Translate this page into:
Antibiotic stewardship initiative in a Medicine unit of a tertiary care teaching hospital in India: A pilot study
For correspondence: Dr Rita Sood, Department of Medicine, All India Institute of Medical Sciences, New Delhi 110 029, India e-mail: ritasood@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The models for implementation of antibiotic stewardship programme (ASP) in the acute care settings of developing countries are lacking. In most of the hospitals, patient turnover is high and a proper system for recording antibiotic-related information and tracking hospital-acquired infections is not in place. This pilot study was conducted in a tertiary care teaching hospital in north India to assess the feasibility of implementation of an ASP in a Medicine unit and to evaluate the effect of implementation as per the criteria applicable in this set up.
Methods:
A pre-post-quasi-experimental non-randomized study was conducted in two phases. In the first phase, current practices in the Medicine wards were observed. In the second phase, the ASP was implemented in a single Medicine unit, along with prospective audit and feedback, tracking of the process, as well as outcome measures. Patient risk stratification, blood culture on day one, day 3 bundle, dose optimization, de-escalation and intravenous to oral conversion of antibiotics were the key elements focused upon.
Results:
There was a significant improvement in the appropriateness of antibiotic prescription (66 vs. 86%, P<0.001) and reduction in the mean number of antibiotics used per person (4.41 vs. 3.86, P<0.05) along with decrease in the duration of hospital stay (17 vs. 14 days, P<0.05). There was a significant improvement in sending of blood cultures on day one during the stewardship phase (P<0.001).
Interpretation & conclusions:
The ASP approach used in our pilot study may be feasible and beneficial. However, it needs further confirmation in other settings and on a large scale.
Keywords
Antibiotic appropriateness
day 3 bundle
days of therapy
developing countries
patient risk stratification
Continuous and indiscriminate use of antimicrobials has led to the emergence of multidrug-resistant (MDR) organisms1. In the early 21st century, the world health bodies have finally acknowledged that combating antimicrobial resistance (AMR) is a need of the hour and have recommended antibiotic stewardship programmes (ASPs) as a way forward, to increase the shelf life of existing antibiotics by their judicious use23. India took a landmark step towards combating the spread of AMR in the form of Chennai Declaration in 20124. The Global Antibiotic Resistance Partnership (GARP) was initiated by the Center for Disease Dynamics, Economics and Policy. It works to develop policy recommendations, particularly for implementation in low- and middle-income countries. India is also a member country of GARP along with seven other nations5.
It has been shown that the AMR is rising alarmingly in India6. As per the study, 56,500 neonatal sepsis deaths were attributable to resistance to the first-line antibiotics in India in 2012. Extended-spectrum beta-lactamase (ESBL)-producing organisms are prevalent in most of the Indian hospitals, making the use of reserved antibiotics such as carbapenems necessary6. Studies have identified ESBLs in 70-100 per cent of Enterobacteriaceae in India7. Extensive uncontrolled use of carbapenem group of antibiotics to tackle ESBL producers, has resulted in carbapenem resistance in the form of New Delhi metallo-beta-lactamase 1 in India8. MDR pathogens are a significant cause of hospital-acquired infections (HAIs) as shown in a large study done between 2004 and 2007 in seven hospitals in Indian cities9. The rates of methicillin-resistant Staphylococcus aureus (MRSA) infections among different studies from India have remained largely variable10.
The purpose of antimicrobial stewardship is to maintain a balance between optimal clinical outcomes and inadvertent consequences of antimicrobial use11. This includes reducing the toxicity, the selection pressure and the emergence of resistance. ASP is best implemented in conjunction with infection prevention and control. ASP is also important in improving appropriateness of antimicrobial treatment for improving clinical and microbiological outcome12. A few Indian studies have demonstrated131415. However, the models for the implementation in the developing countries, especially in the public sector settings, are lacking. The Indian Council of Medical Research (ICMR) has taken many initiatives towards ASP1617.
With this background, a pilot study was planned on the lines of a quality improvement (QI) project in the Medicine unit of a tertiary care teaching hospital in north India, to evaluate the appropriateness of current usage of antibiotics in Medicine wards; and to study the effect of implementation of an ASP in a single unit of the Medicine wards relevant to appropriateness of antibiotic prescription, antibiotic consumption, HAIs, duration of hospital stay and mortality.
Material & Methods
A pre-post-quasi-experimental non-randomized study with prospective audit and feedback during ASP implementation. It was conducted in Medicine wards and Medicine intensive care unit (ICU) of All India Institute of Medical Sciences (AIIMS), New Delhi, India, a tertiary care teaching hospital from April 2015 to August 2016. The study was planned as a pilot study to explore the feasibility of implementation of ASP best applicable in the context of our health system. The study protocol was approved by the Institutional Ethics Committee. Written informed consent was obtained from each patient.
The study was conducted in two phases. Phase 1 was conducted in all the three Medicine units to get an idea about the prescribing patterns in the Medicine department as a whole. The phase 2 i.e. implementation of ASP was restricted to a single unit (Unit II) for intensive implementation. A single unit was the ideal focus point to work as a team and bring about a positive change, to begin with initially. Fig. 1 shows the study design. Twice weekly visits were made to the ward on fixed days and newly admitted patients prescribed antibiotics were included unit-wise. The first visit was for inclusion and the second visit for follow up.
