Translate this page into:
Development of polymerase chain reaction-based diagnostic tests for detection of Malsoor virus & adenovirus isolated from Rousettus species of bats in Maharashtra, India
Reprint requests: Dr Devendra T. Mourya, ICMR-National Institute of Virology, 20-A, Dr. Ambedkar Road, Pune 411 001, Maharashtra, India e-mail: directorniv@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Bats are recognized as important reservoirs for emerging infectious disease and some unknown viral diseases. Two novel viruses, Malsoor virus (family Bunyaviridae, genus, Phlebovirus) and a novel adenovirus (AdV) (family, Adenoviridae genus, Mastadenovirus), were identified from Rousettus bats in the Maharashtra State of India. This study was done to develop and optimize real time reverse transcription - polymerase chain reaction (RT-PCR) assays for Malsoor virus and real time and nested PCR for adenovirus from Rousettus bats.
Methods:
For rapid and accurate screening of Malsoor virus and adenovirus a nested polymerase chain reaction and TaqMan-based real-time PCR were developed. Highly conserved region of nucleoprotein gene of phleboviruses and polymerase gene sequence from the Indian bat AdV isolate polyprotein gene were selected respectively for diagnostic assay development of Malsoor virus and AdV. Sensitivity and specificity of assays were calculated and optimized assays were used to screen bat samples.
Results:
Molecular diagnostic assays were developed for screening of Malsoor virus and AdV and those were found to be specific. Based on the experiments performed with different parameters, nested PCR was found to be more sensitive than real-time PCR; however, for rapid screening, real-time PCR can be used and further nested PCR can be used for final confirmation or in those laboratories where real-time facility/expertise is not existing.
Interpretation & conclusions:
This study reports the development and optimization of nested RT-PCR and a TaqMan-based real-time PCR for Malsoor virus and AdV. The diagnostic assays can be used for rapid detection of these novel viruses to understand their prevalence among bat population.
Keywords
Adenovirus
bat
diagnostic assays
Malsoor virus
polymerase chain reaction
Rousettus
Bats are known to be reservoirs of many zoonotic viruses. Hunting of bats for food and encroachment of humans upon bat habitat has led to increased human contact with bats. This is how zoonotic disease outbreaks can begin, which ultimately leads to emergence of new infectious diseases in new species12345. Bats are widely recognized as important reservoirs for emerging infectious diseases and some other unknown viral diseases, which can cross species barriers6. A large number of novel zoonotic viruses have been isolated from bats7. It is now widely known that viruses in bats are of high prevalence, particularly from the families Rhabdoviridae, Coronaviridae, Astrovirdae, Paramyoxviridae, Filoviridae, Reoviridae, Adenoviridae and Herpesviridae8. A compiled database of viruses in bats and rodents indicated that bats are special in their ability to host more zoonotic viruses per species as compared to rodents9. The fruit bats are known to be responsible for emergence of novel viruses. Rousettus genus is a member of the suborder Megachiroptera10. They are sometimes referred to as dog-faced fruit bats or flying foxes. The genus consists of ten species that range over most of Africa to Southeast Asia and the islands of the South Pacific10. Due to the abundance of these bats, particularly in the tropics, bat-human interface is the important niche for pathogen spill-over and emergence. Sensitive and specific diagnostic tests to discriminate bat-borne viruses are essential for disease surveillance and to improve the prevention and control of potential future outbreaks caused by bat-borne viruses.
