Translate this page into:
Comparative study of clinical features of patients with celiac disease & those with concurrent celiac disease & type 1 diabetes mellitus
Reprint requests: Dr Sanjay Kumar Bhadada, Department of Endocrinology, Postgraduate Institute of Medical Education and Research, Chandigarh 160 012, India e-mail: bhadadask@rediffmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Celiac disease (CD) and type 1 diabetes mellitus (T1DM) share a common genetic locus and clinical manifestations. The present study was planned to compare clinical, biochemical and hormonal profiles of patients with CD and CD with T1DM.
Methods:
Records of CD patients with age ≤20 yr, available anthropometric measurements, haematological, biochemical and hormonal workup with tissue transglutaminase IgA antibody and duodenal biopsy (Marsh grade) were screened. The patients were divided into two groups i.e., CD alone (Group A) and concurrent CD with T1DM (Group B).
Results:
One hundred and nine patients of CD (57 male) with a mean age of 14.9±2.9 yr were evaluated. Of these, 86 (78.9%) patients had CD alone and 23 (13 females) (21.1%) patients had CD with T1DM. The age at diagnosis and the lag duration for the diagnosis of CD were 11.5±4.6 versus 13.8±3.4 yr (P<0.05) and 48.8 ±43.3 versus 20.2±31.8 months (P<0.05) in groups A and B, respectively. The most common histopathological grade was type 3b (59.2%) in group A and type 2 (42.1%) in group B. Short stature (87% vs. 40.9%; P<0.01), anaemia (80.9% vs. 45%, P<0.01) and delayed puberty (61.9% vs. 29.4%; P<0.01) were more common in group A.
Interpretation & conclusions:
Patients with CD alone have a longer lag time to diagnosis and consequent sequel in the form of anaemia, short stature and delayed puberty, as compared to patients with concurrent CD and T1DM.
Keywords
Anaemia
celiac disease
delayed puberty
diabetes mellitus
short stature
Celiac disease (CD) is being increasingly recognized, and the prevalence of CD in north India has been reported to be around 1 per cent1. Prevalence of CD based on cross-sectional and population-based studies in the Western world varies from 0.3 to 2 per cent in adults to as high as 6 per cent in children23. The prevalence of CD is higher in patients with pre-existing autoimmune disorders such as type 1 diabetes mellitus (T1DM), autoimmune hypothyroidism and premature ovarian failure as compared to the healthy population. We reported the prevalence of CD in T1DM patients in north India as 11.1 per cent, majority (90.5%) of whom had pre-existing diabetes and CD was diagnosed later4.
CD and T1DM share common genetic loci on short arm of chromosome 6567 and coexist because of common genetic background. Two most important predisposing factors for CD are the presence of susceptible human leucocyte antigen (HLA) alleles i.e., HLA-DQ2 and DQ8 found in nearly 98 per cent of patients and exposure to gluten8. About 95 per cent individuals who develop diabetes have either DR3-DQ2 or DR4-DQ8, similar to approximately 40 per cent of the general population9.
CD may manifest at any age in either sex. Its clinical presentation varies from the silent asymptomatic form diagnosed during routine screening in association with the various autoimmune disorders, to the symptoms such as diarrhoea, constipation, short stature, anaemia and fractures101112. However, T1DM is usually symptomatic in children with osmotic symptoms or acute metabolic complication in the form of ketoacidosis. Patients with pre-existing T1DM and CD may remain undiagnosed as some of the common symptoms associated with CD may be considered as a part of the chronic complications of diabetes such as nocturnal diarrhoea, alternate diarrhoea and constipation, weight loss, anaemia, poor growth and delayed puberty.
In view of scarcity of data and the common clinical manifestations and genetic background of CD and T1DM, the present study was planned to compare clinical, biochemical and hormonal profile in patients with CD alone and concurrent CD with T1DM.
Material & Methods
This retrospective study was conducted at the department of Endocrinology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. The data of all consecutive patients (CD alone and CD with T1DM below 20 yr of age) who came during April 2008 and March 2013, were included and analyzed. The study was cleared by the institute's Ethics Committee. Written informed consent was obtained from all patients or from their parents. Patients with age ≤20 yr and diagnosed with CD were divided into two groups on the basis of the absence (Group A) or presence of T1DM (Group B).
Diagnostic criteria: The diagnosis of CD was made as per modified European Society of Pediatric Gastroenterology, Hepatology and Nutrition criteria13, and the diagnosis of T1DM was as per the American Diabetes Association criteria14. Short stature was defined as (i) height ≥2.5 standard deviation (SD) below the mean for chronological age and mid-parental height, or (ii) height below 5th percentile for chronological age using the Centers for Disease Control and Prevention growth charts15. Anaemia was defined as per the WHO criteria (haemoglobin <13 g/dl in males and <12 g/dl in females)16. Hypothyroidism was diagnosed on the basis of elevated thyroid-stimulating hormone (TSH) and low T4 levels for the laboratory reference. Delayed puberty was diagnosed on the basis of absence of secondary sexual characteristics by the age of 14 yr in boys and 13 yr in girls.
