Translate this page into:
A prospective study of non-tuberculous mycobacterial disease among tuberculosis suspects at a tertiary care centre in north India
For correspondence: Dr Surendra Kumar Sharma, Department of Molecular Medicine, Jamia Hamdard Institute of Molecular Medicine, Jamia Hamdard (Deemed-to-be-University), Hamdard Nagar, New Delhi 110 062, India e-mail: sksharma.aiims2@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The burden of non-tuberculous mycobacterial (NTM) disease is increasing worldwide. The disease shares clinicoradiological features with tuberculosis (TB), Nocardia and several fungal diseases, and its diagnosis is frequently delayed. The present study was performed to determine the frequency of NTM disease among TB suspects in a tertiary care centre in north India.
Methods:
In this prospective study, mycobacterial culture isolates from pulmonary and extrapulmonary specimens among TB suspects were tested with immunochromatographic assay (ICA). All ICA-negative isolates were considered as NTM suspects and further subjected to 16S-23S rRNA internal transcribed spacer gene sequencing for confirmation and species identification. Patients with active disease were treated with drug regimen as per the identified NTM species. Follow up of patients was done to determine clinical, radiological and microbiological outcomes.
Results:
Of the 5409 TB suspects, 42 (0.77%) were diagnosed with NTM disease. Patients with active disease consenting for treatment were treated and followed up. Thirty four patients had NTM pulmonary disease (NTM-PD) and the remaining eight had extrapulmonary NTM (EP-NTM) disease. Mycobacterium intracellulare and M. abscessus, respectively, were most frequently isolated from NTM-PD and EP-NTM patients. Fifteen NTM-PD and seven EP-NTM patients successfully completed the treatment. Ten patients died due to unrelated causes, five were lost to follow up and another four declined the treatment.
Interpretation & conclusions:
Our study showed that the frequency of NTM disease was low among TB suspects at a large tertiary care centre in north India and this finding was similar to other Indian studies. More studies need to be done in other parts of the country to know the geographical variation in NTM disease, if any.
Keywords
Extrapulmonary disease
Mycobacterium abscessus
Mycobacterium avium intracellulare complex
Mycobacterium kansasii
NTM
pulmonary disease
Non-tuberculous mycobacteria (NTM) are ubiquitously present in the environment. Despite this, the NTM disease occurs infrequently. As compared to tuberculosis (TB), person-to-person transmission is not known to occur except in patients with cystic fibrosis (CF) due to Mycobacterium abscessus1. Similar to TB, lung is the most common site of involvement in NTM disease. The pulmonary disease frequently occurs in the previously damaged lungs due to bronchiectasis, TB, CF, chronic obstructive pulmonary disease and pneumoconiosis2. NTM disease may also manifest as disseminated type, especially in individuals with congenital or acquired immune defects such as HIV/AIDS and as localized disease such as cutaneous involvement34. Children frequently present with cervical lymphadenitis5.
The exact burden of NTM disease is not known because it is not a reportable disease worldwide including India. Recent years have witnessed an increased reporting of NTM disease worldwide, especially from high-income countries2 because of several reasons which include (i) increase in environmental NTM sources, (ii) increase in susceptible populations, (iii) low TB disease burden leading to decreased herd immunity, (iv) heightened awareness about NTM disease, (v) availability of better molecular tools for diagnosis of NTM disease, and (vi) hitherto unidentified cause(s) for the increased NTM infections. There is a scarcity of published reports from high TB burden countries including India. The present report describes a prospective study of NTM patients found among TB suspects in a tertiary care hospital in north India.
Material & Methods
The study was conducted prospectively in the department of Internal Medicine at the All India Institute of Medical Sciences (AIIMS), New Delhi, India, between August 2014 to November 2016. Patients included in this study were of either gender, aged more than 12 yr irrespective of their HIV status. Written informed consent was taken from all the study participants, and the study was approved by the Institutional Ethics Committee.
Sample collection and laboratory processing: Pulmonary and extrapulmonary specimens received from the TB suspects presenting to various outpatient departments and those hospitalized for diagnosis were processed at the Intermediate Reference Laboratory (IRL) of National Capital Region, New Delhi located in the department of Internal Medicine at AIIMS, New Delhi. The IRL is accredited by the Ministry of Health & Family Welfare, Government of India. The American Thoracic Society (ATS) guidelines6 definitions were used for sample collection and processing. In pulmonary patients, sputum specimens and induced sputum and/or bronchoalveolar lavage (BAL) fluid specimens in those patients who were not able to produce sputum were obtained for diagnosis. From patients who were producing sputum, the specimens were tested twice using two samples obtained on different occasions. For extrapulmonary specimen such as pus, lymph node aspirate and/or biopsy, pleural, pericardial, cerebrospinal fluids, bone marrow specimens were obtained and processed as detailed below.
The acid-fast bacilli (AFB) smear was prepared directly from each sample; the smear microscopy was done using Ziehl-Neelsen staining method as per the standard protocol, and the smear was evaluated under the light emitting diode microscope (Carl Zeiss Microscopy, Germany)7. The remaining sample was subjected to standard decontamination protocol (NALC-NAOH method)8 and the processed sample was subsequently used for culture inoculation on solid (Lowenstein-Jensen) and liquid culture (automated MGIT 960) media.