During hunting for highly infectious pathogens such as Ebola-Reston, Marburg, Nipah and other possible viruses in Mahabaleshwar, Maharashtra State of India, two viruses, adenovirus (AdV) and Malsoor virus, were identified from Rousettus leschenaulti bats by the ICMR-National Institute of Virology (NIV), Pune, India. Adenoviruses are members of the family Adenoviridae and genus Mastadenovirus. They are non-enveloped viruses with a double-stranded, linear DNA genome. Mastadenoviruses infect only mammals and can be distinguished from members of other AdV genera traditionally by serology and more recently by genome organization characteristics and phylogenetic distances11. Malsoor virus, a novel Phlebovirus belonging to family Bunyaviridae has three genetic segments. The small segment (S) codes for the viral N protein and a non-structural protein (NSs) and uses an ambisense coding strategy. The medium-sized segment (M) codes for a precursor of the viral glycoproteins and NSm components. The product of the largest segment (L) is the viral RNA polymerase. It is genetically closely related to highly pathogenic Phleboviruses such as severe fever with thrombocytopenia syndrome virus and Heartland virus (Missouri virus)11.
Both of these are novel viruses and have been detected in India. However, there is no information available related to their predominance in bats and disease-causing potential in humans. In this context, it becomes essential to develop diagnostic assays to identify these agents from bats, which would help in estimating the prevalence of these viruses. This study reports the development and optimization of the real-time reverse transcription polymerase chain reaction (RT-PCR) and RT-PCR assays for Malsoor virus and real-time PCR and nested PCR for AdV isolated from Rousettus bats.
Material & Methods
During a survey of bats from Maharashtra state, India, from 2011-2014, two virus isolates were obtained from R. leschenaulti species of bats by the NIV, Pune team1213. These virus isolates were propagated in VeroCCL81 cells (ATCC CCL, 81 obtained from National Centre for Cell Science, Pune, India), and were used in this study for the development of diagnostic assay for Malsoor virus and AdV.
Prior approval was obtained from the Institutional Animal Ethical Committee of NIV, Pune, to conduct this study.
Nucleic acid extraction: Total RNA was extracted from 140 μl of tissue culture grown (VeroCCL81 cells) Malsoor virus stock using a QIAamp viral RNA kit (QIAGEN, Inc., Valencia, CA, USA), according to the manufacturer's protocol. RNA was eluted in 50 μl of nuclease-free water. DNA was extracted from tissue culture grown (VeroCCL81 cells) AdV stock using QIAamp DNA mini Kit (QIAGEN, Inc., Valencia, CA, USA), according to the manufacturer's protocol. DNA was eluted in 50 μl of nuclease-free water. Both RNA and DNA were stored at −20°C until further use.
Oligonucleotide design targeting Malsoor and adenovirus: Alignment of all the sequences from NCBI GenBank database (https://www.ncbi.nlm.nih.gov/genbank/) for polymerase gene for AdV and S-segment for Malsoor virus was performed for designing primers and probes. The probe was labelled with carboxyfluorescein at the 5′ ends and black hole quencher (BHQ) at the 3′ ends.
To amplify the in vitro transcript of Malsoor virus, a standard T-7 promoter sequence (IDT, USA) was attached to the forward primer of S-segment. Similarly, using the available polymerase gene nucleotide sequence of adenoviruses, primer/probes were designed (IDT, USA). Parameters such as G+C content, primer length, melting temperatures, sequence complexity, self-complementarity and secondary structure formation were taken into consideration. PCR primers and probes were custom designed using OLIGO Calc Oligonucleotide properties calculator software (http://www.basic.northwestern.edu/biotools/oligocalc.html). The primer sequences used for RT-PCR, nested RT-PCR and real-time RT-PCR for Malsoor and nested PCR and real-time PCR for AdV are summarized in Tables I and II.

Standardization of nested RT-PCR for Malsoor and nested PCR for adenovirus: Amplification of partial S-segment of Malsoor virus was carried out using gene-specific primers (NExtF192 and NExtR648). The first reverse transcription was carried out at 50°C for 30 min, 94°C for two minutes followed by 40 cycles of 94°C for 15 sec, 49°C for 30 sec, 68°C for five minutes and a final extension at 68°C for 10 min. The above reactions were carried out using SuperScript® III Single-Step RT-PCR system with Platinum® Taq Highfidelity (Invitrogen, USA), according to manufacturer's instructions. Nested PCR was carried out using 1 μl of the first PCR product with the same buffer volumes and temperature conditions as for the first PCR. Nested PCR was carried out using primers (NIntF227 and NIntR541).