Diagnostic workup: The patients who presented with symptoms suggestive of CD were screened with tissue transglutaminase IgA-antibodies (tTGAb) and those found to have tTGAb positivity underwent endoscopy. The mucosal details in the descending part of the duodenum were recorded, and three biopsies were taken during the endoscopic procedure. The biopsy specimens were submitted to routine histological examination and haematoxylin-eosin staining. The degree of intestinal villous atrophy and crypt hyperplasia was evaluated, and the results compatible with Class II and III (a, b or c) of Marsh classification17 as modified by Oberhuber et al18 were considered diagnostic for CD. Routine haemogram and biochemical tests, including fasting calcium profile, liver and renal function tests, were performed. The hormonal workup included the thyroid and gonadal function tests (where applicable), serum cortisol and glycated haemoglobin (HbA1c).
Assay & intervention: TSH, T4, luteinizing hormone, follicle-stimulating hormone, 17-β estradiol, testosterone and cortisol were measured by electrochemiluminescence immunoassay sandwich method (ECLESYS-2010, Roche Diagnostics, Germany). HbA1c levels were measured by high-performance liquid chromatography method (ion exchange chromatography, D-10, Bio-rad, USA). Anti-tTGAb was done using enzyme-linked immunosorbent assay (D-tek, Blue Well, Belgium). All the patients in either group received standard treatment.
Statistical analysis: SPSS (Release 10.01, pc windows; SPSS Inc., Chicago, IL, USA) was used for data analysis. Normality of data was checked by Kolmogorov–Smirnov test of normality. For normally distributed data, means were compared using Student's t test for two groups. For skewed data, Mann–Whitney test was applied for comparison between two groups and proportions were compared using Chi-square test or Fisher's exact test whichever was applicable.
Results
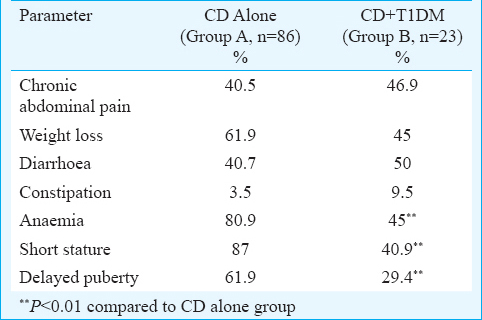
One hundred and nine patients (M: F 57: 52) with age ≤20 yr (mean age 14.9±2.9 yr) with available complete anthropometric, haematological and biochemical details were analyzed. CD alone was seen in 86 (78.9%) patients (Group A) and 23 (21.1%) patients (Group B) had concurrent CD with T1DM. The mean age (±SD) at presentation was 15.1±2.9 and 13.7±3.4 yr and the age at diagnosis of CD was 13.8±3.4 and 11.5±4.6 yr in group A and B, respectively. The lag time between the symptoms suggestive of CD and the diagnosis of CD was 48.8±43.3 months (Group A) versus 20.2±31.8 months (Group B) (P<0.05). The histopathological grading (Marsh classification) of duodenal biopsy specimens of the patients in the two groups is shown in Table I. Among the presenting manifestations, the gastrointestinal symptoms in the form of diarrhoea, constipation and weight loss were similar in the two groups, however, short stature (87% vs. 40.9%, P<0.01), anaemia (80.9% vs. 45%, P<0.01) and delayed puberty (61.9% vs. 29.4%, P<0.01) were significantly more common in group A as compared to group B (Table II).
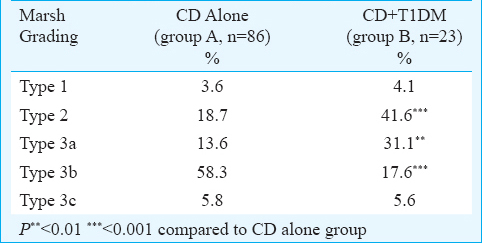
Amongst the biochemical parameters, the haemoglobin level was significantly lower in group A compared to group B (8.3±2.4 g/dl vs. 10.1±3.2 g/dl, respectively, P<0.018). The proportion of patients with elevated transaminases were not different between the two groups. The mean tTGAb levels were 118.3±112.9 and 139.5±111.1 mIU/l (P=0.43) in groups A and B, respectively. The presence of endocrine abnormalities in the form of hypothyroidism, hypoadrenalism and rickets was not significantly different in the two groups (Figure).