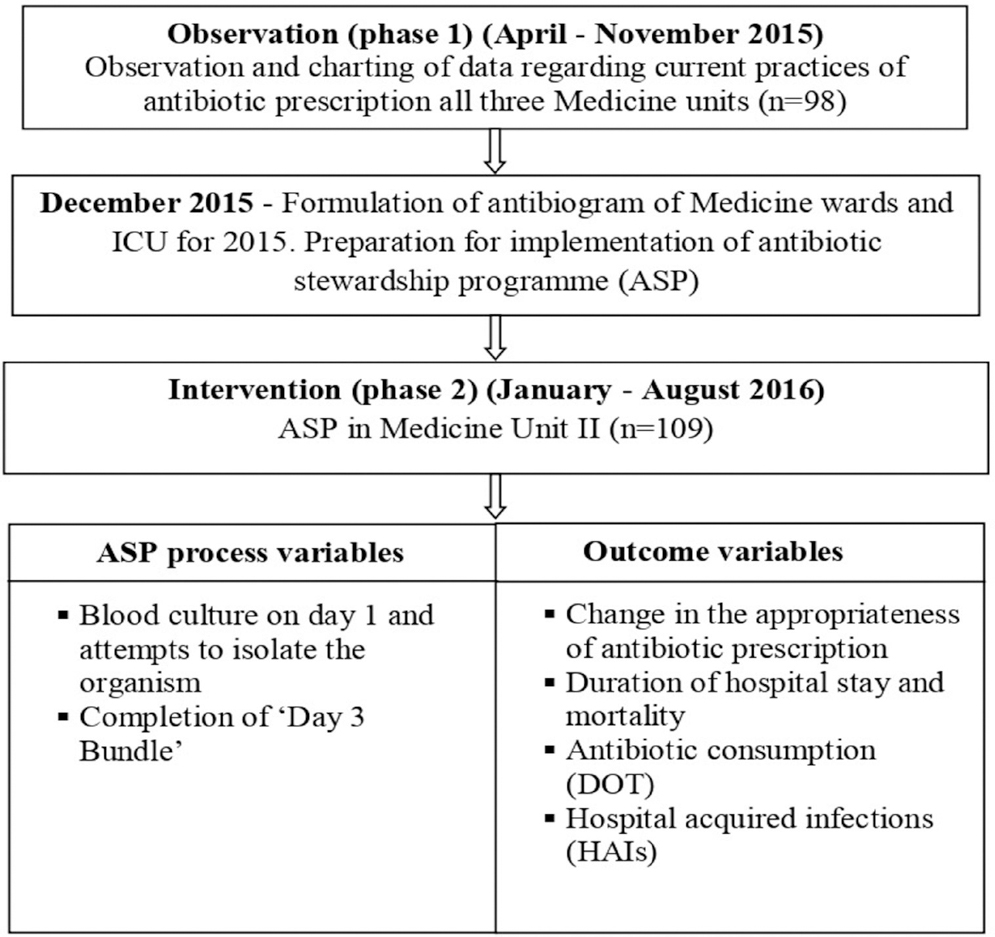
- Flow chart of the study design. ICU, intensive care unit; DOT, days of therapy.
During both the phases, appropriateness of use of antibiotic was assessed under the four criteria as elaborated in Table I. There is no previously established universal consensus definition for appropriate antibiotic usage. The criteria have been formulated keeping in mind the core pharmacy-driven interventions required of a stewardship programme such as dose adjustment, dose optimization, intravenous (iv) to oral conversion and prescription conforming to established syndrome-specific guidelines as reinforced by the Centers for Disease Control and Prevention (CDC)2. The indications for starting antibiotics was determined on the basis of the provisional diagnosis at initial evaluation documented in the records, as adjudged by the clinical history and baseline investigations available. The patient risk stratification was implemented to identify patients with the risk of infection by MDR organisms (Table II).
| Criteria | Sub-criteria | Definition |
|---|---|---|
| Empirical antibiotic selection |
Site of infection | Clinical judgment based on history and examination Laboratory parameters available at admission Radiological evidence |
| Patient risk stratification (As proposed in the National Policy for Containment of Antimicrobial Resistance)18 | History of contact with the health care system within the past 90 days History of antibiotic use within the past 90 days Any invasive procedures done on the patient Co-morbidities |
|
| Evidence based guidelines1920 | Global or National as hospital specific guidelines were not available | |
| Local microbiological data | The antibiotics for which the hospital microbiological culture results are usually reported, were preferable | |
| Dose optimization | Assessed for dose, frequency and renal modification when required | All renal dosage adjustments were assessed based on creatinine clearance calculated by Cockcroft-Gault (CG) equation or MDRD eGFR equation |
| De-escalation or escalation of therapy | Culture reports, clinical condition and surrogates for infection markers like procalcitonin. | Duration of therapy was considered under de-escalation. If the patient was improving but received prolonged days of antibiotics beyond recommended, it was considered inappropriate. Similarly, if the patient was worsening but no attempt was made at antibiotic escalation when there was scope, it was considered inappropriate. |
| iv to oral conversion (As proposed by the Centers for Disease Control and Prevention)21 | Patient must meet the following criteria: • Receiving oral or gastric tube intake • Taking other oral medications • Absence of (i) Mucositis (ii) Malabsorption syndrome or gastrointestinal motility disorder (iii) Severe nausea, vomiting or diarrhoea (iv) Continuous nasogastric suctioning |
Antibiotics with 100% bioavailability: Amoxicillin Amoxicillin/Clavulanate Azithromycin Cefpodoxime Ciprofloxacin Clindamycin Doxycycline Levofloxacin Linezolid Moxifloxacin Trimethoprim/ sulphamethoxazole |
MDRD, Modification of diet in renal disease; eGFR, estimated glomerular filtration rate
| Patient type 1 (Community acquired infection) |
Patient type 2 (Health care associated/hospital acquired infection) |
Patient type 3 (Health care associated/hospital acquired infection) |
|---|---|---|
| No contact with health care system (within last 90 days) No prior antibiotic treatment (within last 90 days) No procedures done (within last 90 days) Patient young with no or few co-morbidities (Non-ESBL/MSSA) |
Contact with health care system (recent hospital admission, nursing home visit, dialysis) Recent antibiotic therapy Minimum procedures done (iv cannula, central line, intubation, etc.) Elderly patients (>65 yr) with few co-morbidities (more prone for MRSA/ESBL organisms) |
Long hospitalization and/or invasive procedures Recent and multiple antibiotic therapies Patient old (>65 yr) with multiple co-morbidities Major invasive procedures done (Laparotomy etc.) Structural lung disease, AIDS, Neutropenia other severe immunodeficiency (Prone for ESBL/MRSA as well as carbapenamese producing organisms) (Inherently resistant organisms like Pseudomonas, Acinetobacter) |
ESBL, Extended-spectrum beta-lactamase, MSSA, methicillin sensitive Staphylococcus aureus; MRSA, methicillin resistant Staphylococcus aureus; AIDS, acquired immune deficiency syndrome
Source: Adapted from Ref. 19
The observational phase data (phase 1) (n=98) on use of antibiotics on all the three Medicine units indicated overall appropriateness of 68 per cent. The sample size for the intervention phase was calculated, assuming a 20 per cent absolute increase in the appropriate antibiotic usage, with a two-sided α error of 5 and 90 per cent power. Thus, at least 84 patients were required during the implementation of the ASP (phase 2). In all, 109 patients were included during phase 2.
