of the recently diagnosed, treatment-naïve RA patients with wide ranging disease durations and compared with those who had attained remission DAS28-C-reactive protein (CRP)(3) ≤2.6 and not on steroid or biologics therapy in the preceding six months. The drug-naïve patients were considered, as the immunological changes in these individuals reflect the true changes of RA. In the second phase, treated RA patients were followed up to third and sixth month to assess the sustained remission4, to identify patients for drug tapering or withdrawal and those requiring targeted therapies.
Material & Methods
This prospective observational study included newly diagnosed and treated RA patients who fulfilled 2010 American College of Rheumatology (ACR)/EULAR 2010 criteria5. The patients were recruited consecutively on their routine visit to the department of Rheumatology & Clinical Immunology, ChanRe Rheumatology & Immunology Center & Research, Bengaluru, India, during the study period 2016-2017. Both the groups (treatment naïve and on treatment) included patients >18 yr of age. Patients with any other overlapping connective tissue disease or other conditions like infections were excluded from the study. The remission group included patients who had DAS28-CRP(3) ≤2.6 and on stable DMARDs and not on steroids or biologics in the past six months. In newly diagnosed RA group, patients who were predominantly on nonsteroidal anti-inflammatory drugs and not exposed to DMARD or biologics in the past three months were recruited. The patients on steroid were excluded from the study. The study was approved by the Institutional Ethics Committee, and written informed consent was obtained from all the patients.
The demographic details of patients such as age, gender and duration of disease were collected. Rheumatoid factor (RF) and anti-citrullinated protein (anti-CCP) antibodies test results were extracted from clinical records. A joint assessor evaluated and documented the clinical parameters such as total, swollen and tender joint counts of RA patients. DAS28-CRP(3) was calculated by three variable method at each visit6. The patients in remission were followed at third and sixth month for disease activity. Patients with DAS28-CRP(3) score ≤2.6 at the time of recruitment were considered to be in remission7. Newly diagnosed patients with DAS28-CRP(3) score >2.6, of variable disease duration and not exposed to steroid or DMARD in the past six months were considered as treatment-naïve/active patients. Patients with DAS28-CRP(3) score ≤2.6 at all the three visits (recruitment, third and sixth month follow up) were considered to be in sustained remission24. DAS28-CRP(3) scores >2.6 at any point of follow up (third or sixth month) were considered as patients with relapse of RA.
The laboratory parameters were analyzed at ChanRe Diagnostic Laboratory, Bengaluru, and the following biochemical, cytokine and immune phenotypes were considered: total lymphocyte counts, neutrophils and lymphocytes measured by automated 5-part cell counter (Sysmex XS 1000i, Sysmex Corporation, Japan) and CRP by nephelometry (Beckman Coulter, USA). Neutrophil to lymphocyte ratio (NLR) was calculated. NLR was classified into the following three groups: ≤2, >2 to ≤4 and >4 for association studies8. Our earlier studies showed that NLR could serve as a predictive marker of sustained remission8 and NLR 1.4 could classify remission patients9. Whole blood samples (6 ml) were collected at the recruitment phase from each patient for quantitation of cytokines namely interleukin-6 (IL-6), IL-10 and tumour necrosis factor alpha (TNF-α), and cell surface markers of CD3+, CD4+, FOXP3+ (CD4+ CD25+ FOXP3+) and CD19+. The ratios calculated were CD3+/CD19+ and FOXP3+/CD4+.
ELISA for cytokines: Serum was separated and stored at −20°C for the assessment of cytokines, namely IL-6, IL-10 and TNF-α. These were measured by ELISA, following the manufacturer's instructions (BD Biosciences, USA). The assay detection limit was 2.2 pg/ml for IL-6 and 2 pg/ml for IL-10 and TNF-α. Based on the limits, the levels of these cytokines were categorized as below detection limit (BDL) and above detection limit values.
Flow cytometry for cell typing:Whole blood samples were used for quantification of peripheral cellular phenotypes by flow cytometry (FC500, Beckman Coulter, USA) and analyzed by CXP 2.2 software (Beckman Coulter, USA). Lymphocyte subset count of CD45+, CD3+ and CD19+ were determined using the CYTO STAT Tetra CHROME CD45-FITC/CD56-RD-1/CD19-ECD/CD3-PC5 Monoclonal Antibody Reagents (6607073, Beckman Coulter, USA), according to the manufacturer's instructions. Red blood cells (RBCs) were lysed using OptiLyse C solution (A11895, Beckman Coulter, USA), and Flow-Count Fluorospheres (7547053, Beckman Coulter, USA) were used to obtain absolute cell counts with at least 105 gated events.