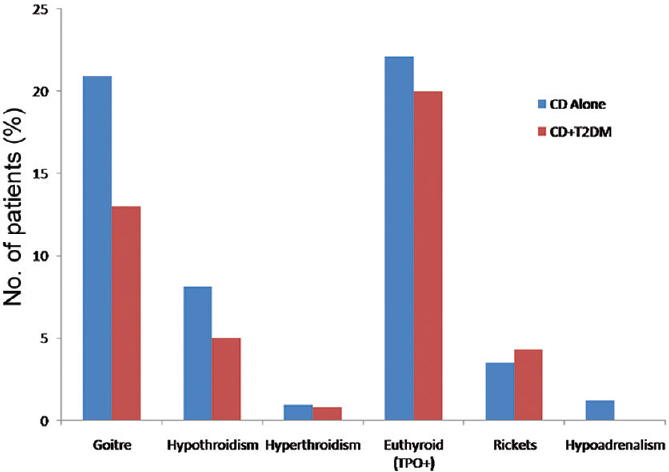
- Endocrinological abnormalities in the two groups of patients.
Discussion
The present study highlights the differences in the clinical presentation and biochemical parameters between patients with CD alone in comparison with the patients having concurrent CD and T1DM. The age at diagnosis and the lag time to the diagnosis of CD were significantly higher in patients with CD alone. Patients with CD have more advanced disease at presentation, with the consequent sequel of anaemia, short stature and delayed puberty, unlike CD with T1DM.
CD was diagnosed earlier in patients with T1DM in comparison to patients with CD alone by virtue of routine screening for CD in patients with pre-existing T1DM. The delay in diagnosis of CD in CD alone patients could be due to lack of awareness among the physicians, low index of suspicion and absence of structured and routine screening programmes for CD. In addition, relatively subtle, non-specific and atypical gastrointestinal manifestations of CD are frequently assumed to be because of diabetic autonomic neuropathy in T1DM. The prevalence of silent CD increases with the passage of time and less than one-sixth of the patients have clinically manifest CD at the time of diagnosis of T1DM and most patients are detected on screening419. In a community-based study from the USA, the mean age at diagnosis of CD was observed to be 36.7 yr though a majority of patients had been diagnosed to have T1DM below the age of 18 yr20. The lag period between the symptoms and the diagnosis of CD was observed significantly longer in patients with CD alone in the present study because of the non-specific symptoms of CD as well as low index of suspicion.
T1DM was observed in 21.1 per cent patients with CD in the present study as compared to 11.2 per cent of patients who were known to have CD at the time of diagnosis of T1DM in our previous study4. This could be due to the inherent bias, being a hospital-based study rather than a community survey. The prevalence of CD in patients with known T1DM has been reported to be 1.6-12.3 per cent from various parts of the world212223.
Short stature was twice more common in patients with CD alone than CD with T1DM in the present study. CD has been recognized as the most common cause of short stature in north India2425. The possible explanation for higher occurrence of short stature in CD alone patients at presentation could be due to the delay in recognizing CD in the absence of T1DM. CD was diagnosed on routine screening in majority of the patients who had pre-existing T1DM. Patients with both CD and T1DM had an earlier diagnosis and commencement of treatment of CD with gluten-free diet (GFD), better compliance to GFD and a regular follow up, as compared to the patients with CD alone. The short stature in CD is otherwise multifactorial, including malabsorption leading to protein-calorie malnutrition, defective absorption of calcium and vitamin D, growth hormone resistance, hypothyroidism and hypogonadism, which further attenuate the pubertal growth spurt17. However, constitutional delay in growth cannot be ruled out in these patients as this was a retrospective analysis.
Anaemia was nearly twice more common in CD alone patients (80.9%) and this could possibly due to the low index of suspicion, delay in the diagnosis of CD corroborated by more severe histopathological Marsh grading as compared to CD with T1DM patients as reported in literature also26. Anaemia in CD patients is attributed to iron deficiency, because the site of maximal iron absorption is the duodenum, which is also the predominant site of mucosal damage in CD27. The other reasons for anaemia in CD patients are vitamin B12 or folate deficiency due to associated parietal cell antibody causing pernicious anaemia, concurrent hypothyroidism, systemic inflammation and hyposplenism2728. However, the type of anaemia was not characterized in the present study as serum iron, vitamin B12 and folate levels were not measured.
Delayed puberty was more commonly observed in CD patients as compared to patients with CD and T1DM. Puberty is a physiological state determined by multiple factors such as body weight, bone age, nutrition and optimum hormonal milieu. Thus, delayed puberty in the present study could be due to longer duration of undiagnosed and untreated CD, a lower body mass index as also depicted by presentation with significant weight loss. However, the data regarding the family history of delayed puberty, skeletal skiagram for bone age in either of the groups were not available. Another possible reason for delayed puberty could be prevalent hypothyroidism. However, the presence of overt hypothyroidism in the two groups was similar.