Implementation of antibiotic stewardship programme: The ASP team consisted of the senior and junior residents posted in Medicine Unit II during the period of study, along with the investigator and faculty from the departments of Medicine and Microbiology. Prospective audit and feedback, which is the core element of any ASP, was implemented. As the team of residents rotated every two months, data were collected by the investigator regularly during the implementation of the ASP. The processes as well as the outcome measures were actively conveyed to the team during the monthly meetings. Feedback was taken from the team. The reasons and difficulties in documentation in the ASP form were analyzed and corrected. The programme was formulated on the basis of CDC core elements of hospital ASP2. A bedside proforma was designed exclusively for clear documentation of antibiotic-related information. The team was sensitized to the elements of ASP through monthly sessions, interaction and feedback and one-to-one sessions during weekly visits and to the concept of antibiotic rounds.
Certain tools were helpful in the implementation of the ASP. Antibiogram of the Medicine wards and of the ICU combined was constituted for the year before ASP implementation. The antibiotic susceptibility patterns for the three main sites i.e. blood, respiratory secretions (sputum, endotracheal aspirate/tracheal aspirate, bronchoalveolar lavage) and urine were constituted. This was utilized as a guide in choosing empirical therapy. Syndrome-specific guidelines were shared and discussed in group sessions for the three most commonly encountered conditions in the ward [pneumonia, urinary tract infections (UTIs) and meningitis].
The elements of ASP which were focussed upon are as below:
Patient risk stratification18: This was the first step which was required to be fulfilled during the implementation of ASP. The patients were stratified as types 1, 2 and 3. Although no specific antibiotic protocol was formulated, the patient risk stratification was to help in recognition of patients requiring the use of broad-spectrum (MRSA/ESBL) coverage in the empirical therapy. The patient risk stratification was also used for deciding further escalation in case of no response and also in de-escalation of therapy.
Cultures Blood and site of infection: Before the initiation of antimicrobial therapy, at least two samples of blood were taken from separate venipuncture sites for culture22. After the patient risk stratification for deciding on the empirical therapy, the next important step advocated was the sending of the first set of cultures on day one of patient admission (before the antibiotic administration). Culture from the suspected site of infection as well as one blood culture was also required to be sent.
Day 3 bundle: This was implemented to improve the reassessment of in-patient empirical antibiotic prescriptions23. Four measures were selected for documentation in the bedside proforma. This was required to be completed within three days after the start of therapy.
Escalation/de-escalation of therapy: De-escalation was encouraged based on culture reports, clinical condition and surrogates for infection markers such as procalcitonin. The team was educated and reinforced about the suggested duration of antibiotic therapy based on the specific indication in accordance with the guidelines1920. Similarly, if the patient was worsening, antibiotic escalation was encouraged when there was scope.
Intravenous (iv) to oral switch: The team was educated about the antibiotics with good oral bioavailability and criteria to be fulfilled21 for consideration of switching of antibiotic therapy from the iv to oral. If the criteria were met and the patient was receiving any of those antibiotics, the patient was switched to oral therapy. The same was reinforced for documentation in the 'day 3 bundle' also.
Automatic stop order: This was advocated for only one antibiotic i.e. azithromycin. Azithromycin was chosen as it was observed to be one of the inappropriately prescribed antibiotics in phase 1 with respect to duration of therapy. The maximum treatment duration was five days for most of the indications. Hence, it was a suitable first choice antibiotic for the implementation of the stop order for this pilot study. An automatic stop order after five days was to be given by the residents whenever azithromycin was prescribed.
Dose optimization: The team was educated about the loading dose and the maintenance doses for commonly used antibiotics as well as appropriate renal-modified doses. Novel dosing strategies for certain antibiotics in the form of continuous or prolonged iv infusion were also discussed.