Expression of FOXP3 was quantified using CD3, CD4, CD8, CD25 and FOXP3 monoclonal antibodies. Cells were stained for cell surface markers with anti-human CD3 phycoerythrin - Fluor 610 (Pefluor610) (eBioscience, 61-0038-42, Thermo Fisher Scientific, USA), anti-human CD4 fluorescein isothiocyanate (FITC) (eBioscience, 11-0048-42), anti-human CD8 phycoerythrin - cyanine 7 (PC7) (eBioscience, 25-0087-42) and anti-human CD25 PC5 (eBioscience, 15-0259-42). The cells were washed and fixed using fixing/permeabilization buffer (eBioscience, 00-5523-00). These were subsequently stained for intracellular FOXP3 expression with anti-human FOXP3 phycoerythrin (PE) (eBioscience, 12-4776-42), as per the manufacturer's instructions. A total of 105 to 106 events were collected with an acquisition time of 300 seconds and analyzed using the CXP software. Cells were gated for lymphocytes and further for CD3+ and CD4+. For the analysis of FOXP3+, gating for CD4+ cells was analyzed for co-expression of CD25+ FOXP3+ to detect CD4+ CD25+ FOXP3+ Treg population.
Statistical analysis: The demographic, clinical, cytokine and cellular phenotype characteristics of the patients with RA were compared between DAS28-CRP(3) remission (≤2.6) and treatment-naïve (>2.6) groups. Comparison of continuous variables with normal distribution was done by independent t test, data without normal distribution by Mann-Whitney test and counts data by Fisher's exact test. Comparison of sustained remission and relapse patients was also performed. Logistic regression was done to identify baseline study variables associated with sustained remission. RA patients with complete data of DAS28-CRP(3) assessment at recruitment and third and sixth month follow up visits were considered for regression analysis. As the comparison among disease activity groups involved multiple risk factors for a single outcome, Bonferroni-adjusted P value was used (α/no. of variables tested, 0.05/13=0.0038). Statistical Package for the Social Sciences version 22.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis.
Results
Seventy two patients with RA who fulfilled the ACR/EULAR 2010 classification criteria were included in the study. Fifty two patients in remission [DAS28-CRP(3) ≤2.6] and 20 newly diagnosed DMARD-naïve active disease patients [DAS28-CRP(3) >2.6] were enrolled. The flow chart summarizing the study design is shown in Figure 1. Among the participants, 11 were male and 61 were female with a mean age±SD of 46.54± 10.6 yr. The median (range) duration of illness was 45 (3-240) months. The flow cytometric analyses of cell populations are presented in Figures 2 and 3. The average CRP level noted in remission patients was 4.24±3.07 mg/l. Patients with active disease had 18.33±18.02 mg/l CRP levels. Forty two patients in remission were RF test positive and 10 were RF test negative. Eighteen active disease patients were RF test positive and two were test negative. Anti-citrullinated protein antibody levels were evaluated in 20 patients and all had >5 values (9 remission and 11 active disease patients).