Amplification of polymerase gene of AdV was carried out using specific primers (PolF outer and polRouter)14. The first PCR cycle was denaturation at 94°C for five minutes, followed by 35 cycles of oneminutes steps at 94, 55 and 72°C each and a final five minutes extension at 72°C. The above reactions were carried out using Platinum Taq DNA polymerase (Invitrogen, USA), according to manufacturer's instructions. Nested PCR was carried out using 1 μl of the first PCR product with the same buffer volumes and temperature conditions as for the first PCR. Nested PCR was carried out using primers (polRTF1 and polRTR1).
DNA sequencing of the amplified products was carried out using an ABI 3100 Automated DNA Sequencer and the Big Dye Terminator® Kit (Life Technologies, NY, USA). Both forward and reverse primer sequences were used for making a consensus sequence.
TaqMan real-time PCR optimization for Malsoor and adenovirus: In the initial optimization, TaqMan-based real time RT-PCR (qRT-PCR) was carried out with Malsoor virus-specific primer and probes and SuperScript® III Platinum One-Step qRT-PCR system (Invitrogen, USA). Two primer and probe sets were used for qRT-PCR. In brief, 5 μl of Malsoor viral RNA was added to 20 μl of the mixture containing 10 pM of each primer in one-step qRT-PCR. Every sample was run in triplicate. The thermal cycling conditions for Malsoor real-time RT-PCR using the first set of primers (NF1184, NR1279, NProbe1225) were 50°C for 30 min, 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 52°C for one minute. The thermal cycling conditions for Malsoor real-time RT-PCR using the second set of primers (Phlebo_S_F_169, Phlebo_S_R_239, Phelbo_S_Probe_191) were 50°C for 30 min, 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 60°C for one minute. The Malsoor viral RNA was used initially for standardization of real-time RT-PCR. Further, in vitro transcribed RNA was serially diluted and used for sensitivity of the assay. Similarly, the qPCR assay was optimized for adenovirus using same reagents and specific primers and probes. Real-time PCR for AdV was optimized using tissue culture-isolated AdV DNA. Five microlitres DNA was added to optimized reaction mix containing the primer, probe mix for polymerase gene. Thermal cycling conditions followed were 95°C for five minutes, 95°C for 15 sec and 50°C for one minute. These conditions were repeated for 40 cycles.
In vitro transcription of Malsoor viral RNA: To determine the sensitivity of the TaqMan assay for Malsoor virus, S-gene-specific RNA was synthesized by in vitro transcription. A T-7 promoter sequence was attached to the forward primer of S-segment, and viral RNA was amplified by SuperScript® III One-Step RT-PCR System with Platinum® Taq (Invitrogen, Carlsbad, CA, USA) using Malsoor virus ‘S’-segment-specific primers,10 pmoles each (NT7proExtF192 and NExtR1487). In parallel, nuclease-free water was used as no template control. The desired PCR product was purified using QIAquick column extraction kit (Qiagen, Hilden, Germany). The purified PCR products were used for in vitro transcription of the S-segment using a Riboprobe® Kit (Promega, Madison, USA), according to manufacturer's protocol.
The synthesized RNA was treated with DNAse I (Qiagen, Hilden, Germany) to remove the remaining DNA molecules. The RNA was purified using Tripure reagent (Roche, USA) and further purified with RNAeasy purification Kit (Qiagen, Hilden, Germany) and eluted in diethyl pyrocarbonate (DEPC)-treated water.
Cloning of adenovirus polymerase gene: Bat AdV DNA was amplified using primers polF outerF+polR outerR (557 bp). This polymerase gene PCR product was cloned into pGEMT easy vector. Transformed clones were selected by colony PCR (Invitrogen, USA) and confirmed by DNA sequencing using BDTv 3.1 kit (Thermofisher, USA).