Monitoring for adverse drug reactions (ADRs): The ASP team was vigilant regarding the development of antibiotic-related adverse effects as well as to actively document any such information. In suspected cases of antibiotic-associated diarrhoea, stool sample for Clostridium difficile toxin A and B was sent.
Antibiotic consumption measurement: Antibiotic use was measured as days of therapy (DOT) standardized to 1000 patient days. One DOT is any dose of antibiotic that is received during a 24 h period3.
Statistical analysis: Statistical analysis was done using STATA 14 software (StataCorp LLC, TX, USA). For continuous variables, mean and standard deviation were calculated. For categorical variables, frequency and percentage were calculated. Quantitative variables were compared using t test or Wilcoxon rank-sum test analysis as appropriate. Categorical variables were compared using Chi-square/Fisher's exact test.
Results
The observation phase (phase 1) consisted of 98 patients with a mean age of 48.8 yr and the intervention phase (phase 2) consisted of 109 patients with a mean age of 48.4 yr. There were 30 (30.61%) and 38 (34.86%) patients with ICU stay in phases 1 and 2, respectively. There was no difference in the baseline characteristics of the patients included in both the phases as summarized in Table III. On the basis of history and initial investigations, the most common site of infection was found to be the lower respiratory tract as shown in (Table IV). There was no significant difference in the indications for starting antibiotics between the two groups. However, the difference was significant (P<0.05) when the data from Unit II was compared with phase II data.
| Characteristic | Observation (Units I, II and III) (n=98) (phase 1) |
Intervention (Unit II) (n=109) (phase 2) |
Observation (Unit II) (n=39) (phase 1) |
|---|---|---|---|
| GCS (at presentation) | 12.10 | 12.12 | 12.33 |
| Intubation at admission | 35 (35.71) | 40 (36.70) | 12 (30.77) |
| SBP (mm Hg) | 113.26 | 113.64 | 113.923 |
| Inotrope requirement at admission | 27 (27.55) | 26 (23.85) | 16 (41.03) |
| RR (per min) | 20.67 | 21.61 | 20.67 |
| Renal dysfuntion | 42 (42.86) | 50 (45.87) | 21 (53.85) |
| MODS | 6 (6.12) | 13 (11.93) | 2 (5.13) |
| Hb (g/dl) TLC (per cu mm) Platelets (per cu mm) |
10.34 15,436 1,71,274 |
10.80 13,590 1,96,495 |
10.25 13,758 1,76,128 |
| Urea (mg/dl) Serum creatinine (mg/dl) |
86.18 2.67 |
81.13 2.31 |
98.128 3.115 |
| Total bilirubin (mg/dl) | 1.68 | 2.74 | 2.19 |
| Patient risk stratification | |||
| type 1 type 2 type 3 |
18 (18.37) 61 (62.24) 19 (19.39) |
15 (13.76) 69 (63.30) 25 (22.94) |
9 (23.08) 22 (56.41) 8 (20.51) |
Values in parentheses are percentages. GCS, Glasgow Coma Scale; SBP, systolic blood pressure; RR, respiratory rate; MODS, multiple organ dysfunction syndrome (defined as patients with ≥3 organ dysfunction)
| Type | Observation (Units I, II, III) (n=98) (phase 1) |
Intervention (n=109) (phase 2) |
Observation Unit II (n=39) (phase 1) |
|---|---|---|---|
| SSTI | 7 (7.1) | 3 (2.7) | 6 (15.3) |
| Sepsis (Unknown focus) | 12 (12.2) | 7 (6.4) | 4 (10.2) |
| CAP | 41 (41.8) | 55 (50.4) | 14 (35.9) |
| Aspiration pneumonia | 4 (4.0) | 11 (10.0) | 0 |
| HAP | 0 | 2 (1.8) | 0 |
| UTI | 12 (12.2) | 9 (8.2) | 7 (17.9) |
| Acute febrile illness | 8 (8.1) | 6 (5.5) | 3 (7.6) |
| Others | 14 (14.2) | 16 (14.6) | 5 (12.8) |
Values in parentheses are percentages. SSTI, skin and soft tissue infection; CAP, community acquired pneumonia; HAP, hospital acquired pneumonia; UTI, urinary tract infection
Process measures: There was significant difference in between the two phases in the sending of blood culture on day one (P<0.001). There was improvement in the number of attempts to isolate causative organisms from other sites as well during phase 2. However, the yield of the cultures was less, and the difference was not significant. Among the 109 patients included in phase 2, 'day 3 bundle' was completed in 70 (64.2%) patients with the initial antibiotic plan documented in 104 (95.4%), culture reports documented in 103 (94.5%), review of the antibiotic plan/adaptation documented in 79 (72.4%) and the iv to oral consideration in 81 (74.3%) patients. The month-wise completion of 'day 3 bundle' during the eight months of implementation of ASP is shown in Fig. 2 as indicated by the percentage of patients. During the implementation of ASP, the number and the type of decisions taken during days 1-3, days 4-7 and days 8-14 were compiled for each month. As shown in Fig. 3, the number of decisions taken was more during the ASP phase, implying that there was a more frequent review of the antibiotics. There were more de-escalation and iv to oral conversion decisions taken. The data on adverse drug reactions (ADRs) attributed to antibiotics could not be acquired as there was inadequate documentation with regard to this observation. Overall, ADRs were documented in only eight patients during both the phases, three during phase 1 and five during phase 2. Most common (n=4) was antibiotic-associated diarrhoea. In none of the cases, stool sample for C. difficile toxin A and B was positive.