The Xpert MTB/RIF test was performed using the G4 version of cartridges according to the manufacturer's instructions (Cepheid, Sunnyvale, USA) as described previously9. The samples were subsequently inoculated into Mycobacterium Growth Indicator Tube (MGIT 960), a non-radiometric automated isolation system (Becton Dickinson, USA)8. The MGIT tube contained 7 ml of 7H9 medium, supplemented with 0.8 ml of oleic acid-albumin-dextrose-catalase and polymyxin B-amphotericin B-nalidixic acid-trimethoprim-azlocillin, and the tube was inoculated with 0.5 ml of processed sample8. The mycobacterial positive cultures were tested to differentiate between M. tuberculosis and NTM using immunochromatographic assay (ICA) kit (SD MPT64TB Ag kit, Standard Diagnostics, South Korea). ICA-negative culture isolates were further subjected to NTM species identification. The mycolic acid extraction and high performance liquid chromatographic (HPLC) analysis were performed at the National Tuberculosis Institute, Bengaluru, using a previously described protocol10. The species identification of NTM isolates was done by the line probe assay (LPA) as per the manufacturer's protocol using GenoType® Common Mycobacteria/Additional Species kit (HAIN LifeScience, Germany). The genomic mycobacterial DNA extraction of culture isolates for targeted gene sequencing was done by heat lysis method and amplified using standardized protocols11. Further, for the confirmation of species of NTM isolates, targeted amplification of 16S-23S rRNA internal transcribed spacer (ITS) region was performed by polymerase chain reaction (PCR). The amplified 16S-23S rRNA ITS region was sequenced using DNA sequencing with ABI Prism 3130xl Genetic Analyzer (Applied Biosystems, USA). The PCR primers used for the amplification for 16S-23S rRNA ITS region were ITS (Forward) 5'-GAAGTCGTAACAAGGTAGCCG-3' and ITS (Reverse) 3'-GACAGCTCCCCGAGGCTTATCGCA-5' (Sigma-Aldrich, USA)11. The PCR product was further examined by gel electrophoresis in two per cent agarose gel. The cycle sequencing was carried out using BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, USA), and the products were loaded in the sequencer. The sequencing results were analyzed using BioEdit, a biological sequence alignment editor software (Carlsbad, California, USA), and the alignment was done using Clustal W (Dublin, Ireland).
HIV testing: All patients underwent HIV-1 and HIV-2 testing by the ELISA technique as previously described12 at the Integrated Counselling and Testing Centre of the hospital after counselling and obtaining written informed consent.
Clinical features and radiological investigations: Relevant clinical features and laboratory data of all diagnosed patients were recorded in the source notes, subsequently transferred to the case record form and finally entered into the Excel spreadsheet. All relevant clinical and laboratory details were recorded. Radiological findings were independently scored by a radiologist and a chest physician, and in case of discordance, an alternative radiologist resolved the scoring.
Treatment and follow up: All except one patient had active NTM disease and were offered treatment. Benefits of drug treatment versus prolonged treatment and their adverse events (AEs) were discussed with patients before starting the treatment. The drug regimen consisted of oral administration of rifabutin (R) 300 mg/day (typically administered for M. avium complex disease), clarithromycin (C) 500 mg twice daily and ethambutol (E) 15 mg/kg/day up to a maximum of 1.2 g/day. In M. abscessus disease, along with RCE, some additional antibiotics were included in the regimen. All patients with NTM pulmonary disease (NTM-PD) were treated for one year following culture conversion and extrapulmonary NTM (EP-NTM) patients were treated according to clinical, radiological and microbiological responses and were followed up periodically for AEs and serious adverse events (SAEs).
Results
Pulmonary and extrapulmonary specimens from 5409 TB suspects were tested and 42 (0.77%) patients were diagnosed with NTM disease and were treated and followed up till December 2017 (Fig. 1). Clinical and microbiological characteristics of these patients are detailed in Table I. Males (24:18) were frequently affected with this disease. The mean age of NTM patients was 39.7 (SD: 14.4) yr. Of the 42 patients, 34 (80.9%) had NTM-PD. Various predisposing risk factors for NTM-PD are described in Table II. Thirty three (97%) patients had bronchiectasis and 32 of these had a history of pulmonary TB. Two patients had bronchial asthma and one of these had allergic bronchopulmonary aspergillosis with bronchiectasis (ABPA).

- Flowchart of patient recruitment and follow up in the study. All drugs were administered orally; dosages: rifabutin=300 mg daily, clarithromycin=500 mg twice daily and ethambutol=15 mg/kg/day and in Mycobacterium abscessus patients, additional drugs such as linezolid, amikacin, ciprofloxacin, co-trimoxazole and imipenem were also administered. MTB, M. tuberculosis; AFB, acid-fast bacilli; LPA, line probe assay; ICA, immunochromatographic assay; CM/AS, common mycobacteria/additional species; HPLC, high-performance liquid chromatography; ITS, internal transcribed spacer; NTM, non-tuberculous mycobacteria; NTM-PD, NTM pulmonary disease; EP-NTM, extrapulmonary NTM.