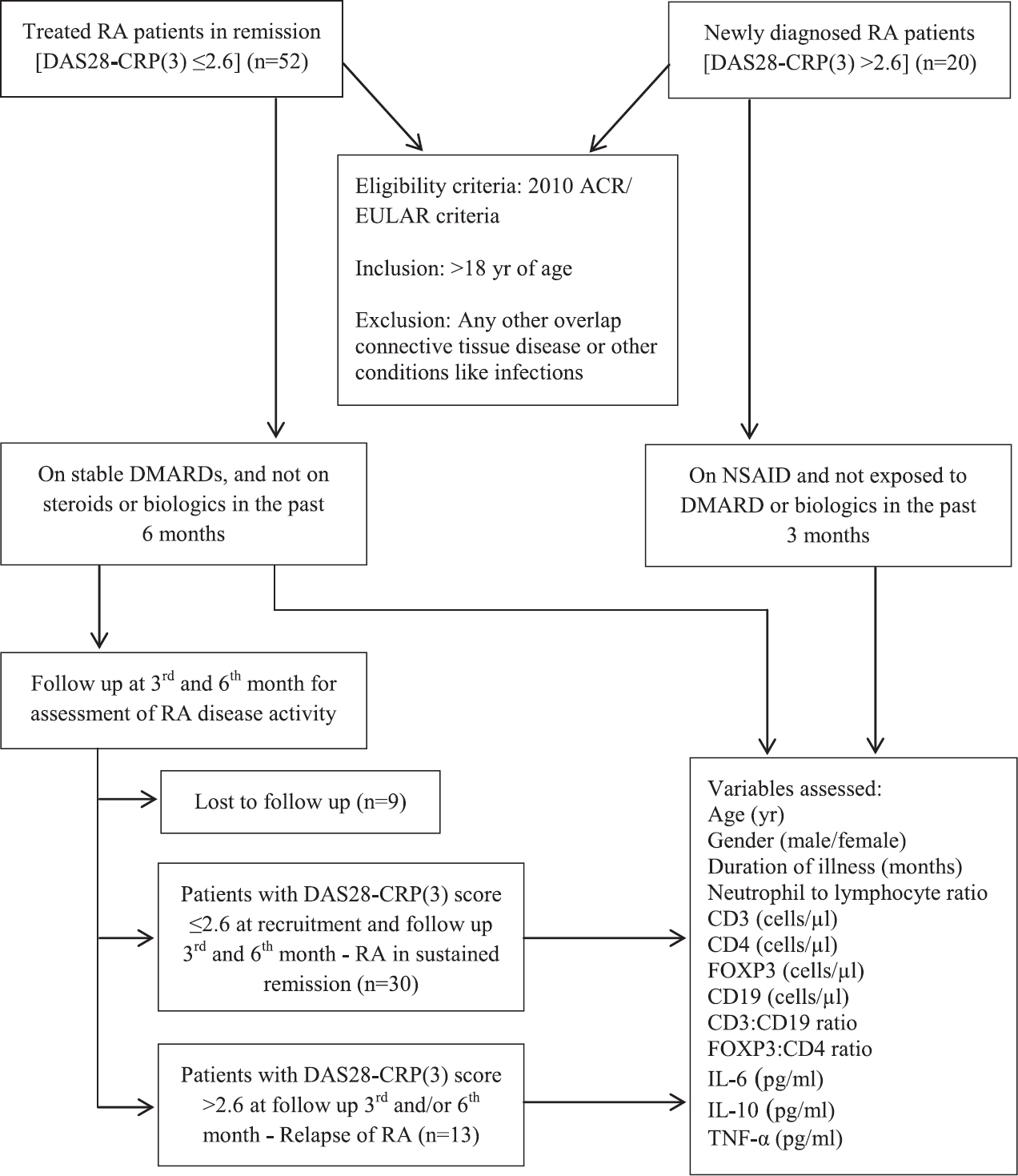
- Flow chart summarizing the study design in rheumatoid arthritis (RA) patients. DAS28-CRP(3), disease activity score 28-C-reactive protein(3); ACR, American College of Rheumatology; EULAR, European League Against Rheumatism; DMARDs, disease modifying anti-rheumatic drugs; NSAID, nonsteroidal anti-inflammatory drugs; IL, interleukin; TNF-α, tumour necrosis factor-alpha.
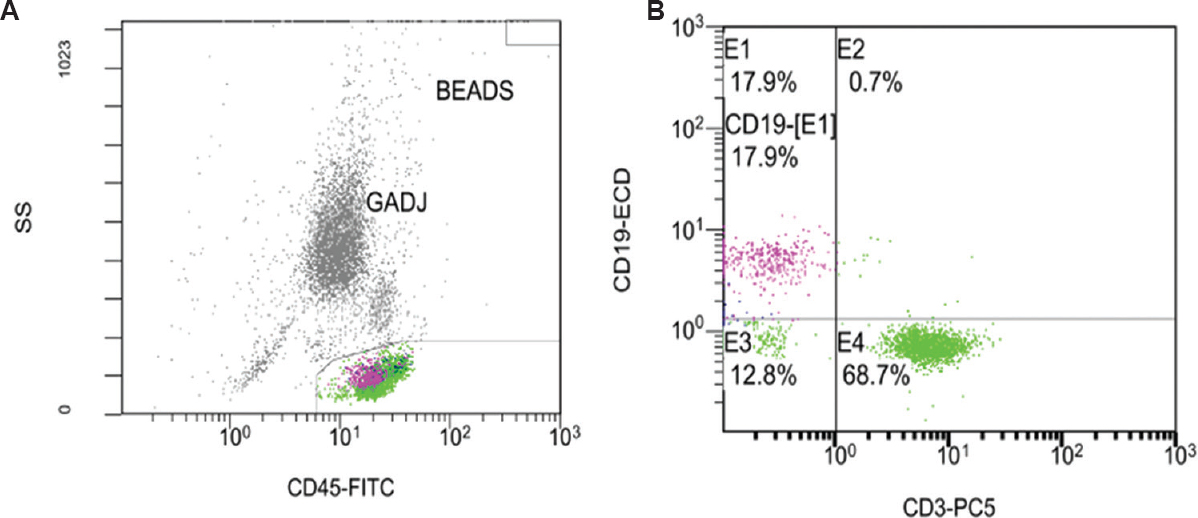
- A representative flow cytometric dot plot of CD19+ and CD3+ cell subsets expression in rheumatoid arthritis patients. (A) CD45+ cells (general leukocyte marker) of the peripheral blood sample were gated in CD45-FITC vs. side light scatter (SS) dot plot. (B) CD3-PC5 vs. CD19-ECD cellular expression was then gated to differentiate CD3+ T cell (E4) and CD19+ B cell (E1) population. GADJ, gate adjust.
