Translate this page into:
Comparison of injectable doxorubicin & its nanodrug complex chemotherapy for the treatment of 4-nitroquinoline-1-oxide induced oral squamous cell carcinoma in rats
Reprint requests: Dr Mahmoud Sina, Department of Oral & Maxillofacial Pathology, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran e-mail: pursina1@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Combination treatments of chemotherapy and nanoparticle drug delivery have shown significant promise in cancer treatment. The aim of the present study was to compare the efficacy of a nanodrug complex with its free form in the treatment of tongue squamous cell carcinoma induced by 4-nitroquinoline-1-oxide in rats.
Methods:
In this study, 75 male Sprague-Dawley rats were divided into five groups. Oral squamous cell carcinoma (OSCC) was induced by using 4- nitroquinoline-1-oxide (4NQO) as a carcinogen. Newly formulated doxorubicin (DOX)-methotrexate (MTX)-loaded nanoparticles, and free DOX-MTX were administrated intravenously to rats. During the study, the animals were weighed once a week. At the end of the treatment, rats’ tongues were evaluated histopathologically.
Results:
There was significant difference between the mean weight of rats in groups A and B (P=0.001) and also groups A and K (P<0.001). No significant association was found between the mortality rate of groups. The difference between the severity of dysplasia of treated and untreated groups was significant (P<0.001).
Interpretation & conclusions:
Our study showed that DOX-MTX nanoparticle complex was more effective than free DOX-MTX in chemotherapy treatment of oral squamous cell carcinoma in rat models. Further investigations are necessary to clarify the advantages and disadvantages of the nanoparticle complex and its potential therapeutic application for different types of cancer.
Keywords
4-nitroquinoline-1-oxide
doxorubicin
nanoparticle
oral
rat
squamous cell carcinoma
Oral cancer is one of the most prevalent cancers in the world12. Though the five-year survival rate of cancer has improved, but still remains in the range of 53-60 per cent. Most oral squamous cell carcinoma (OSCC) cases are not diagnosed until they reach an advanced stage, which is one of the major reasons for minimal improvements in survival rate over the years34. Treatment modalities for OSCC include surgery, radiotherapy and adjuvant systemic therapy (chemotherapy and/or target agents); various combinations of these modalities may also be used depending on the pathological findings and disease presentation125.
Chemotherapy is the most common anticancer therapy. Cancer cells may have or develop resistance before or during chemotherapy. The resistance is correlated with a variety of biochemical changes, including increased efflux of cytotoxic drugs, enzymatic detoxification of cytostatic drugs67 and overexpression of the glutathione transferases8. A combination of chemotherapy and nanoparticle drug delivery has shown significant promise in cancer treatment. In addition, nanoparticle drug delivery enhances therapeutic effectiveness and reduces side effects of the drug by improving their pharmacokinetics. There are also some challenges and design specifications that need to be considered in optimizing nanoparticle-based combination chemotherapy9.
Doxorubicin (DOX) is one of the most active anticancer drugs. Some evidence supports the view that non-tumour tissues like the heart are also damaged by DOX10. Therefore, increasing the DOX efficacy in cancer cells and minimizing its related unwanted toxicities have been at the forefront of scientific research11. Methotrexate (MTX) is the central drug in the management of rheumatoid arthritis and other chronic inflammatory disorders. It is widely used either in monotherapy or in association with other synthetic and biologic disease modifying anti-rheumatic drugs1213.
The purpose of this study was to develop a new form of DOX-MTX-loaded nanoparticles as a new combination chemotherapy and nanoparticle drug delivery system for oral cancer therapy with the ability of enhancing intracellular uptake in 4-nitroquinoline-1-oxide (4-NQO)-induced OSSC in rats.
Material & Methods
This experimental study was carried out at the Research Center Tabriz University of Medical Sciences and Department of Oral Pathology, Faculty of Dentistry, Tabriz, Iran. The 4-NQO was purchased from Sigma (Poole, UK) and other chemicals were purchased from Merck (Darmstadt, Germany).