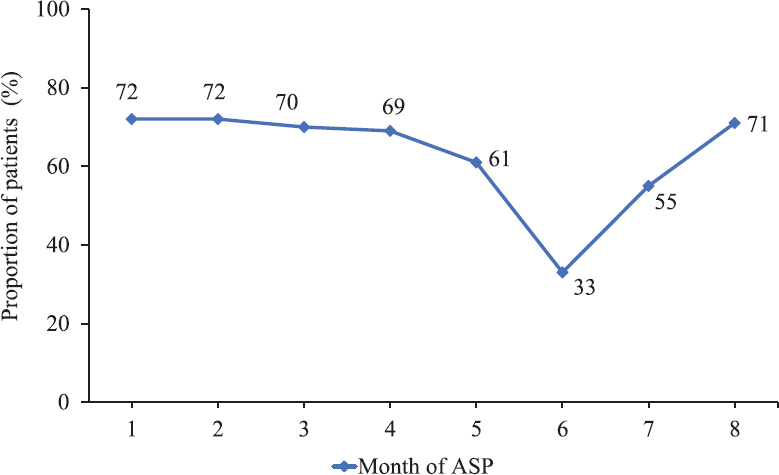
- Completion of ‘Day 3 Bundle’ during the implementation of antibiotic stewardship program (ASP) (proportion of patients expressed as percentage).
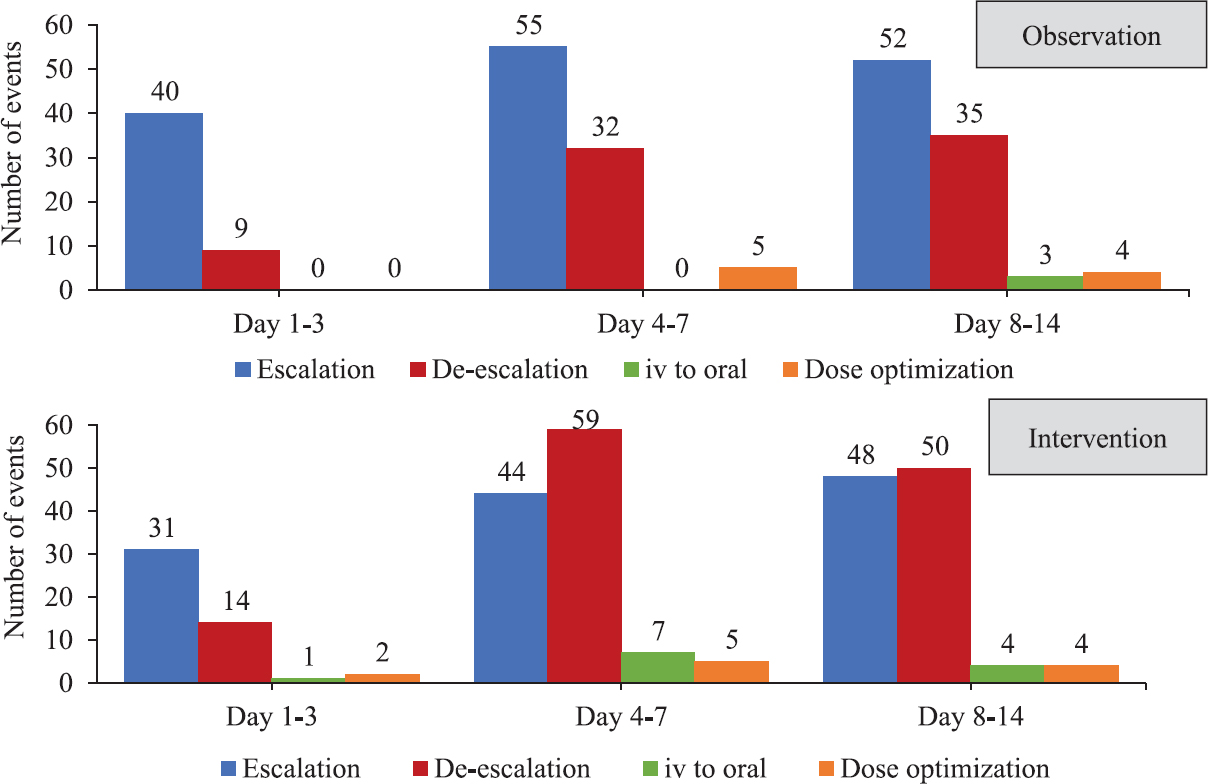
- Type of antibiotic decisions taken during day 1-3, day 4-7 and day 8-14 of patient admission during phase 1 and phase 2. The numbers represent the number of events.
Outcome measures: There was a significant improvement in the mean percentage appropriate antibiotic use per person from 66.82 to 86.82 per cent (P<0.001). The mean number of antibiotics used per person significantly decreased (P<0.05) from 4.41 to 3.86. There was no significant difference in the mortality between the two groups. These findings along with the final outcomes of the patients in the two groups are shown in Tables V and VI. Piperacillin-tazobactam was the most common antibiotic used during both the phases followed by levofloxacin. The use of first-line antibiotics such as ceftriaxone and azithromycin increased in the ASP phase compared to phase 1. The observations related to antibiotic consumption are shown in Table VII. The comparison of the DOT/1000 days of the various antibiotics through iv and oral route separately is shown in Fig. 4. The risk reduction for HAIs for phase 2 in comparison with phase 1 was 43 per cent. The data for the HAIs were obtained mainly from the documentation in the files. Hospital-acquired pneumonia, ventilator-associated pneumonia and UTIs were the HAIs recorded during observation phase and the bedside ASP forms during ASP phase.