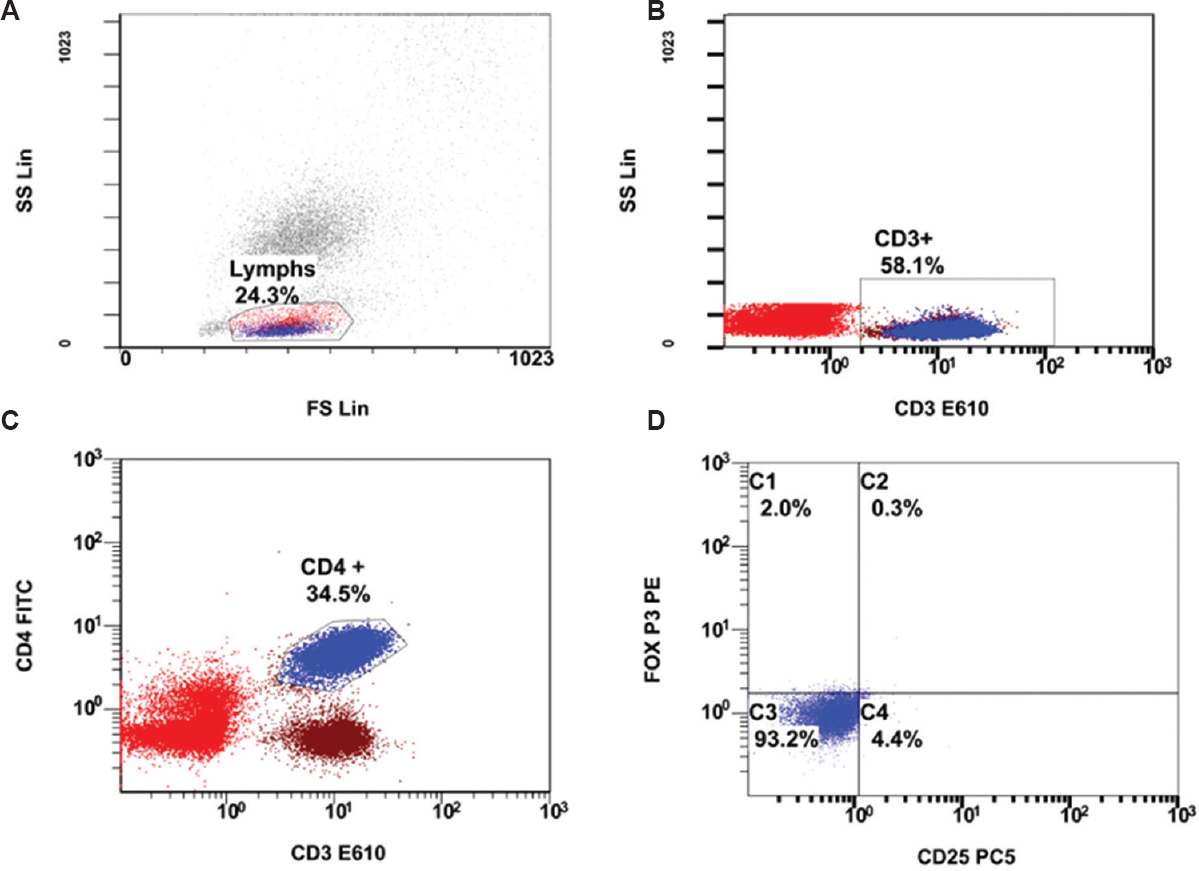
- Flow cytometric analysis of CD4+CD25+FOXP3+ cells. (A) Lymphocytes are gated in forward light scatter (FS) vs. side light scatter (SS) dot plot, then (B) CD3+ cells gated in CD3- E610 vs. SS, followed by (C) CD4+ gating in CD3- E610 vs. CD4- FITC. (D) CD4+ cells gated are plotted CD25-PC5 vs. FoxP3-PE for assessing CD4+CD25+FoxP3+ (C2) cell expression.