The nanoparticles were synthesized as suggested by Salehi et al14. Seventy five male Sprague-Dawley rats weighing 150±15 g were randomly divided into five groups. The animals were housed in standard polycarbonate cages in a temperature-controlled animal room (22±2°C) with a 12/12 h light/dark cycle during the experiments. The animals were provided a standard rat pellet diet ad libitum and 4-NQO was used as a carcinogen15. Fifteen animals were placed in each group, and the groups were as follows: Groups A and B were the treatment groups; group K was carcinoma control group and group C was the nanodrug control group. Rats in groups A, B and K received 4-NQO at the concentration of 30 ppm in their drinking water for 14 wk. Rats in groups A and C received the DOX-MTX-loaded nanoparticles intravenously and group B rats received free DOX-MTX at the dose 1.5 mg/kg of body weight once a day on days 2, 5 and 8 of the study. Group K rats did not receive any treatment. Group N was as the normal control group; the rats of this group did not receive any carcinogen or treatment material. All animals were weighed before, and after the study and weight changes were calculated. The death rate of the animals was also recorded during the study. All experiments were conducted after the approval of the study by the Ethics Committee of Tabriz University of Medical Sciences, Iran.
Histological examinations: At the end of the interventional period, the animals were euthanized under anaesthetic conditions (pentobarbital, 150 mg/kg IP)16. Tongue tissue samples were taken from each animal and were immediately fixed in 10 per cent phosphate-buffered formalin. The 5 μm thick microscopic sections were prepared after embedding of tissue samples in paraffin. The sections were stained using a hematoxylin-eosin (H & E) staining method, and histological evaluations were performed with light microscopy. In the stained slides, the thickness of tongue epithelium at five different fields was measured by software Motic-image plus-2 (Motic, PR China), and the mean was considered as well as the thickness of epithelium layer. Pathological changes including dysplasia, hyperplasia, hyperkeratosis, parakeratosis, tumour-like cells and pearl body existence were determined in the epithelium and were scaled as mild, moderate, severe, carcinoma in situ and OSCC.
Ki-67 immunostaining: Sections of 4 μm thickness were cut from representative paraffin blocks of groups A, B, C and K rats and Ki-67 immunohistochemistry staining was performed using a cell proliferation detection kit (mouse monoclonal antibody Ki-67 protein; DO-7, Novocastra Lab, Newcastle, UK). The Ki-67 positive cells appeared brown. The immunohistochemical slides were evaluated (for Ki-67) using a light microscope (Olympus BX40, Tokyo, Japan). The average number of epithelial proliferative cells was estimated through counting positive cells in five random neighbouring medium-power fields (×400) that included 100 cells (at least 500 cells in each slide) and dividing the total by five. Only distinct nuclear staining of invasive carcinoma cells was used for scoring, which was determined semi-quantitatively as nil (10% or less immunopositive cells), low (11-25%), moderate (26-50%) or high (>50% cells)1718.
Statistical analysis: The data were analyzed using the Statistical Package for Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance was used to compare weight changes, followed by multiple comparisons with the Tukey post-hoc test. Chi-square test was used for analysis of pathological changes, and death rate.
Results & Discussion
Studies with different approaches have been carried out to achieve the purpose of increasing the effectiveness of DOX in killing cancer cells611. Following the example of the previous studies15192021, in this study, the 4-NQO-induced OSCC animal model was chosen for evaluating the effectiveness of new DOX- and MTX-combined nanodrug in comparison to a free DOX-MTX drug. In this regard, weight changes, death rate, pathological changes and cellular proliferation were the parameters used for the evaluation. OSCC carcinogenesis usually develops through a multistep process that begins from hyperplasia and passes to mild, moderate and severe dysplasia before OSSC15. Our macroscopic and microscopic findings confirmed that 4-NQO induced typical cancerous lesions in the tongue epithelium of rats in OSCC groups. After treatment, the changes in the treatment groups varied in tongue tissue samples from mild dysplasia to carcinoma in situ. The body weight loss was observed in the groups B and K rats while the rats in groups A, N and C gained weight at the end of the study. The maximum amount of weight gain was observed in groups N and A rats (Fig. 1). Comparison of the weight changes between groups showed a significant difference between groups A and B rats (P=0.001) and also groups A and K rats (P<0.001). There was no significant difference in weight between groups K and B rats, and between group A and groups C and N.