| Characteristics | Number of patients (%) |
|---|---|
| Pulmonary NTM disease | 34 (80.9) |
| Gender | |
| Male | 17 (50) |
| Female | 17 (50) |
| Symptoms† | |
| Cough with expectoration | 25 (60.6) |
| Cough without expectoration | 2 (39.9) |
| Haemoptysis | 18 (54.5) |
| Chest pain | 13 (39.4) |
| Fever | 25 (75.7) |
| Dyspnoea | 17 (51.5) |
| Significant weight loss | 15 (45.4) |
| Radiological features | |
| Bronchiectasis | 33 (97) |
| Cavity | 9 (27) |
| Consolidation | 2 (6) |
| Collapse/atelectasis | 1 |
| Microbiological species | |
| Mycobacterium intracellulare | 11 (32.3) |
| M. abscessus | 9 (26.5) |
| M. kansasii | 4 (11.7) |
| M. simiae | 5 (14.7) |
| M. gordonae | 3 (8.8) |
| M. chimaera | 1 |
| M. senegalense | 1 |
| Extrapulmonary NTM disease | 8 (23.5) |
| Gender | |
| Male | 7 (87.5) |
| Female | 1 |
| Clinical features | |
| Right suppurative knee joint arthritis | 1 |
| Left ear discharge | 1 |
| Post-surgery abdominal wall abscess in mesh used for hernia repair | 1 |
| Injection abscess in the right buttock | 1 |
| Right breast abscess | 1 |
| Post-surgery osteomyelitis of the left femur | 1 |
| Peri-anal pus discharge | 1 |
| Microbiological species | |
| M. abscessus | 6 (75) |
| M. intracellulare | 1 |
| M. parascrofulaceum | 1 |
*Of the total 42 NTM patients, in one patient with bronchial asthma, M. simiae was repeatedly isolated. He did not have active disease. Significant weight loss, >10% of body weight loss over six months; †Some patients may have more than one pulmonary symptoms. NTM, non-tuberculous mycobacteria
| Risk factors | Number of patients (%) |
|---|---|
| NTM-PD | 34 |
| Bronchiectasis | 33 |
| Post-tuberculosis | 32 (97) |
| Bronchial asthma* | 2 (5.9) |
| ABPA | 1 |
| EP-NTM† | 8 |
| Healthcare associated factors | 4 (50) |
| Post-orthopaedic surgical intervention§ | 2 (25) |
| Mesh infection following para-umbilical hernia repair | 1 |
| Right gluteal muscle injection abscess | 1 |
| HIV/AIDS** | 1 |
| Left ear discharge‡ | 1 |
| Right nipple trauma sustained during breast feeding §§ | 1 |
*One patient had history of bronchial asthma but was asymptomatic for NTM disease and another had asthma with ABPA and bronchiectasis; †In one patient with disseminated NTM disease no underlying immune defect could be found despite extensive investigations; §One patient had post-operative osteomyelitis of the left femur and another had post-fracture suppurative right knee joint arthritis; **CD4 cell count: 644 cells/μl; ‡Trauma occurred while ear cleaning, resulting in chronic suppurative otitis media and conductive deafness. Chronic suppurative otitis media with deafness and lower motor neurone type of facial nerve palsy and left optic neuropathy due to orbital cellulitis; §§Patient also had vitamin D deficiency and chronic hypersensitivity pneumonitis due to exposure to domestic pigeons. NTM, non-tuberculous mycobacterial; NTM-PD, NTM pulmonary disease; ABPA, allergic bronchopulmonary aspergillosis; EP-NTM, extrapulmonary NTM
Of the eight patients with EP-NTM disease, HIV/AIDS was present in one patient and his CD4 cell count was 644 cells/μl, four had hospital-acquired NTM disease (detailed in Table II) and another two had NTM disease following trauma.
M. intracellulare (11/34; 32.3%) was the most common NTM isolate followed by M. abscessus (9/34; 26.5%) in NTM-PD. In one patient with bronchial asthma, M. simiae was repeatedly isolated. This patient was not treated as he did not have active disease. M. simiae was isolated in another four patients and M. gordonae was isolated from three patients. Six of the eight patients with EP-NTM disease had M. abscessus, and of the remaining two, one had M. intracellulare and another had M. parascrofulaceum. Figures 2-10 show clinical and radiographic images of some of the patients. None of the patients with NTM disease had MTB co-infection.
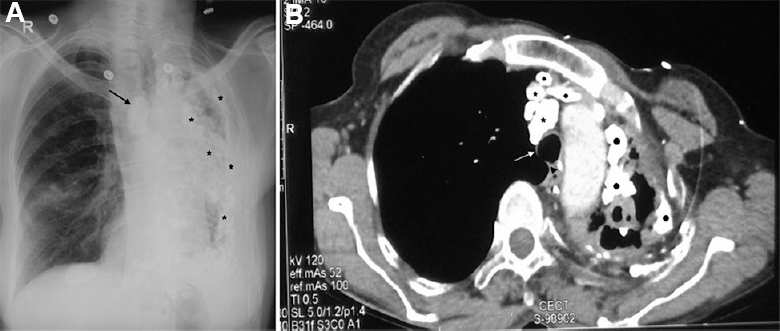
- A 52 yr old female, presented with shortness of breath and cough with expectoration. Mycobacterium kansasii was repeatedly isolated from sputum. (A) Her chest skiagram (posteroanterior projection) shows sequelae of pleuro-pulmonary tuberculosis in the form of mediastinal shift to the left, lymph nodal (arrow) and extensive pleuroparenchymal calcifications (asterisks) along with marked volume loss (atelectasis) of the left lung. The right lung shows compensatory hyperinflation. (B) Computed tomography (CT) chest of the same patient showing tracheal shift to the left (white arrow pointing to tracheal bifurcation and black arrow head to displaced carina) and multiple calcifications in lymph nodes (asterisks). Filled black circles show pleuroparenchymal calcifications. Hyperinflation of the right lung is also seen.