Remission patients were either on single or combination DMARDs. Four patients (7.69%) received single DMARD, 37 (71.15%) two DMARDs and 11 (2.15%) three DMARDs. All the patients on single DMARDs were given methotrexate. Double DMARD patients received a combination of methotrexate-hydroxychloroquine (n=31; 59.22%), methotrexate-leflunomide (n=3; 5.77%) and leflunomide-hydroxychloroquine (n=3; 5.77%). Three combination DMARDs received were methotrexate-leflunomide-hydroxychloroquine (n=10; 19.23%) and methotrexate-hydroxychloroquine-sulphasalazine (n=1; 1.99%).
Table I provides the details of comparison between remission and active disease groups. The treatment-naïve patients were newly diagnosed and had significantly (P<0.001) lesser (15 months) duration of illness compared to remission patients (60 months). Other demographic/clinical variables and cell phenotypes such as age, gender, NLR, CD3+, CD4+, FOXP3+, CD19+, FOXP3+/CD4+ and CD3+/CD19+ ratio did not differ significantly between the study groups. However, the active/treatment-naïve patients demonstrated non-significantly higher levels of cellular phenotypes of CD3+, CD4+, FOXP3+ and CD19+ compared to the remission group. Similar results were seen for FOXP3+/CD4+ and CD3+/CD19+ ratios. Treatment-naïve patients had significantly increased IL-6 and IL-10 levels when compared to remission group (Table II). TNF-α was BDLs in both remission and active disease groups.
| Demographic and immune variables# | Remission-treated patients (n=52) | DMARD-naÏve patients (n=20) |
|---|---|---|
| DAS28-CRP(3) groups | ≤2.6 | >2.6 |
| Age (yr) | 47.31±10.05 | 44.55±11.96 |
| Gender (male/female) | 10/42 | 1/19 |
| Duration of illness (months) | 60 (7-240) | 15 (3-120)*** |
| Neutrophil to lymphocyte ratio | 2.33 (1.09-6.65) | 2.44 (1.44-5.82) |
| CD3 (cells/μl) | 701.5 (176-2130) | 804.5 (374-1485) |
| CD4 (cells/μl) | 434.72 (142.65-1420.98) | 508.40 (198.99-850.88) |
| FOXP3 (cells/μl) | 0.209 (0-9.26) | 0.32 (0-3.41) |
| CD19 (cells/μl) | 137 (10-321) | 151 (27-481) |
| CD3:CD19 ratio | 4.74 (1.61-54.4) | 5.1 (0.89-18.22) |
| FOXP3:CD4 ratio | 0 (0-0.05) | 0.001 (0-0.012) |
#Continuous variables represented as mean±SD for normal data and median (range) for data without normal distribution. Counts are given for categorical variables. ***P<0.001 compared to treated patients. DMARD, disease-modifying anti-rheumatic drug; CRP, C-reactive protein; DAS, disease activity score
| Cytokines (pg/ml)# | Remission-treated patients DAS28-CRP(3) ≤2.6 (n=52) | DMARD-naïve patients DAS28-CRP(3) >2.6 (n=20) | P |
|---|---|---|---|
| IL-6 | |||
| Below detection limit (≤2.2) | 37 | 4 | <0.001 |
| Above detection limit (>2.2) | 15 | 16 | |
| IL-10 | |||
| Below detection limit (≤2) | 48 | 11 | 0.001 |
| Above detection limit (>2) | 4 | 9 | |
| TNF-α | |||
| Below detection limit (≤2) | 51 | 20 | 1 |
| Above detection limit (>2) | 1 | 0 |
#Assay detection limit of the cytokine kit taken to classify the cytokine groups. IL, interleukin; TNF-α, tumour necrosis factor-alpha
Of the 52 patients in remission, 43 returned for follow up visits during the study period and were assessed for sustained remission. Thirty RA patients had sustained remission and 13 had relapse. Comparison of clinical and immune parameters showed that none of the variables differed significantly between the patients with sustained remission and relapse (Table III). The univariate logistic regression to identify baseline demographic, clinical, cytokines and cell phenotypes associated with sustained remission are given in Table IV. NLR was the only variable associated with sustained remission. The corresponding increased likelihood for achieving sustained remission noted in RA patients with baseline NLR ≤2 and >2 to ≤4 were 10.5 and 2.63 times compared to those with NLR >4. However, the latter point estimate did not achieve statistical significance. The corresponding amount of variability explained by the variable was 15.8 per cent (Nagelkerke R2=0.158). The variables namely age, gender, duration of illness, CD3+, CD4+, FOXP3+, CD19+, CD3+/CD19+, FOXP3+/CD4+ and cytokines - IL-6 and IL-10 were not found to be associated with sustained remission. The patients with reduced levels of IL-10 cytokine showed 2.55 times increased likelihood for attaining sustained remission. In contrast, patients were 40 per cent less likely to attain sustained remission if they had reduced levels of IL-6. NLR was the only variable within the cut-off P=0.2 and hence multivariate logistic regression was not performed.