| Measure | Observation (n=98) (Units I, II, III) (phase 1) |
Intervention (Unit II) (n=109) (phase 2) |
95% CI | Observation (Unit II) (n=39) (phase 1) |
95% CI |
|---|---|---|---|---|---|
| Blood culture on day 1 | 30 (30.6) | 76 (69.7)*** | - | 9 (23.08)††† | - |
| Final outcome | |||||
| Death Discharge | 53 (54.0) 45 (45.9) |
52 (47.7) 57 (52.9) |
- | 16 (41.03) 23 (58.97) | - |
| Hospital acquired infections | |||||
| None At least 1 |
60 (61.2%) 38 (38.7%) |
80 (73.3)† 29 (26.6) |
0.317-1.030 | 21 (53.85)† 18 (46.15) |
0.197-0.903 |
***P<0.001 compared to phase 1; P†<0.05; †††<0.001 compared to phase 2
| Outcome measure | Observation (Units I, II, III) (n=98) (phase 1) |
Intervention (Unit II) (n=109) (phase 2) |
Observation (Unit II) (n=39) |
|---|---|---|---|
| Mean no. of days in ICU | 9.90 | 9.05 | 9.76 |
| Mean total days of stay per person | 17.34 | 14.09* | 17.64 |
| Mean number of antibiotics used per person | 4.41 | 3.86* | 4.46 |
| Mean % appropriate antibiotic use per person | 66.82 | 86.82*** | 64.61 |
P *<0.05; ***<0.001 compared to observation group
| Antibiotic | Observation (Unit I, II, III) (n=98) |
Intervention (Unit II) (n=109) |
Difference (%) |
Observation (Unit II) (n=39) |
Difference (%) |
|---|---|---|---|---|---|
| Ceftriaxone | 90 | 161 | 71 (+ 78) | 36 | 125 (+347) |
| Teicoplanin | 234 | 101 | 133 (- 56.8) | 239 | 138 (-58.4) |
| Levofloxacin | 385 | 303 | 82 (- 21.2) | 492 | 189 (-38.4) |
| Piperacillin-tazobactam | 436 | 399 | 37 (- 8.4) | 453 | 54 (-11.9) |
| Cefoperazone-sulbactam | 198 | 139 | 59 (- 29.7) | 207 | 68 (-32.8) |
| Clindamycin | 194 | 158 | 36 (-18.5) | 235 | 77 (-32.7) |
| Meropenem | 145 | 70 | 75 (-51.7) | 228 | 158 (-69.2) |
| Colistin | 49 | 33 | 16 (-32.6) | 58 | 25 (-43.1) |
| Amikacin | 62 | 20 | 42 (-67.7) | 4 | 16 (+400) |
| Vancomycin | 24 | 50 | 26 (+108) | 36 | 14 (+38.8) |
| Linezolid | 81 | 97 | 16 (+19.7) | 55 | 42 (+76.3) |
| Metronidazole | 124 | 117 | 7 (-5.6) | 161 | 44 (-27.3) |
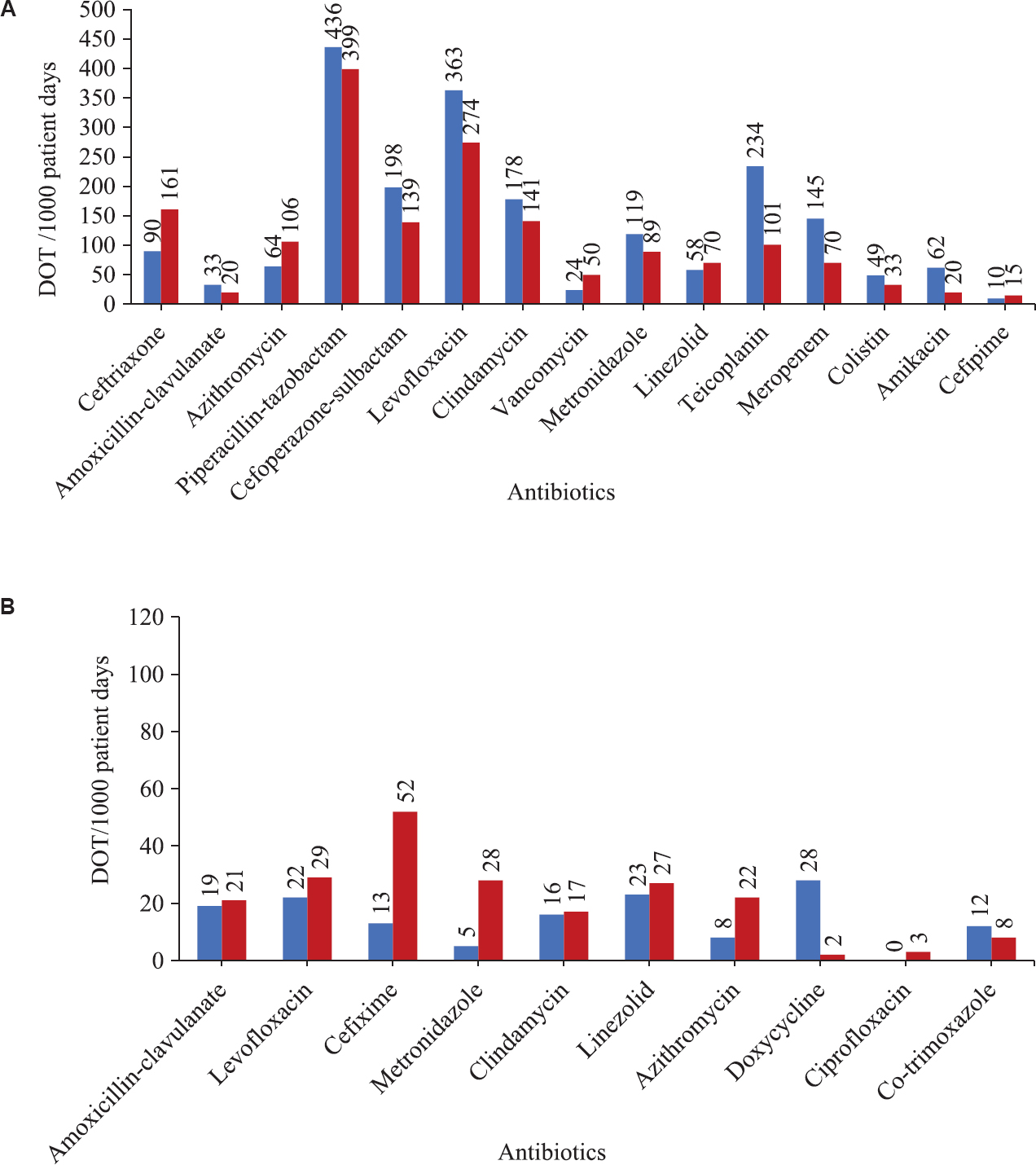
- Comparison of the antibiotic consumption measured as days of therapy (DOT) per 1000 patient days for intravenous (A) and oral (B) antibiotics.