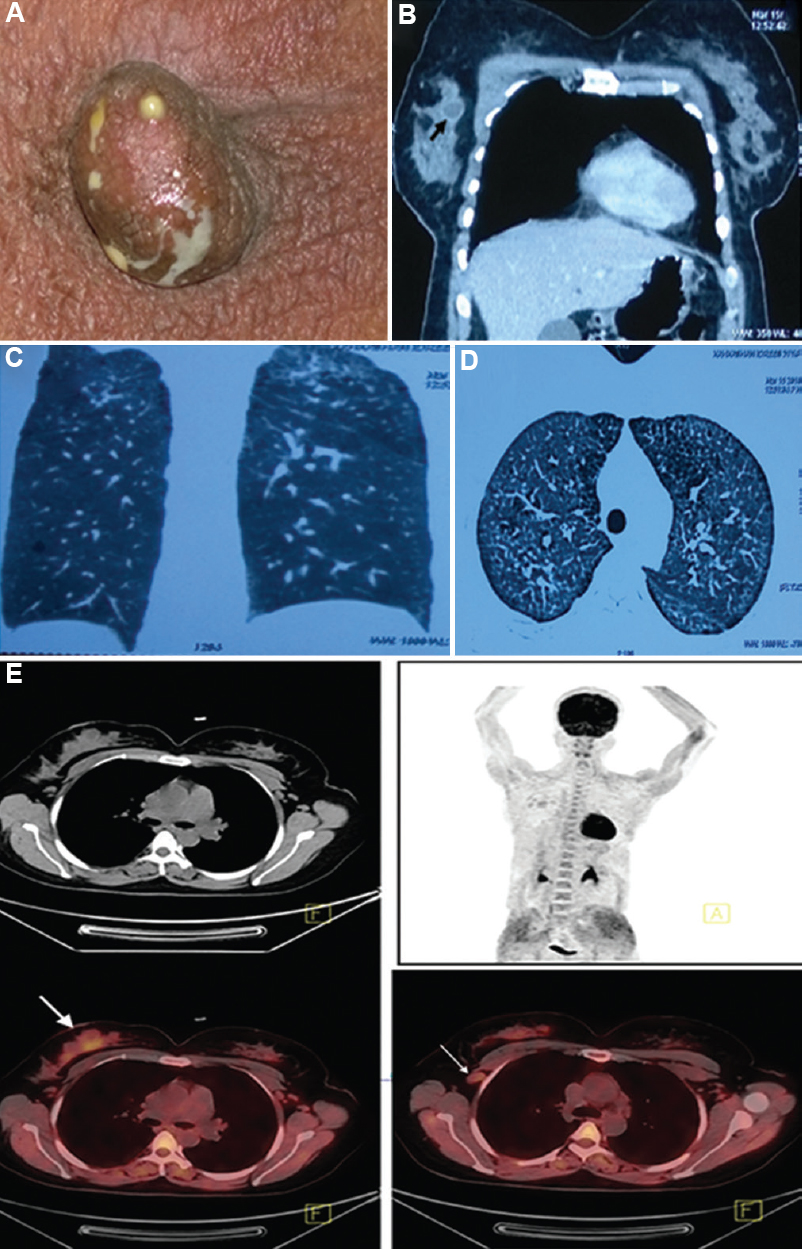
- (A) A 35 yr old female presented with discharge from the right nipple, Mycobacterium abscessus was isolated from the pus on several occasions prior to treatment. (B) CT chest showing enhancement of the margin of the abscess (black arrow) with intravenous contrast. (C and D) Coronal and axial sections of the CT of the chest show changes of chronic hypersensitivity pneumonitis due to exposure to domestic pigeons. (E) FDG PET-CT images of the same patient with discharge from the right nipple, showing FDG-avid right breast lesion (thick arrow) and right axillary lymphadenopathy (thin arrow). FDG, 18F labelled 2-deoxy-D-glucose; PET, positron emission tomography.
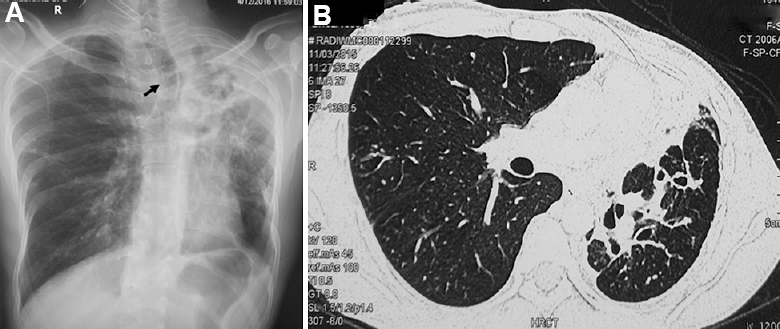
- (A) Chest skiagram of a 30 yr old male showing atelectasis of the lung with marked mediastinal shift (black arrow points to the tracheal shift). (B) Axial section of CT chest of the same patient showing atelectasis of the left upper lobe with architectural distortion of airways resulting in bronchiectasis.
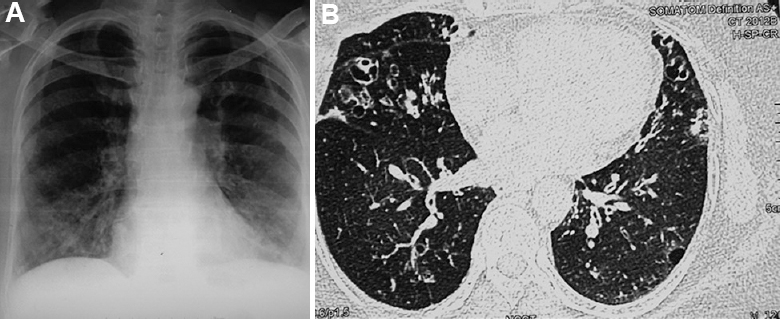
- (A). Chest radiograph of a 63 yr old female showing bilateral bronchiectasis in a patient with asthma and allergic bronchopulmonary aspergillosis. (B) Axial section on high resolution CT chest showing bilateral bronchiectasis in the right middle lobe, lingula and lower lobes. Mycobacterium simiae was repeatedly isolated from the sputum.