Cloned AdV polymerase gene was used for the optimization of diagnostic real-time PCR and sensitivity testing.
Sensitivity and specificity of the assay: Sensitivity assay assessment using in vitro-transcribed RNA (10−1-10−10 RNA copies) was carried out for Malsoor virus by RT-PCR and real-time RT-PCR. The sensitivity testing of the conventional PCR and real-time PCR for AdV was carried out using serial dilutions of cloned DNA. Data were analyzed for both viruses to determine the highest dilution of transcripts and plasmids capable of giving an amplification signal above the threshold and expected product size.
The specificity assay for primers of RT-PCR and real-time RT-PCR of Malsoor virus was performed using dengue 2, chikungunya and Ingwavuma viral RNA along with positive controls of Malsoor virus-infected PS, RD, BHK21, Bat embryo cell line and Vero E-6 cells. Nuclease-free water was used as negative control. Specificity of AdV PCR and real-time PCR assays was evaluated in terms of an ability to detect other Mastadenovirus represented by human AdV (NIV isolate no. 924621), as well as the presence or absence of non-specific amplification in various control specimens such ascontrol mouse tissue DNA (potential experimental model), control bat tissue DNA (field specimens) and Vero CCL81 tissue culture fluid (TCF) as negative control.
Screening of bat samples for bat adenovirus and Malsoor virus: A total of 69 (61 Rousettus & 8 Pteropus) bats collected during field excursions to Mahabaleshwar, Wagheshwar and Lawle of Maharashtra State were screened for the presence of bat AdV and Malsoor virus by optimized real-time PCR and nested PCR.
Results
Oligonucleotide design targeting Malsoor and adenovirus: The alignment of the highly conserved region of the S-segment among various Phlebovirus isolates was used for designing primers. Nested RT-PCR was designed to amplify 314 bp of nucleoprotein gene. The two primer-probe pairs were designed on the basis of a partial polymerase gene fragment sequence from the Indian bat AdV isolate (unpublished data NIV, 2010). AdV-nested PCR was designed to amplify 186 bp polymerase gene.
Standardization of nested RT-PCR for Malsoor and nested PCR for adenovirus: The standardization of nested RT-PCR was performed using the Malsoor virus isolate that produced the expected size of 456 bp in the first PCR and 314 bp in the nested PCR. Nested RT-PCR for Malsoor virus was standardized at primer concentration of 15 pmole, MgSO4 at 1.3 mM concentration and annealing temperature at 56°C. Forward and reverse sequences generated were aligned to make a consensus sequence, and the sequences for the partial NS protein of Malsoor isolate were submitted to the NCBI databank (acc. no. KF186499, KF186496.1).
For standardization of nested PCR, adenoviral isolate was used that amplified expected product size of 557 bp in the first PCR and 186 bp in the nested PCR. The PCR products were confirmed by DNA sequencing. The sequence generated from the first PCR productfor the polymerase gene was submitted to the NCBI databank (acc. no. HQ529709).
TaqMan real-time PCR optimization for Malsoor and adenovirus: Initially, two sets of primers were designed for Malsoor virus real-time RT-PCR. The first set of primers detected the target gene up to 10−5 dilution and the second set of primers detected the target gene up to 10−7 dilution with lower Ct values (threshold cycle, Ct=35) and better amplification at highest dilution of template. Of the two primer/probe combinations evaluated for AdV, the second set of primers demonstrated the expected amplification curve for isolated adenoviral DNA up to 10−7(Ct=37) at highest dilution of template.