| Demographic and immune variables# | Sustained remission (n=30) | Relapse (n=13) |
|---|---|---|
| DAS28-CRP(3) groups | ≤2.6 | >2.6 |
| Age (yr) | 46.4±10.56 | 48.54±5.3 |
| Gender (male/female) | 4/26 | 2/11 |
| Duration of illness (months) | 54 (7-240) | 60 (24-120) |
| Neutrophil to lymphocyte ratio | 2.21 (1.09-4.42) | 2.75 (1.4-6.65) |
| ≤2 | 14 | 2 |
| >2-≤4 | 14 | 8 |
| >4 | 2 | 3 |
| CD3 (cells/µl) | 708 (293-2130) | 710 (176-1313) |
| CD4 (cells/µl) | 409.5 (175.77-1420.98) | 473.79 (142.65-847.12) |
| FOXP3 (cells/µl) | 0.248 (0.00-9.26) | 0.398 (0.00-7.4) |
| CD19 (cells/µl) | 145.8±74.84 | 164.62±87.65 |
| FOX P3:CD4 ratio | 0.001 (0.00-0.05) | 0.001 (0.00-0.016) |
| CD3:CD19 ratio | 4.84 (1.61-54.4) | 4.77 (2.19-16.74) |
| IL-6 (pg/ml) | ||
| Below detection limit (≤2.2) | 20 | 10 |
| Above detection limit (>2.2) | 10 | 3 |
| IL-10 (pg/ml) | ||
| Below detection limit (≤2) | 28 | 11 |
| Above detection limit (>2) | 2 | 2 |
#Continuous variables represented as mean±SD for normal data and median (range) data for those without normal distribution. Counts are given for categorical variables
| Demographic and immune variables | OR | 95% CI | P | Variance explained (Nagelkerke R2) |
|---|---|---|---|---|
| Age (yr) | 0.974 | 0.906-1.048 | 0.485 | 0.016 |
| Gender | ||||
| Reference: Female | - | - | - | 0.001 |
| Male | 0.846 | 0.135-5.317 | 0.859 | |
| Duration of illness (months) | 1.003 | 0.988-1.018 | 0.711 | 0.005 |
| Neutrophil to lymphocyte ratio | ||||
| Reference: >4 | - | - | 0.118 | 0.158 |
| ≤2 | 10.5 | 1.029-107.166 | 0.047 | |
| >2-≤4 | 2.625 | 0.359-19.182 | 0.342 | |
| CD3 (cells/µl) | 1 | 0.998-1.002 | 0.961 | 0 |
| CD4 (cells/µl) | 1 | 0.997-1.002 | 0.617 | 0.008 |
| FOXP3 (cells/µl) | 0.835 | 0.593-1.174 | 0.299 | 0.036 |
| CD19 (cells/µl) | 0.997 | 0.989-1.005 | 0.467 | 0.017 |
| CD3:CD19 ratio | 1.049 | 0.9-1.222 | 0.544 | 0.02 |
| FOXP3:CD4 ratio# | 0.059 | - | 0.945 | <0.001 |
| IL-6 (pg/ml) | ||||
| Reference: Above detection limit (>2.2) | - | - | - | |
| Below detection limit (≤2.2) | 0.6 | 0.134-2.681 | 0.504 | 0.015 |
| IL-10 (pg/ml) | ||||
| Reference: Above detection limit (>2) | - | - | - | |
| Below detection limit (≤2) | 2.545 | 0.318-20.38 | 0.379 | 0.025 |
#Point estimate of FOXP3:CD4 has high SE and 95% CI. OR, odds ratio; CI, confidence interval; SE, standard error
Discussion
In the present study, patients with NLR <2 had 10 times lesser chance of relapse. IL-6 and IL-10 levels were significantly different in patients who were in remission and on treatment compared to DMARD-naïve patients. Though not significant, but patients with lower IL-6 had decreased likelihood of sustained remission, whereas lower IL-10 was associated with increased likelihood of sustained remission.