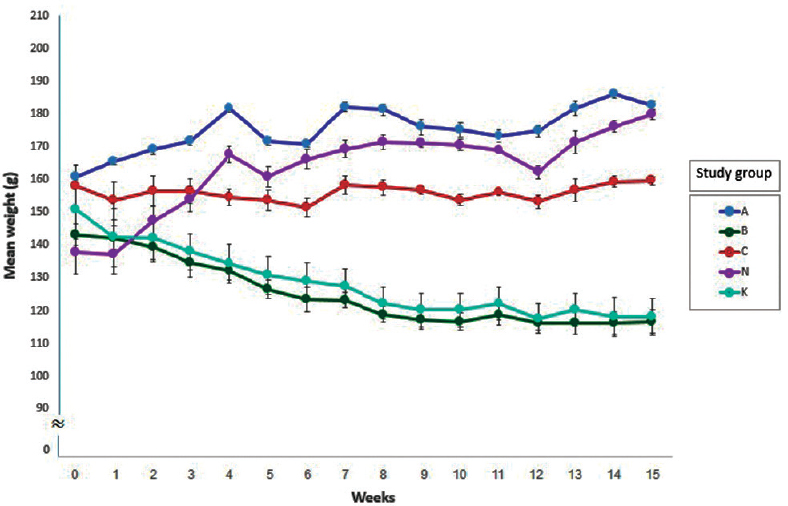
- Mean weight (±SEM) of the study groups in the 15 wk period.
All rats in groups C and N (cancer free) were alive till the end of study. The DOX-MTX-nanoparticle group (group C) was considered for assessing possible unwanted side effects of the DOX-MTX-nanoparticle. Results showed a higher weight gain of the rats of this group, and there were also no deaths and no detectable pathological or proliferative changes in tongue tissue samples of this group. However, more investigations about cardiotoxicity, hepatotoxicity and other probable toxic effects of the new formulation are needed to understand the side effects of this nanoparticle. The overall weight gain of the OSCC group treated with the DOX-MTX-nanoparticle (group A) was higher, and the death rate was lower than that of other OSCC groups. There were no progressive carcinogenesis changes (carcinoma in situ and OSCC) in tissue samples of this group.
In histopathological evaluations of tissue samples in the control group (group K), OSCC was the most frequent (81.8%) pathological change (Fig. 2); whereas mild and moderate dysplasia were the most frequent changes in OSCC groups treated by DOX-MTX nanoparticles and free DOX-MTX, respectively. Groups C and N rats were without lesion. No significant difference was observed between the two treatment groups (groups A & B). There were overall better results of treatment with the DOX-MTX-nanoparticle group (group A) in comparison to the DOX-MTX free group (group B) (Table). Our results were in agreement with previous studies on nanodrug effectiveness on cancer tissues. Wohlfart et al22 showed that DOX-loaded poly (isohexyl cyanoacrylate) nanoparticles were more efficient than the free drug, and therefore suggested the nanoparticles for the non-invasive therapy of human glioblastomas. Maeng et al23 demonstrated the efficacy of DOX-loaded superparamagnetic iron oxide nanoparticles for chemotherapy in liver cancer. Liu and Zhang24 concluded that lipid-based nanocarriers played significant role in the formulation of anticancer drugs to improve therapeutics. All of these studies including the present one suggest that nanoparticles are both useful and efficient in treating cancers over the free drug alone.

- Well-differentiated squamous cell carcinoma, islands of malignant squamous epithelium and dysplastic epithelial cells invade the lamina propria with keratin pearl formation (arrow a). Arrow b shows the invaded malignant squamous cells (H & E, ×400).