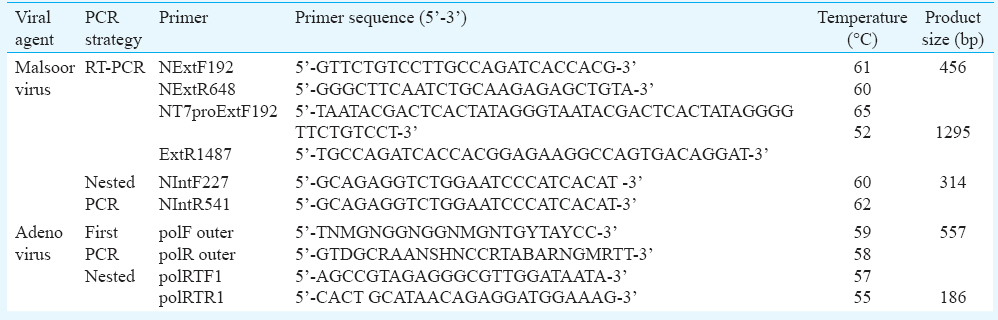
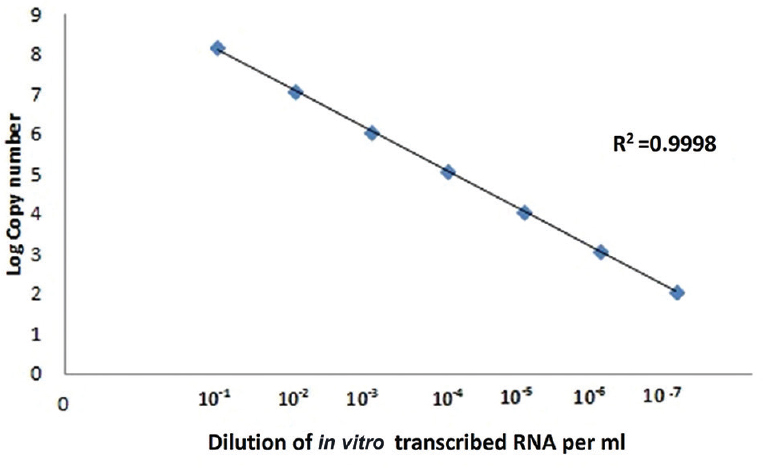
Sensitivity of RT-PCR, nested PCR and real-time PCR for Malsoor virus and adenovirus: In vitro transcription of Malsoor virus partial ‘S’ gene segment was carried out, and the expected product size of 1295 bp of Malsoor virus RNA was observed (Fig. 1). RNA diluted serially was used to determine the sensitivity of real-time RT-PCR. Real-time RT-PCR was found to be sensitive up to 10−7 dilution i.e., 100 copies of RNA (Fig. 2), while RT-PCR was sensitive up to 10−8 dilution i.e., 10 copies of RNA (Fig. 3). This proved that RT-PCR was more sensitive than real-time RT-PCR for Malsoor virus.

-
In vitro transcription of Malsoor virus segment product.
- Standard curve of Malsoor virus; log copy number of in vitro-transcribed RNA versus dilution of RNA.
- Sensitivity of Malsoor virus; nested reverse transcription polymerase chain reaction.
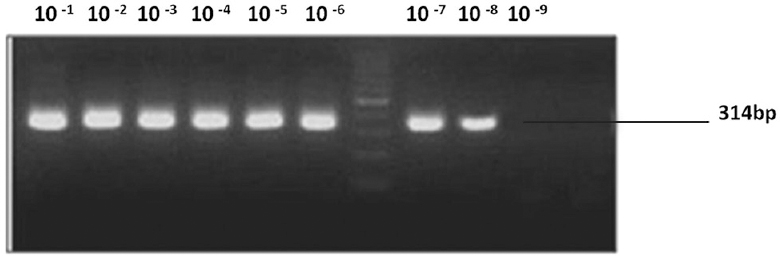
AdV primer sets, polF outer+polR outer, detected dilutions of DNA upto 10−6, and product of the expected size was observed in nested PCR. Nested primer set polRTF1 + polRTR1 amplified all dilutions of DNA upto 10−9 (Fig. 4).

- Sensitivity of adenovirus; nested polymerase chain reaction.