The RA is both immunologically and clinically a heterogeneous disease. Although cells such as CD3, CD4, CD19 and FOXP3-positive were higher in patients with active disease, but were not significantly different because of their increased dispersion. A previous study has found these immune cells to be significantly increased in RA with active disease than healthy controls10. In contrast, Niu et al11 observed reduced FOXP3+ regulatory T (Treg) cells in patients with active disease in comparison to healthy controls. Khattab et al12 demonstrated that CD4+ CD25+ CD127low Treg cells were significantly lower in RA patients with higher disease activity compared to patients with lower activity or controls and were negatively correlated with disease activity. The current study showed higher FOXP3+ cells in patients with increased disease activity. The univariate analysis of FOXP3+ did not demonstrate any predictive value. The number of CD3+, CD4+, CD19+ and FOXP3+ cells did not differ significantly between the patients with stable disease and those who had disease relapse. Walter et al13 did not find any difference in the ex vivo phenotype (CD4, CD25, CD127, CD39 or CD161) or pro-inflammatory cytokine profile [IL-17, interferon-gamma (IFN-γ) or TNF] among the RA and healthy controls with regard to the frequency of Treg cells. Treg enrichment into the joint compartment is not specific to inflammatory arthritis, as it is similarly enriched in osteoarthritis14. Kikuchi et al15 demonstrated that the proportion of Treg cells among CD4+ cells correlated well with clinical response in RA patients who received tocilizumab treatment. Present study and literature findings cited suggest that the increase in Treg cells may be in response to elevated inflammation. Other factors like autoantibody and B cells activity may influence the Treg cells population, rather than having a direct impact on causation of RA. Based on the literature evidence and present study findings, it appears that Treg cells may not serve as a biomarker of normalization of immune response in RA.
Although the cytokine levels significantly differed between patients with active RA and those having remission, these were not significantly different between the patients who had sustained remission and those with relapse. The detectable levels of IL-6 predicted sustained remission (40% risk of relapse in below detectable limit) and the BDL of IL-10 suggested a possibility of sustained remission. Nishimoto et al16 observed that lower serum levels of IL-6 and matrix metalloproteinase-3 were useful markers for identifying patients who could discontinue the tocilizumab treatment, without acute disease flare. Though contrasting observation was noted in the present study, the associated confidence intervals were not significant. The IL-10 seems to play a dual role in RA by simultaneously suppressing pro-inflammatory cytokines and enhancing humoral autoimmune response. The study conducted to evaluate the safety and therapeutic efficiency of IL-10 in RA has corroborated the opposing roles of the cytokine17. The opposing role of IL-10 in RA pathogenesis limits its use as a predictor of immune response. Consideration of other factors such as seropositivity may enhance the predictive potential of IL-10 and this needs further study. CD3 and CD19 ratio, reflecting the balance between circulating T and B cells, showed wide variations.
In the present study the NLR <2 was significantly associated with sustained remission in RA patients than those who had relapse, suggesting that the ratio could classify the patients into sustained remission as also shown in a previous study8. This observation was in line with other studies1819. Zengin et al20 have shown significant difference in both the NLR and platelet to lymphocyte ratio (PLR) between patients with active disease and remission (P<0.001) in both anti-TNF- and cDMARD-treated patients.
Ghang et al21 reported that the NLR was significantly higher during flares in patients who received tocilizumab (anti-IL-6) therapy, however, ESR levels (n=9) were within the normal limit or decreased (n=4) and was noted in one patient in the flare group. This observation suggests that NLR is independent of cytokines influencing inflammatory parameters. NLR, representing the subclinical inflammation, may predict the possible relapse better than the other inflammatory parameters9. The present study findings corroborated with the previous observations22 suggesting that the NLR might reflect the homeostatic status of the immune system more significantly than other immunocompetent cells.