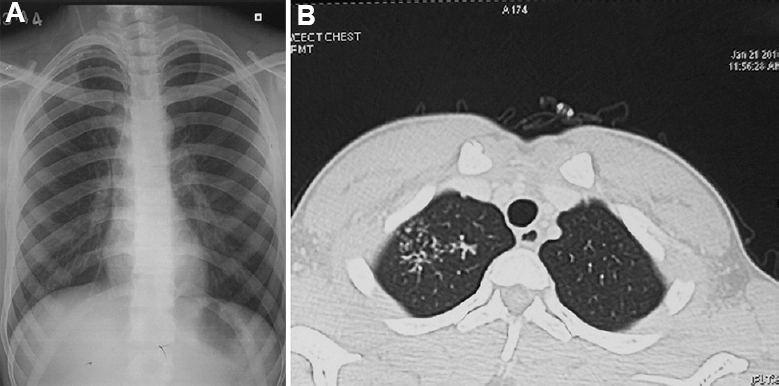
- A 18 yr old male presented with cough and expectoration, sputum examination revealed Mycobacterium intracellulare. (A) Chest radiograph showing infiltration in the right upper lobe. (B) Axial section of CT chest showing tree-in-bud appearance in the right upper lobe.
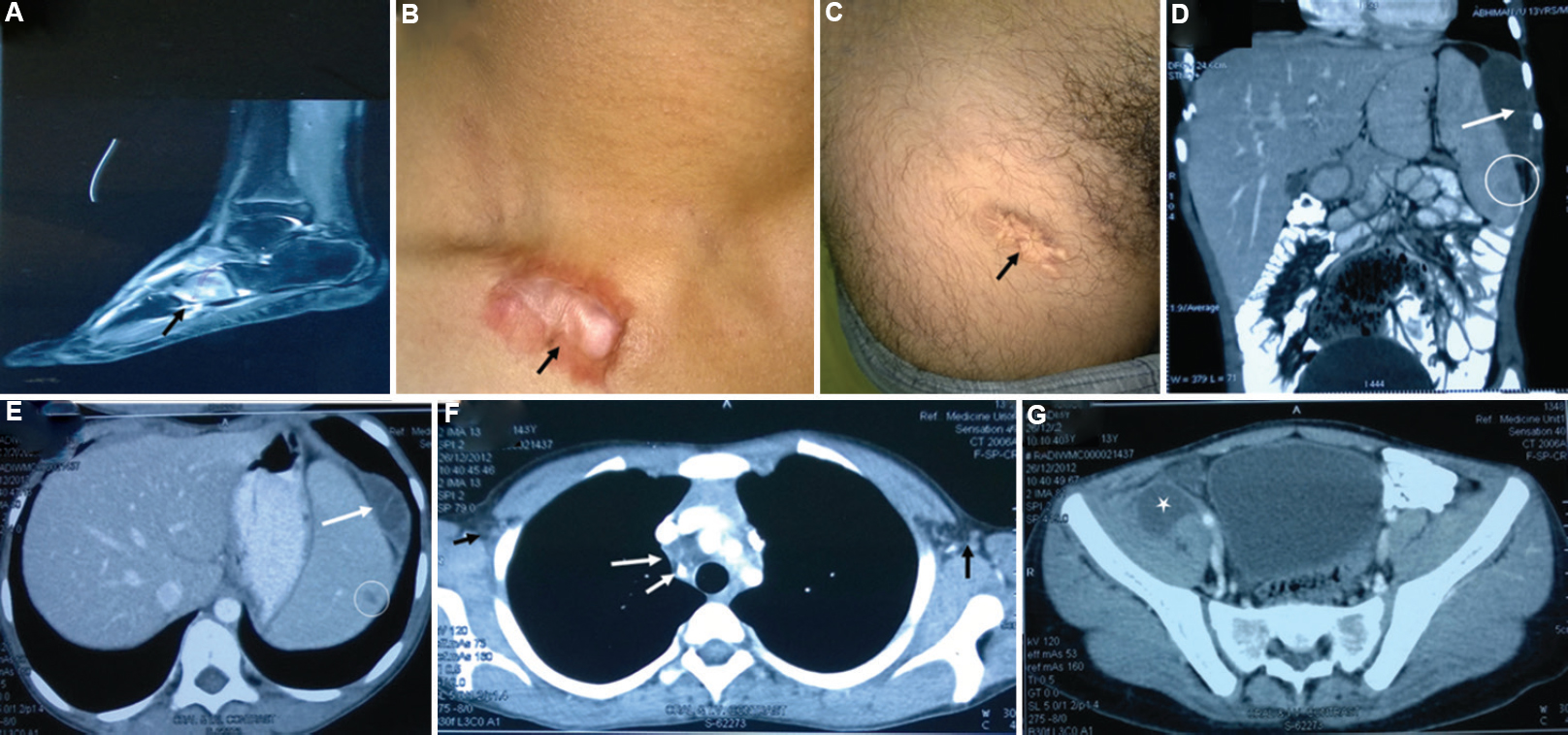
- The patient had disseminated Mycobacterium intracellulare infection; no immune defect could be detected. He was successfully treated. (A) The MRI scan picture shows osteomyelitis of foot bone (black arrow). (B) Black arrow shows healing of cutaneous lesion by keloid formation. (C) Upper part of thigh shows another healed skin lesion (black arrow). (D and E) Hypodense lesions in the spleen (white open circles) and peri-splenic abscess (white arrows). (F) Bilateral conglomerate necrotic axillary (extreme-left and -right arrows) and right paratracheal lymph nodes (long and short arrows in the centre of CT image), calcification is also noted in the lymph nodes. (G) Iliopsoas abscess on the right side (white asterisk).
