Translate this page into:
Cholera outbreak in Aurangabad, Maharashtra, western India
For correspondence: gopalvk58@hotmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Globally, there are nearly 1.7 billion cases and 1.6 million deaths due to diarrhoeal diseases every year.
The burden is greatest in low-income populations with poor access to safe water, sanitation and urgent medical care1. In India, the problem is widespread with diarrhoeal diseases being the most common among the outbreaks reported across the country2. Among the causative agents, diarrhoeal outbreaks due to enteric viruses, namely rotavirus A, B and C, (RVA, RVB, RVC), norovirus, adenovirus and astrovirus, are frequently reported345678. Among the As Cryptosporidium is a parasite please modify bacterial to non-viral agents, Vibrio cholerae, Cryptosporidium spp., Shigella spp., non-typhoidal Salmonella spp. and diarrhoeagenic Escherichia coli have been documented19.
An outbreak of acute diarrhoeal disease started on November 10, 2017 in the cantonment area of Aurangabad city in Maharashtra State of India. On the first day of the outbreak, a total of 100 patients were hospitalized for diarrhoeal treatment, and therefore, the identification of the specific index case was not possible. The total population of the cantonment area was reported to be 16,000. A total of 7447 cases of acute diarrhoea were identified during the outbreak period of 12 days with an attack rate of 46.5 per cent. Of these, 5825 and 1622 cases were from outpatient and inpatient departments, respectively, and 90 per cent were older than 14 yr of age. The highest number of cases (n=2390) was reported on November 12, and no new cases were identified after November 22, 2017 (Fig. 1). Most of the patients presented with foul-smelling profuse watery diarrhoea, severe abdominal colic, vomiting and history of fever with chills in Cantonment General Hospital. All the patients were treated with oral rehydration therapy, intravenous fluids, antiemetics and antimicrobial agents including metronidazole and quinolones. The findings of laboratory investigations carried out on the stool specimens of the diarrhoeal patients and water samples collected from different houses and water storage tanks during the outbreak period are reported here.
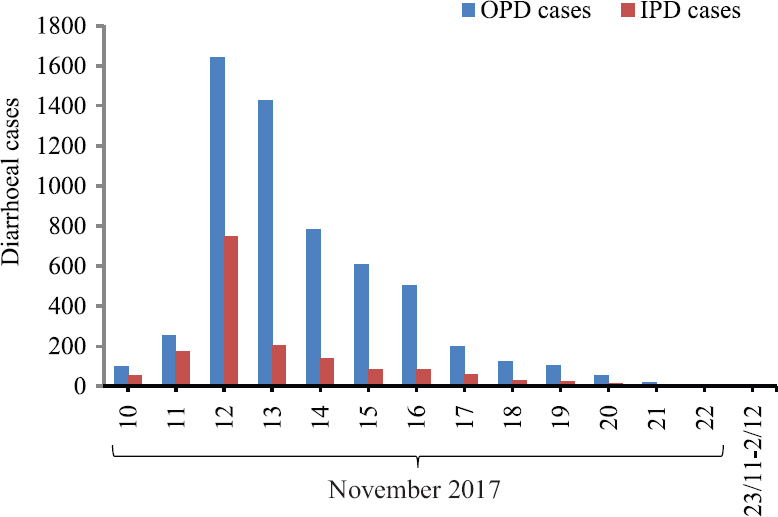
- Day-wise distribution of acute diarrhoeal disease cases reported during the outbreak period in Aurangabad city of Maharashtra State, India. OPD, outpatient department; IPD, inpatient department.
Stool specimens (n=46) along with their case report forms (CRFs) were collected from the patients (n=46) admitted between November 13-15, 2017 for acute diarrhoea at the cantonment hospital, Aurangabad. These 46 patients were residents of Pensionpura (n=10), Gawalipura (n=16), Guddigudam (n=10), Shantipura (n=7) and Padegaon (n=3) areas of Aurangabad, respectively. At the time of sample collection, one aliquot of stool sample was introduced into a tube containing Cary-Blair medium (HiMedia Laboratories, Mumbai). These stool specimens were transported in cold chain to ICMR-National Institute of Virology, Pune, for virological and bacteriological investigations.