Discussion
In our study, patient risk stratification was adapted to identify patients at risk of having infection by MDR organisms. The maximum number of patients belonged to patient type 2, followed by 3 and the least to patient type 1, in both the phases. This was because the study was conducted in a tertiary care hospital; most of the cases were acute and were referred from other places and already had antibiotic exposure.
The most common indication for the start of antibiotic was pneumonia during both the phases followed by UTI in phase 1 and sepsis with unknown focus in phase 2. This was different from a study conducted in six acute care hospitals in the United States, in which antibiotic use was primarily for respiratory (27.6% of prescriptions) followed by gastrointestinal (13.1%) infections24.
The most common antibiotic prescribed was piperacillin-tazobactam followed by levofloxacin. This was observed in both the phases. This was similar to the study done in the acute care hospitals in the US in which fluoroquinolones, vancomycin and antipseudomonal penicillins were the most frequently used antibiotics, specifically for respiratory indications24. The use of vancomycin was much less in our study as nearly 44 per cent patients had renal dysfunction at presentation with the mean creatinine of 2.67 and 2.31 mg/dl during phases 1 and 2, respectively.
Most of the earlier studies on ASP have measured antibiotic consumption using defined daily dosage (DDD)/1000 days. In our study, DOT/1000 days was measured because it was not influenced by changes in the recommended DDD or variations between the DDD and the preferred daily dose and by dose-adjustment in renal insufficiency25. The use of first-line antibiotics such as ceftriaxone and azithromycin increased in the ASP phase compared to phase 1. The use of higher antibiotics such as colistin, teicoplanin and levofloxacin decreased. This may indicate the benefit of patient risk stratification in avoiding the inadvertent use of higher antibiotics. In a study done to measure the antimicrobial use in 130 US hospitals25, antimicrobial drug consumption was measured using DOT per 1000 patient days and DDD per 1000 patient days. In comparison, the baseline antibiotic consumption from the observation phase (measured as DOT per 1000 days) in our study was higher for broad-spectrum antibiotics, including piperacillin-tazobactam, cefoperazone, levofloxacin and penems, which is a cause for concern. This might be because our study was limited to medicine wards having many patients with infection.
There was a significant decrease in the mean number of antibiotics used per person. A study conducted in north India showed a decrease in mean number of antibiotics used per patient from 2.37 to 1.97, after ASP efforts15. There was a significant improvement in the number of blood cultures sent on day one, before the start of antibiotics and during the ASP phase.
The opportunities for de-escalation were based on the judgment of the clinical condition of the patient. No predefined objective criteria were set for the assessment of the same. There was a favourable response towards de-escalation during the intervention phase, as seen by the type of antibiotic decisions taken during the first two weeks of patient admission. There were 76 de-escalation decisions taken during the observation phase, whereas 123 decisions on de-escalation either in the form of stopping of an antibiotic or change from broad to narrow spectrum were taken during the implementation of the stewardship. This implied that there was a frequent review of the antibiotics during phase 2 and that the implementation of 'day 3 bundle' was beneficial, as indicated by the de-escalation decisions which increased from 32 to 59 during days 4-7.
The conversion from iv to oral therapy was tracked separately and those patients who satisfied the criteria for iv to oral switch and on antibiotics with good oral bioavailability were considered. 'Day 3 bundle' was implemented exclusively for the improvement in the review of empirically started antibiotics and thus increased the opportunities for antibiotic de-escalation during the stewardship phase. There was a significant improvement in the appropriate antibiotic usage per person during the intervention phase. The overall total appropriate antibiotic usage increased by nearly 20 per cent. Various studies have shown that nearly 30–50 per cent of the antibiotic use in hospitals is unnecessary or inappropriate2627. The baseline inappropriateness found during the observation phase was 31 per cent. A study conducted in a tertiary care teaching hospital in central India revealed that appropriate antibiotic usage was only 30 per cent28. Another study conducted in north India, showed a baseline irrational antibiotic usage between 39 and 79 per cent15. In our study, the baseline appropriateness observed was higher at nearly 67 per cent. A further 20 per cent improvement above this level indicated that the ASP efforts were effective. There was a significant decrease in the mean total stay in the hospital between the two groups (17 vs. 14 days). In the study for community-acquired pneumonia patients, the median length of stay was reduced using education intervention and apprenticeship (16.5-13 days)29.