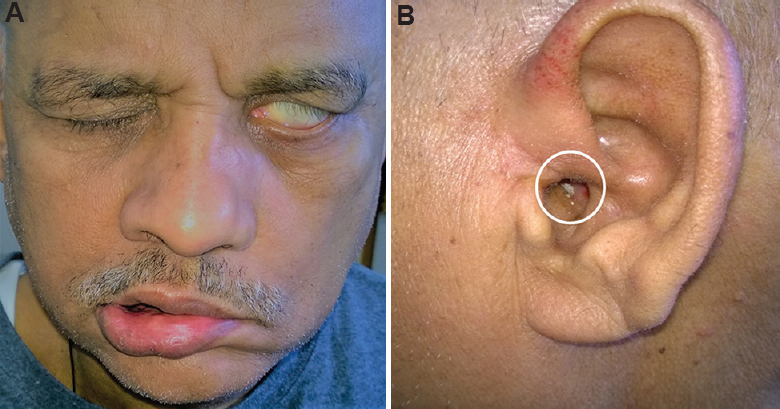
- (A) Clinical picture of a 58 yr old patient with Mycobacterium parascrofulaceum disease. The patient had lower motor neurone type of left facial nerve palsy. The disease produced left orbital cellulitis resulting in left-sided blindness, mastoiditis, involvement of apex of the petrous bone and meningitis. (B) Pus discharge (white open circle) from the left ear is visible which disappeared on follow up.
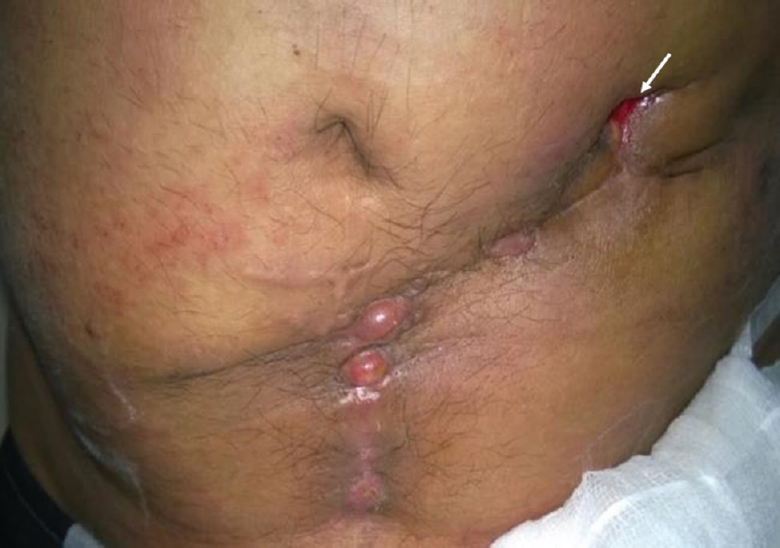
- Clinical photograph of a 35 yr old male, showing discharging sinus (white arrow) in the abdominal wall in a patient infected with Mycobacterium abscessus following left inguinal hernia repair with mesh.
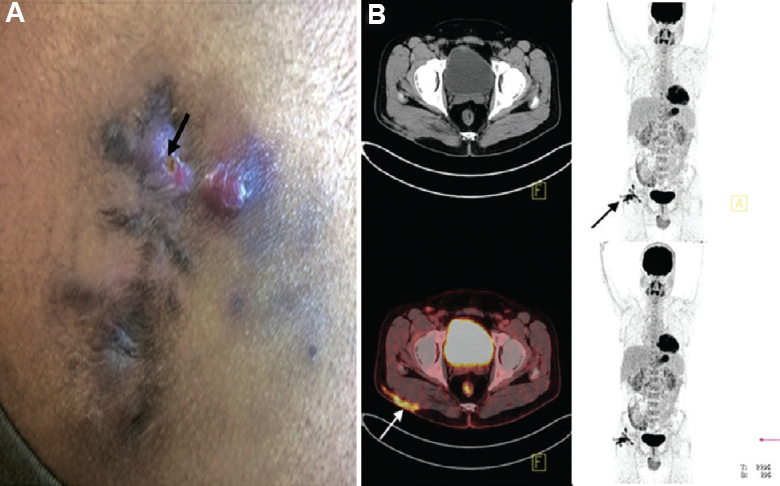
- (A) Clinical photograph of a 30 yr old male, showing right-sided post-injection gluteal abscess (black arrow) in a patient with NTM infection. (B) Transaxial fused FDG PET-CT image of the same patient, at the level of acetabulum showing FDG accumulation in the subcutaneous thickening and stranding (arrow) involving the underlying right gluteus muscle superficially in right gluteal region.