All specimens were tested for RVA using commercial enzyme-linked immunosorbent assay (Premier Rotaclone, Meridian Bioscience, Inc., USA) and for RVB, RVC, norovirus, adenovirus and enterovirus using reverse transcription-polymerase chain reaction (RT-PCR)/PCR4510111213.
Standard protocols were adapted for bacterial isolation and identification1415. For identification of V. cholerae, standard procedures including oxidase production and salt tolerance tests were used1516. Further, the isolates with yellow colonies on thiosulphate-citrate-bile salts-sucrose (TCBS) agar were subjected to agglutination tests with V. cholerae polyvalent O1 antiserum, followed by Ogawa and Inaba serotype-specific antisera16 (Denka Seiken, Japan). The biotype confirmation was carried out using standard phenotypic tests (chick red cell agglutination, Voges-Proskauer test and sheep blood haemolysis)14. Antimicrobial susceptibility testing of the isolates was carried out using azithromycin (15 μg), tetracycline (30 μg), nalidixic acid (30 μg), ampicillin (10 μg) and cefotaxime (30 μg) (BD BBL™ Sensi-Disc, Becton Dickinson, USA) using Mueller-Hinton agar by Kirby-Bauer disk diffusion method17. E. coli ATCC 25922 reference strain was used as a control, and the Clinical Laboratory Standards guidelines were followed for interpretation of the results18.
Water samples collected on eight different days from 152 houses in the outbreak area were tested at the State Public Health Laboratory, Pune, for bacteriological indicators of contamination using the methodology reported earlier14. Similarly, a total of nine water samples collected from municipal water storage tank and water-supplying tankers in the Aurangabad city collected on seven different days were also tested.
All the stool specimens were tested negative for RVA, RVB, RVC, norovirus, adenovirus and enterovirus with a single exception each to RVA and RVC. Six representative stool specimens subjected to electron microscopic studies showed absence of any viral agent. Six of the 46 specimens showed yellow-coloured colonies on TCBS agar plates and were confirmed as V. cholerae O1 Ogawa by slide agglutination test using type-specific antiserum. The identification of the V. cholerae isolates was reconfirmed at the State Public Health Laboratory, Pune. All the isolates agglutinated chick red blood cells, showed mild haemolysis of sheep blood and produced acetoin, thereby confirming the presence of biotype as El Tor. Antimicrobial testing of all six isolates of V. cholerae showed susceptibility to azithromycin, tetracycline and resistance to nalidixic acid, ampicillin and cefotaxime. All 46 stool specimens showed negative results for isolation of Salmonella, Shigella spp. and E. coli O157:H7.
Forty nine of the 152 water samples (32%) collected from different households showed >16 coliform count per 100 ml of water, and 24 and seven samples were also positive for thermotolerant coliforms and E. coli, respectively19. Of these 49 samples, 12, 10, 8, 7, 4 and 8 were collected from Tophkhana, Guddigudam, Pensionpura, Gawalipura, Mission Compound and other regions of the cantonment area, respectively. All the nine samples collected on seven different days from the municipal water storage tank and water tankers showed absence of coliforms.
Cholera is an acute diarrhoeal disease caused by ingestion of toxigenic strains of V. cholerae serogroups O1 and O139 in contaminated water or food. By definition, cholera is endemic when the causative organisms reside in the local environment and the occurrence of the disease in humans is not dependent on the importation of cholera from outside9. Maharashtra is classified as an endemic region for cholera, and it has also been reported from Aurangabad in the past2021. In the present study, V. cholerae O1 Ogawa biotype El Tor was isolated in 13.1 per cent specimens. Administration of antibiotics immediately after admission may be the reason behind low isolation rate.