Optimized real-time PCR using bat AdV polymerase gene-specific primer/probe combination wassensitive up to 10−6 dilution which could successfully detect upto 457 copies of viral DNA (Fig. 5).
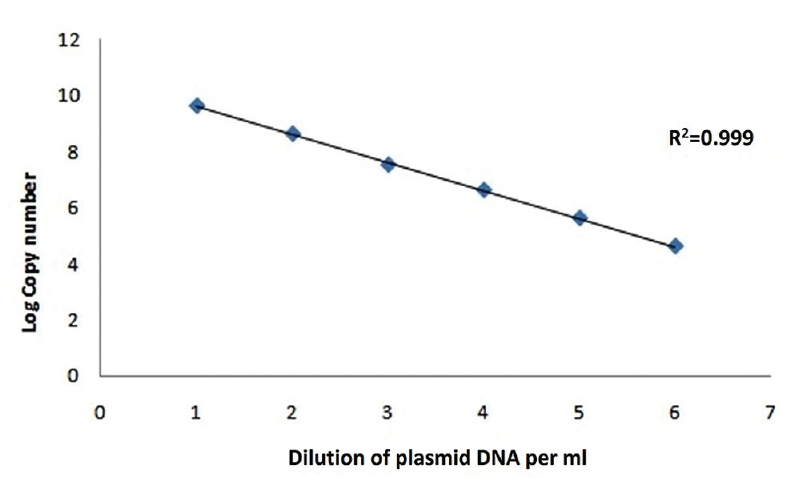
- Standard curve of adenovirus; plasmid log copy number versus plasmid dilutions.
Specificity of RT-PCR, nested PCR and real-time PCR for Malsoor virus and adenovirus: The primers designed for RT-PCR and real-time PCR showed specific amplificationfor Malsoor viral RNA only. The nested RT-PCR and real-time PCR were highly specific and could distinguish Malsoor virus from other dengue, chikungunya and Ingwavuma viral RNAs used in this study.
Specificity of nested PCR and real-time PCR was evaluated in terms of an ability to detect other Mastadenovirus represented by human AdV (NIV isolate no. 924621), as well as the presence or absence of non-specific amplification in various control specimens, namely, control mouse tissue DNA (potential experimental model), control bat tissue DNA (field specimens) and Vero CCL81 TCF as negative control. Both nested PCR and real-time PCR did not show any non-specific amplification.
Screening of bat samples: Liver/spleen samples of 69 bats (61 Rousettus & 8 Pteropus) were found to be negative for AdV DNA by real-time PCR. However, four liver/spleen samples of Rousettus bats were tested positive for novel bat AdV by nested PCR. Analyzed sequences showed 81 per cent identity with bat AdV FBV1 pol gene for DNA polymerase, partial coding sequence (CDS) (AB303301.1).
Of the liver/spleen samples of 69 bats, 19 were found to be positive for Malsoor virus by nested RT-PCR and three were found to be positive by real-time RT-PCR. Sequence analysis showed highest homology with known sequences of Phleboviruses and was found to be distinctly close to Heartland and severe fever with thromocytopenia syndrome (SFTS) viruses12.
Discussion
A large number of viruses have been isolated from different species of Rousettus bats including European bat lyssavirus, parainfluenza virus, chikungunya virus, Uganda virus and Yogue virus1112. Antibody against severe acute respiratory syndrome corona virus (SARS-CoV)-like viruses from bat was also detected in R. leschenaulti, suggesting that these bats also may support infection with SARS-CoV-like viruses12. Due to the abundance of these frugivorous bats, particularly in the tropics, the bat-human interface is the important niche for pathogen spillover and emergence1415161718.