Our study had several limitations. The conclusions drawn from this pilot study may be less definitive as the variables are less controlled due to lack of randomization. Due to the non-randomized study design, there was a potential for bias from confounding. Furthermore, the socio-economic and cultural background of the patients studied in the two groups was not looked into. The phase 2 i.e. implementation of ASP was restricted to a single unit for intensive implementation. A more focused approach with a larger sample size might have shown better results. Data on ADRs related to antibiotics could not be acquired in our study due to inadequate documentation. Similar studies need to be carried out at multiple centres on a larger scale for a far-reaching impact. The results of our study need to be evaluated in a larger context, and internal and external validity may be best assessed through the replication of the results in a variety of clinical settings.
To conclude, the constitution of local antibiogram is an important step in the implementation of ASP. There is a need for ASP to be institutionalized with hospital-specific guidelines.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ. 2010;340:C2096.
- [Google Scholar]
- 2016. Core Elements of Hospital Antibiotic Stewardship Programs | Get Smart for Healthcare | Centers for Disease Control and Prevention. Available from: http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html
- 2016. Infectious Diseases Society of America: New Antimicrobial Stewardship Guideline. Available from: https://www.idsociety.org/New_Antimicrobial_Stewardship_Guideline_20 16/
- The Chennai declaration: India's landmark national commitment to antibiotic stewardship demonstrates that 'truth alone triumphs' J Antimicrob Chemother. 2013;68:1453-4.
- [Google Scholar]
- 2016. Global Antibiotic Resistance Partnership. Washington D.C., New Delhi: Center for Disease Dynamics, Economics & Policy; Available from: http://www.cddep.org/garp/home
- Access to effective antimicrobials: A worldwide challenge. Lancet. 2016;387:168-75.
- [Google Scholar]
- Emergence of high levels of extended-spectrum-beta-lactamase-producing gram-negative bacilli in the Asia-Pacific region: Data from the study for monitoring antimicrobial resistance trends (SMART) program, 2007. Antimicrob Agents Chemother. 2009;53:3280-4.
- [Google Scholar]
- Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602.
- [Google Scholar]
- Device-associated nosocomial infection rates in intensive care units of seven Indian cities. Findings of the International Nosocomial Infection Control Consortium (INICC) J Hosp Infect. 2007;67:168-74.
- [Google Scholar]
- 2011. Situation Analysis: Antibiotic Use and Resistance in India. Washington D.C: Center for Disease Dynamics, Economics & Policy; Available from: http://c ddep.org/publications/situation_analysis_antibiotic_use_and_resistance_india
- Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;2:CD003543.
- [Google Scholar]
- Appropriateness of antibiotic prescription for targeted therapy of infections caused by multidrug-resistant bacteria: Assessment of the most common improper uses in a tertiary hospital in Southern Italy. Infez Med. 2017;25:224-33.
- [Google Scholar]
- Control of multidrug resistant bacteria in a tertiary care hospital in India. Antimicrob Resist Infect Control. 2012;1:23.
- [Google Scholar]
- “Save antibiotics, save lives”: An Indian success story of infection control through persuasive diplomacy. Antimicrob Resist Infect Control. 2012;1:29.
- [Google Scholar]
- Antibiotic stewardship in a tertiary care hospital of a developing country: Establishment of a system and its application in a unit-GASP initiative. Infection. 2016;44:651-9.
- [Google Scholar]
- Establishing Antimicrobial Resistance Surveillance & Research Network in India: Journey so far. Indian J Med Res. 2019;149:164-79.
- [Google Scholar]
- Policy document on antimicrobial stewardship practices in India. Indian J Med Res. 2019;149:180-4.
- [Google Scholar]
- 2011. National Policy for the Containment of Antimicrobial Resistance, India. New Delhi: Ministry of Health & Family Welfare, Government of India; Available from: https://mohfw.gov.in/sites/default/f iles/3203490350abpolicy%20%281%29.pdf
- 2016. National Treatment Guidelines for Antimicrobial Use in Infectious Diseases. New Delhi: Ministry of Health & Family Welfare, Government of India; Available from: http://pbhealth.gov.in/AMR_guideline7001495889.pdf
- Implementation Resources. Available from: https://www.cdc.gov/antibiotic-use/core-elements/implementation.html
- Current blood culture methods and systems: Clinical concepts, technology, and interpretation of results. Clin Infect Dis. 1996;23:40-6.
- [Google Scholar]
- Design of a 'day 3 bundle' to improve the reassessment of inpatient empirical antibiotic prescriptions. J Antimicrob Chemother. 2008;61:1384-8.
- [Google Scholar]
- Indications and types of antibiotic agents used in 6 acute care hospitals, 2009-2010: A pragmatic retrospective observational study. Infect Control Hosp Epidemiol. 2016;37:70-9.
- [Google Scholar]
- Measurement of adult antibacterial drug use in 130 US hospitals: Comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664-70.
- [Google Scholar]
- A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164:1451-6.
- [Google Scholar]
- Study of prescribing pattern of antimicrobial agents in medicine intensive care unit of a teaching hospital in central India. J Assoc Physicians India. 2012;60:20-3.
- [Google Scholar]
- Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18:389-95.
- [Google Scholar]