Of the 42 NTM patients, 41 were having active NTM disease as per ATS definition6. Therefore, it was decided to treat these patients. M. abscessus was treated with additional drugs such as linezolid, amikacin, ciprofloxacin, co-trimoxazole and imipenem. AEs and SAEs are detailed in Table III.
| Events | n (%) |
|---|---|
| Gastrointestinal disturbances (Anorexia, nausea and abdominal pain) | 13 (30.9) |
| Sensorineural hearing loss (Sensorineural deafness occurred due to amikacin) | 1 |
| Alopecia | 1 |
| Generalized pruritis | 1 |
| Hospitalization | 2 (8.3) |
| Deaths† | 10 (24) |
†Two patient died due to massive haemoptysis and in eight patients cause of death could not be ascertained
Of the 33 patients with active NTM-PD, five were lost to follow up, 10 patients died, two died due to massive haemoptysis and in eight patients exact cause of death could not be ascertained. Three patients declined the treatment and one of these opted for an alternative form of treatment. In 15 NTM-PD patients, there was good response to treatment. All were given one year of treatment after culture conversion. One patient with bronchial asthma and ABPA was hospitalized nearly after one year of cure with acute exacerbation of bronchial asthma and microbiological relapse was ruled out in this case after appropriate microbiological investigations. In EP-NTM patients, treatment duration varied from 1-16 months (median: 12 months; interquartile range: 3-16 months). Surgery was performed in one patient with knee joint arthritis and linezolid was added to his antimycobacterial drug regimen. One patient with chronic osteomyelitis of the left femur had multiple surgeries and received drug regimen consisting of imipenem and injectable amikacin. Later, this patient was also treated with antifungal drugs for mucormycosis infection. In one patient with a history of hernia, the infected mesh was surgically removed and the improvement was observed after one month of the drug treatment. One patient with breast abscess was treated for 11 months. The patient with HIV/AIDS declined the prescribed treatment.
Discussion
With wider availability of molecular diagnostic tests for NTM and their identification at species and sub-species level, there has been a renewed interest in this disease. In the past decade, there has been an increase in the reporting of NTM disease234 but most of these reports are from the industrialized world and approximately 200 NTM species have been described till date13 and all of these may not be clinically relevant. There is a scarcity of NTM reporting from the developing countries with high TB burden. Only a handful of studies have been published from India1415161718192021222324. Ironically, most of these have reported microbiological data from TB laboratories involved in isolating NTM. There is a lack of information on patients' data and in most instances ATS guidelines definition criteria for disease activity have not been applied. Therefore, it was difficult to conclude whether these NTM isolates were just commensals, contaminants or causative agent of disease. In addition, follow up details including drug treatment regimens, AEs and SAEs and disease outcomes were not reported in most of these studies.
A variable prevalence of NTM disease (0.38-27.4%) has been reported from India1415161718192021222324. In the present study, the prevalence of NTM among TB suspects was low (0.77%). Three other studies from large TB centres in India have reported a low NTM disease prevalence among TB suspects, 0.38, 0.8 and 0.86 per cent, respectively161819. A possible explanation for the low NTM disease prevalence in these studies can be the sample collection from a larger population as these healthcare facilities cover large number of districts in their respective States. Another three Indian studies141517 reporting higher NTM prevalence were published before the ATS guidelines6 were released. In other four studies, the prevalence rates were higher as two studies were solely laboratory based2024, and one was a retrospective study on clinical isolates21 and the remaining one was in HIV/AIDS patients only15.
In the present study, of the 42 patients with NTM infection, 41 fulfilled NTM disease criteria as per ATS definition6 and one was asymptomatic. The male involvement occurred more frequently and pulmonary involvement was predominantly (34 out of 42 patients; 81%) observed. This involvement was similar to TB where 80 per cent involvement was observed in HIV-negative individuals25. Bronchiectasis as sequelae of pulmonary TB was the most common predisposing cause. Only one patient in the EP-NTM group was HIV seropositive.
In the present study, M. intracellulare was the most common NTM isolate followed by M. abscessus among pulmonary patients. This finding was similar to worldwide published reports2 in patients with pulmonary disease; however, some geographical diversity of NTM species such as M. xenopi and M. szulgai has been observed26. M. gordonae is frequently a contaminant or colonizer and rarely produces disease. In this study, this organism was repeatedly isolated from three patients and all had active pulmonary disease. One patient opted for an alternative form of treatment and other two patients died without treatment and the exact cause of death could not be ascertained in these patients.
In the present study, the diagnosis of NTM disease was based on patients' clinical characteristics, radiological features and laboratory results including microbiological and molecular tests. It is to be emphasized that no patient with suspected NTM disease should be empirically treated. Further, mere isolation of NTM does not necessarily indicate an active disease. While treating patients with NTM disease, especially patients with disseminated disease, it is crucial to make assessment of co-morbidities, host-related predisposing factors including immunological perturbations. HIV/AIDS must be ruled out as it requires life-long treatment with highly active antiretroviral treatment.
During treatment, careful follow up and monitoring of the side effects were done meticulously. In patients with NTM-PD, persistence of clinical symptoms despite culture conversion may be attributed to the underlying permanent abnormalities of the lungs and these abnormalities may also predispose to relapse of NTM infection. In most patients with EP-NTM disease, the drug treatment along with surgical intervention cured patients except one patient with left femur osteomyelitis due to M. abscessus who required prolonged hospitalization for intravenous antibiotics administration in addition to surgery. It was difficult to treat this patient with antibiotics. Eventually, he was administered antifungal treatment. None of the patients in this study had co-infection of NTM and MTB, one patient had co-infection with mucormycosis. It is suggested that co-infection with TB and fungal infection should be diligently searched and ruled out.