The prevalence of other endocrinopathies, especially thyroiditis, is reported to be high in patients with CD and variably reported as 12.9-14 per cent1929. Overall, hypothyroidism and goitre were present in 8.2 and 19.3 per cent patients, respectively; however these were comparable between the two groups.
Serum anti-tTGAb levels were comparable in the two groups, in spite of more severe duodenal histopathology in CD alone patients. It has been shown that there is no correlation between the serology and the duration of undiagnosed disease, clinical manifestations and the histopathological grade13. Calcium levels were comparable between the two groups as many of the patients were on calcium and vitamin D supplementation.
Our study had several limitations. Retrospective nature of the study limited the retrieval of relevant data regarding all the parameters. IgA deficiency was not looked for in the patients and only IgA anti-tTG levels were measured. The lack of control group was another limitation which could have added a bias as few abnormalities, for instance anaemia were common in the general population.
In conclusion, our study showed that patients with CD alone remained undiagnosed for a longer period and consequently had sequel in the form of anaemia, short stature and delayed puberty at diagnosis as compared to patients with concurrent CD and T1DM.
Conflicts of Interest: None.
References
- Prevalence of celiac disease in the northern part of India: A community based study. J Gastroenterol Hepatol. 2011;26:894-900.
- [Google Scholar]
- American gastroenterological association (AGA) institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981-2002.
- [Google Scholar]
- Celiac disease in the developing countries: A new and challenging public health problem. World J Gastroenetrol. 2007;13:2153-9.
- [Google Scholar]
- Prevalence and clinical profile of celiac disease in type 1 diabetes mellitus in North India. J Gastroenterol Hepatol. 2011;26:378-81.
- [Google Scholar]
- Prevalence of celiac disease in children with type 1 diabetes mellitus increased in the mid-1990 s: An 18-year longitudinal study based on anti-endomysial antibodies. J Pediatr Gastroenterol Nutr. 2008;46:612-4.
- [Google Scholar]
- Co-existence of coeliac disease and insulin-dependent diabetes mellitus in children: Screening sera using an ELISA test for gliadin antibody. Aust N Z J Med. 1992;22:256-60.
- [Google Scholar]
- Prevalence of coeliac disease in diabetic children and adolescents. A multicentre study. Eur J Pediatr. 1988;148:113-7.
- [Google Scholar]
- HLA susceptibility genes in celiac disease: Genetic mapping and role in pathogenesis. Gastroenterology. 1993;105:910-22.
- [Google Scholar]
- Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797-801.
- [Google Scholar]
- Celiac disease: A missed cause of metabolic bone disease. Indian J Endocrinol Metab. 2012;16:780-5.
- [Google Scholar]
- Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990;65:909-11.
- [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care. 2011;34(Suppl 1):S11-61.
- [Google Scholar]
- Nutritional anemias: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1968.
- Morphology of the mucosal lesion in gluten sensitivity. Baillieres Clin Gastroenterol. 1995;9:273-93.
- [Google Scholar]
- The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185-94.
- [Google Scholar]
- Celiac disease in type 1 diabetes mellitus in a North American community: Prevalence, serologic screening, and clinical features. Mayo Clin Proc. 2005;80:1429-34.
- [Google Scholar]
- Prevalence and clinical features of celiac disease in 950 children with type 1 diabetes in France. Diabetes Metab. 2007;33:453-8.
- [Google Scholar]
- Diabetes and autoimmune diseases: Prevalence of celiac disease in children and adolescents with type 1 diabetes mellitus. Arq Bras Endocrinol Metabol. 2008;52:1461-5.
- [Google Scholar]
- Celiac disease in African American children with type 1 diabetes mellitus in inner city Brooklyn. Pediatr Endocrinol Rev. 2008;5(Suppl 4):994-8.
- [Google Scholar]
- Does every short stature child need screening for celiac disease? J Gastroenterol Hepatol. 2008;23(8 Pt 2):e353-6.
- [Google Scholar]
- Changing scenario in aetiological profile of short stature in India-growing importance of celiac disease: A study from tertiary care centre. Indian J Pediatr. 2011;78:41-4.
- [Google Scholar]
- The clinical pattern of subclinical/silent celiac disease: An analysis on 1026 consecutive cases. Am J Gastroenterol. 1999;94:691-6.
- [Google Scholar]
- Anemia in celiac disease is multifactorial in etiology. Am J Hematol. 2007;82:996-1000.
- [Google Scholar]
- Prevalence of thyroid disorders in untreated adult celiac disease patients and effect of gluten withdrawal: An Italian multicenter study. Am J Gastroenterol. 2001;96:751-7.
- [Google Scholar]