Global surveillance of emerging infectious diseases has led to the isolation of numerous viruses in a variety of bat species. When a new virus is identified, PCR detection of viral nucleic acid plays an important role, and lack of early detection of emerging diseases may affect the timely diagnosis of the disease. In the present study, diagnostic assays were developed for some other novel viruses such as Malsoor and bat AdV discovered in India. The disease-causing potential of these viruses in humans is not known. These are novel viruses identified for the first time in Rousettus bats whose prevalence is also unknown. Looking at the close relatedness of Malsoor virus to SFTS and Heartland, which cause lethal disease in humans, it is essential to study the prevalence of this virus in potentially risky areas of India12. Similarly, it has been known that variant of AdV also has the potential to cause infection in humans11. The developed diagnostic assays have a good specificity and sensitivity and can be used in rapid screening of the bats specimens for Malsoor and bat AdV.
Acknowledgment
Authors thank Sarvshri Rajen J Lakra, Prasad Sarkale, Ms Pooja Shinde, Ms Divya Bhattad, Sarvshri Sanjay Gopale, Ganesh Chopade, Manjunath and Uttam K. Shende for technical help, and acknowledge the Principal Chief Conservator of Forests, Maharashtra, for rendering permission to collect samples from bats in Mahabaleshwar, Maharashtra, India.
Conflicts of Interest: None.
References
- Emerging virus diseases: Can we ever expect the unexpected? Emerg Microbes Infect. 2012;1:e46.
- [Google Scholar]
- Agricultural intensification, priming for persistence and the emergence of Nipah virus: A lethal bat-borne zoonosis. J R Soc Interface. 2012;9:89-101.
- [Google Scholar]
- Urban habituation, ecological connectivity and epidemic dampening: The emergence of Hendra virus from flying foxes (Pteropus spp.) Proc Biol Sci. 2011;278:3703-12.
- [Google Scholar]
- Emerging infectious diseases of wildlife – Threats to biodiversity and human health. Science. 2000;287:443-9.
- [Google Scholar]
- Bats: Important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006;19:531-45.
- [Google Scholar]
- 2011. Investigating the role of bats in emerging zoonoses: Balancing ecology, conservation and public health interest. FAO animal production and health manual No. 12. Rome, Italy: Food and Agriculture Organization of the United Nations. Available from: http://www.fao.org/docrep/014/i2407e/i2407e00.pdf
- Virology. What links bats to emerging infectious diseases? Science. 2005;310:628-9.
- [Google Scholar]
- A comparison of bats and rodents as reservoirs of zoonotic viruses: Are bats special? Proc Biol Sci. 2013;280:20122753.
- [Google Scholar]
- The natural history of the Egyptian fruit bat, Rousettus aegyptiacus, in Turkey (Mammalia: Chiroptera) Turk J Zool. 2008;32:11-8.
- [Google Scholar]
- Malsoor virus, a novel bat Phlebovirus, is closely related to severe fever with thrombocytopenia syndrome virus and heartland virus. J Virol. 2014;88:3605-9.
- [Google Scholar]
- Isolation of a novel adenovirus from Rousettus leschenaultii bats from India. Intervirology. 2012;55:488-90.
- [Google Scholar]
- Detection and analysis of six lizard adenoviruses by consensus primer PCR provides further evidence of a reptilian origin for the adenoviruses. J Virol. 2004;78:13366-9.
- [Google Scholar]
- Oral shedding of Marburg virus in experimentally infected Egyptian fruit bats (Rousettus aegyptiacus) J Wildl Dis. 2015;51:113-24.
- [Google Scholar]
- Isolation of a new parainfluenza virus from a frugivorous bat, Rousettus leschenaulti, collected at Poona, India. Am J Trop Med Hyg. 1971;20:125-30.
- [Google Scholar]
- Host phylogeny constrains cross-species emergence and establishment of rabies virus in bats. Science. 2010;329:676-9.
- [Google Scholar]
- Managing emerging diseases borne by fruit bats (flying foxes), with particular reference to henipaviruses and Australian bat lyssavirus. J Appl Microbiol. 2003;94:59S-69S.
- [Google Scholar]