Subspecies identification and drug sensitivity testing (DST) of these NTM isolates were not done. This was a limitation of the study. It is important to identify subspecies of M. abscessus as M. abscessus subsp. abscessus is difficult to treat with various combinations of intravenous and oral antibiotics4. Another limitation of the study was that the speciation of the NTM was done by single target gene sequencing. A multi-locus gene analysis could have facilitated the NTM identification up to subspecies level and could have provided additional information about the drug resistance in NTM due to mutations in certain genes such as erm (41), 16S rRNA and 23S rRNA especially macrolide and amikacin resistance. The major strengths were the prospective nature of the study, use of ATS definition criteria for NTM disease activity and treatment, NTM species identification by various methods including HPLC, LPA and ITS gene sequencing and follow up while patients were on treatment.
In conclusion, our findings showed a low frequency of NTM disease among TB suspects at a large tertiary care centre in north India. Systematic large studies involving different regions of India to determine geographical diversity of NTM distribution with confirmation of diagnosis, species and subspecies identification using currently available molecular laboratory tests should be carried out in future.
Financial support & sponsorship: The study was supported by JC Bose National Fellowship awarded to the first author (SKS) by the Science and Engineering Research Board (SERB; no. SB/S2/JCB-04/2013) of the Department of Science & Technology, Government of India.
Conflicts of Interest: None.
References
- Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: A retrospective cohort study. Lancet. 2013;381:1551-60.
- [Google Scholar]
- Nontuberculous mycobacterial pulmonary disease: An increasing burden with substantial costs. Eur Respir J. 2017;49 pii 1700374
- [Google Scholar]
- Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015;36:91-9.
- [Google Scholar]
- Skin and soft tissue infections due to nontuberculous mycobacteria. Curr Infect Dis Rep. 2018;20:6.
- [Google Scholar]
- The management of non-tuberculous cervicofacial lymphadenitis in children: A systematic review and meta-analysis. J Infect. 2015;71:9-18.
- [Google Scholar]
- An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- [Google Scholar]
- Revised National Tuberculosis Control Programme: Guidelines on programmatic management of drug resistant TB (PMDT) in India. New Delhi: Directorate General of Health Services, Ministry of Health & Family Welfare; 2012.
- MGIT™ procedure manual: For BACTEC™ MGIT 960™ TB system. Franklin Lakes: Becton Dickinson; 2006.
- Evaluating the diagnostic accuracy of xpert MTB/RIF assay in pulmonary tuberculosis. PLoS One. 2015;10:e0141011.
- [Google Scholar]
- Mycolic acid analysis by high-performance liquid chromatography for identification of Mycobacterium species. Clin Microbiol Rev. 2001;14:704-26.
- [Google Scholar]
- Detection and identification of mycobacteria by amplification of the internal transcribed spacer regions with genus- and species-specific PCR primers. J Clin Microbiol. 2000;38:4080-5.
- [Google Scholar]
- Increasing HIV seropositivity among adult tuberculosis patients in Delhi. Indian J Med Res. 2003;117:239-42.
- [Google Scholar]
- Comprehensive subspecies identification of 175 nontuberculous mycobacteria species based on 7547 genomic profiles. Emerg Microbes Infect. 2019;8:1043-53.
- [Google Scholar]
- Species level identification of non-tuberculous mycobacteria from South Indian BCG trial area during 1981. Tubercle. 1985;66:9-15.
- [Google Scholar]
- Isolation of Mycobacterium avium complex and M. simiae from blood of AIDS patients from Sevagram, Maharashtra. Indian J Tuberc. 2005;52:21-6.
- [Google Scholar]
- Atypical mycobacterial infection among HIV seronegative patients in Pondicherry. Indian J Chest Dis Allied Sci. 2006;48:107-9.
- [Google Scholar]
- Non tuberculous mycobacteria isolated from clinical specimens at a tertiary care hospital in South India. Indian J Med Microbiol. 2005;23:172-5.
- [Google Scholar]
- Time to identify and define non-tuberculous mycobacteria in a tuberculosis-endemic region. Int J Tuberc Lung Dis. 2010;14:1001-8.
- [Google Scholar]
- Occurrence of non-tuberculous Mycobacterium in clinical samples-a potential pathogen. Indian J Tuberc. 2013;60:71-6.
- [Google Scholar]
- High prevalence of non-tuberculous mycobacterial disease among non-HIV infected individuals in a TB endemic country - Experience from a tertiary center in Delhi, India. Pathog Glob Health. 2014;108:118-22.
- [Google Scholar]
- Prevalence of nontuberculous mycobacteria among extrapulmonary tuberculosis cases in tertiary care centers in Northern India. Biomed Res Int. 2015;2015:465403.
- [Google Scholar]
- Prevalence and species spectrum of both pulmonary and extrapulmonary nontuberculous mycobacteria isolates at a tertiary care center. Int J Mycobacteriol. 2016;5:288-93.
- [Google Scholar]
- Ocular mycobacteriosis-dual infection of M tuberculosis complex with M fortuitum and M bovis. J Ophthalmic Inflamm Infect. 2017;7:2.
- [Google Scholar]
- Non-tuberculosis mycobacterium speciation using HPLC under revised national TB control programme (RNTCP) in India. J Appl Microbiol. 2018;124:267-73.
- [Google Scholar]
- The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013;42:1604-13.
- [Google Scholar]