The uniform pattern of antimicrobial resistance to three commonly used groups of antibiotics was shown by V. cholerae isolates. On the background of increase in the emergence of antimicrobial resistance among strains of V. cholera22, judicious use of antibiotics is necessary, particularly in cholera where other treatment modalities such as rehydration take precedence. A single municipal water storage tank was supplying drinking water to the cantonment area, military area and rest of the corporation area of Aurangabad city; however, diarrhoeal cases were restricted to the cantonment area only. The water pipeline supplying drinking water to the affected area runs through the Kham River (Personal communication, Cantonment Board, Aurangabad). Although, specific testing for bacterial and viral agents was not performed on the water samples, high coliform counts in piped water samples (32%) and their absence in municipal water storage tank and water-supplying tankers indicated the possibility of faecal contamination of the river water and leakage in the pipeline. The supply of piped water was stopped and alternative supply of the drinking water was provided.
In conclusion, the study showed the absence of known causative enteric viral agents and identification of the V. cholerae O1 Ogawa biotype El Tor as the aetiological agent of the diarrhoeal outbreak. In future, continuous monitoring of the diarrhoeal outbreaks using rapid diagnostic tests in an integrated manner is essential for timely diagnosis and to avoid unnecessary usage of antibiotics leading to antibiotic resistance.
Financial support & sponsorship: Authors acknowledge the ICMR-National Institute of Virology, Pune, for financial support
Conflicts of Interest: None
References
- Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:909-48.
- [Google Scholar]
- Integrated Disease Surveillance Programme. Weekly Outbreaks. Available from: http://idsp.nic.in/index4.php?/lang=1&level=0&linkid=406&lid=3689
- A waterborne outbreak of epidemic diarrhea due to group A rotavirus in Malatya, Turkey. New Microbiol. 2011;34:17-24.
- [Google Scholar]
- Identification of group B rotavirus as an etiological agent in the gastroenteritis outbreak in Maharashtra, India. J Med Virol. 2017;89:2244-8.
- [Google Scholar]
- Group C rotavirus infection in patients with acute gastroenteritis in outbreaks in western India between 2006 and 2014. Epidemiol Infect. 2017;145:310-5.
- [Google Scholar]
- An outbreak of norovirus-associated acute gastroenteritis associated with contaminated barrelled water in many schools in Zhejiang, China. PLoS One. 2017;12:e0171307.
- [Google Scholar]
- A non-enteric adenovirus A12 gastroenteritis outbreak in Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2016;111:403-6.
- [Google Scholar]
- Outbreak of astrovirus in adults with acute gastroenteritis in Korea. J Gastrointest Dig Syst. 2015;S13:1-4.
- [Google Scholar]
- Cholera in India: An analysis of reports, 1997-2006. Bull World Health Organ. 2010;88:185-91.
- [Google Scholar]
- Foodborne outbreak caused by a Norwalk-like virus in India. J Med Virol. 2002;67:603-7.
- [Google Scholar]
- Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and southern hybridization. J Clin Microbiol. 1995;33:64-71.
- [Google Scholar]
- Polymerase chain reaction for detection of adenoviruses in stool samples. J Clin Microbiol. 1990;28:2659-67.
- [Google Scholar]
- Enteroviruses in patients with acute encephalitis, Uttar Pradesh, India. Emerg Infect Dis. 2009;15:295-8.
- [Google Scholar]
- Manual of clinical microbiology (8th ed). Washington, DC: American Society for Microbiology; 2003.
- Diagnostic microbiologic methods in the GEMS-1 case/control study. Clin Infect Dis. 2012;55(Suppl 4):S294-302.
- [Google Scholar]
- Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in the developing world: Bacterial agents of enteric diseases of public health concern. Available from: http://www.who.int/drugresistance/publications/WHO_CDS_CSR_RMD_2003_6/en
- Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493-6.
- [Google Scholar]
- Performance standards for antimicrobial susceptibility testing (26th ed). Wayne, PA: CLSI; 2017. 32:M100-M02-A12, M07-A10, and M11-A8
- Park's textbook of preventive and social medicine (24th ed). India: Bhanot Publishers; 2017.
- Changing epidemiology and antimicrobial resistance pattern of Vibrio cholerae isolates at a tertiary care health laboratory in North India (2011-2015) Trop J Med Res. 2017;20:132-8.
- [Google Scholar]