The present study was a preliminary study and the major limitation was its small sample size. Nine patients in remission did not come for follow up visits during the study period. Sustained remission was assessed after six months follow up, which was considered as a sufficient period as per EULAR 2016 update2 and literature studies4. Multivariate regression analysis could not be performed due to small sample size. Inclusion of other clinical and cellular markers/variables associated with disease pathogenesis could improve the predictability of disease status.
In conclusion, the current preliminary study suggests that the NLR may be a predictive marker of sustained remission with a cut-off <2 in patients with RA. Using additional parameters, which represent different aspects of the disease process, would improve the positive predictive value of this parameter.
Financial support & sponsorship: This work was supported by the Immunology and Arthritis Research and Education Trust (IARET), Bengaluru.
Conflicts of Interest: None.
References
- Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: Exploratory analyses from the BeSt study. Ann Rheum Dis. 2011;70:315-9.
- [Google Scholar]
- EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-77.
- [Google Scholar]
- Is there a feudal hierarchy amongst regulatory immune cells? More than just Tregs. Arthritis Res Ther. 2009;11:237.
- [Google Scholar]
- Sustained remission in rheumatoid arthritis: Latest evidence and clinical considerations. Ther Adv Musculoskelet Dis. 2017;9:249-62.
- [Google Scholar]
- 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569-81.
- [Google Scholar]
- The development of the disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28) Clin Exp Rheumatol. 2014;32:S.
- [Google Scholar]
- Neutrophil-lymphocyte ratio, pain perception, and disease activity score may serve as important predictive markers for sustained remission in rheumatoid arthritis. Reumatismo. 2015;67:109-15.
- [Google Scholar]
- Characterization of neutrophil-to-lymphocyte ratio as a measure of inflammation in rheumatoid arthritis. Int J Rheum Dis. 2017;20:1457-67.
- [Google Scholar]
- Deficient or abundant but unable to fight Estimation of circulating Foxp3+ T regulatory cells and their counteracting FoxP3 In rheumatoid arthritis and correlation with disease activity. Egypt Rheumatol. 2013;35:185-92.
- [Google Scholar]
- Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol Int. 2012;32:2731-6.
- [Google Scholar]
- CD4+ CD25+ CD127low regulatory T Cells as indicator of rheumatoid arthritis disease activity. Egypt J Immunol. 2016;23:87-95.
- [Google Scholar]
- Phenotypic, functional, and gene expression profiling of peripheral CD45RA+ and CD45RO+ CD4+CD25+CD127(low) treg cells in patients with chronic rheumatoid arthritis. Arthritis Rheumatol. 2016;68:103-16.
- [Google Scholar]
- CD4+ CD25+/highCD127low/– regulatory T cells are enriched in rheumatoid arthritis and osteoarthritis joints-analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Arthritis Res Ther. 2014;16:R97.
- [Google Scholar]
- Peripheral blood CD4(+)CD25(+)CD127(low) regulatory T cells are significantly increased by tocilizumab treatment in patients with rheumatoid arthritis: Increase in regulatory T cells correlates with clinical response. Arthritis Res Ther. 2015;17:10.
- [Google Scholar]
- Drug free REmission/low disease activity after cessation of tocilizumab (Actemra) Monotherapy (DREAM) study. Mod Rheumatol. 2014;24:17-25.
- [Google Scholar]
- Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine. 2015;74:27-34.
- [Google Scholar]
- The association between neutrophil/lymphocyte ratio and disease activity in rheumatoid arthritis and ankylosing spondylitis. J Clin Lab Anal. 2016;30:597-601.
- [Google Scholar]
- Comparisons of neutrophil-, monocyte-, eosinophil-, and basophil- lymphocyte ratios among various systemic autoimmune rheumatic diseases. APMIS. 2017;25:863-71.
- [Google Scholar]
- New inflammatory markers in early rheumatoid arthritis. Z Rheumatol. 2018;77:144-50.
- [Google Scholar]
- Neutrophil-to-lymphocyte ratio is a reliable marker of treatment response in rheumatoid arthritis patients during tocilizumab therapy. Mod Rheumatol. 2017;27:405-10.
- [Google Scholar]
- The relationship between hematological indices and autoimmune rheumatic diseases (ARDs), a meta-analysis. Sci Rep. 2017;7:10833.
- [Google Scholar]