About the cell proliferation, a moderate severity of Ki-67 was most common in OSCC treated with free DOX-MTX (Fig. 3). However, the expression of Ki-67 in 35.7 per cent of OSCC cases treated with DOX-MTX nanoparticles was negative. There was also no Ki-67 expression in the group that received DOX-MTX nanoparticles without OSCC. On the other hand, high cellular proliferation with a higher severity was most frequent (81.8%) in the OSCC control group. Ki-67 is a proliferation-associated nuclear antigen expressed in all cycling cells except resting cells in the G0 phase. The Ki-67 antigen is detected in G1, S, G2 and M phases of the cell cycle, but not in the G0 phase25. Ki-67 had predictive and prognostic value and was a feasible marker for independent prediction of treatment response and prognosis in a group of breast cancer patients receiving new drug treatment26. In our study, there was not high proliferation severity in the pathological slides of tissue samples of group rats. The proliferation severity of group B was lower than cancer control group, as expected. Our findings were similar to the results of earlier studies2223 regarding Ki-67 as a marker of improving cellular proliferation.
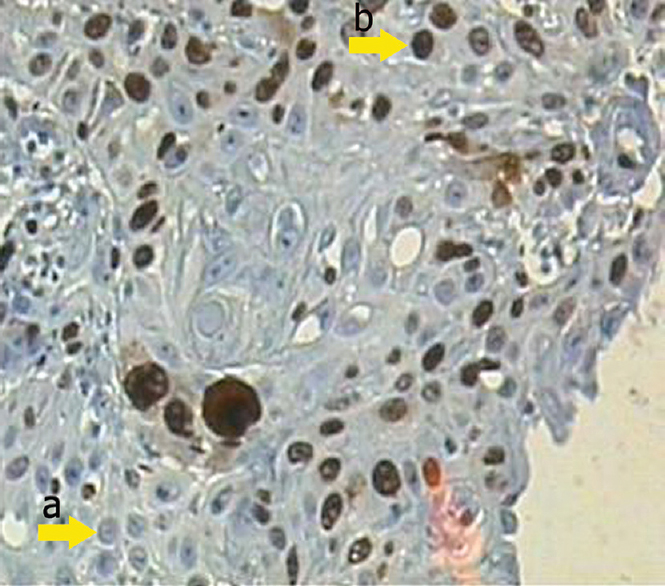
- The moderate Ki-67 expression shows positive nuclear staining belonging to group B by immunohistochemistry (IHC, ×400). Arrows a and b demonstrate the negative and positive nuclear staining, respectively.
In conclusion, our results indicated that the new DOX-MTX nanoparticle complex used in this study was more effective than free DOX-MTX in the treatment of 4-NQO- induced OSCC in rats. However, the severity of histopathological changes and cellular proliferation were not significantly different among the groups. Hence, further investigation is necessary to clarify the advantages and disadvantages of the nanoparticle complex and its potential therapeutic application in different types of cancer.
Acknowledgment
This study was supported by a grant from the Vice Chancellor for Research, Tabriz University of Medical Sciences (Tabriz, Iran). The authors thank the members of the Drug Applied Research Center for technical support.
Conflicts of Interest: None.
References
- Early diagnosis in primary oral cancer: is it possible? Med Oral Patol Oral Cir Bucal. 2011;16:e300-5.
- [Google Scholar]
- Oral cancer aetiopathogenesis; past, present and future aspects. Med Oral Patol Oral Cir Bucal. 2011;16:e306-11.
- [Google Scholar]
- Advances in diagnostic adjuncts for oral squamous cell carcinoma. Open Pathol. 2011;5:3-7.
- [Google Scholar]
- Oral cancer: current role of radiotherapy and chemotherapy. Med Oral Patol Oral Cir Bucal. 2013;18:e233-40.
- [Google Scholar]
- Nanotechnology applied to overcome tumor drug resistance. J Control Release. 2012;162:45-55.
- [Google Scholar]
- Nanoparticle-directed sub-cellular localization of doxorubicin and the sensitization breast cancer cells by circumventing GST-mediated drug resistance. Biomaterials. 2014;35:1227-39.
- [Google Scholar]
- Glutathione transferases and development of new principles to overcome drug resistance. Arch Biochem Biophys. 2010;500:116-22.
- [Google Scholar]
- Nanoparticle-assisted combination therapies for effective cancer treatment. Ther Deliv. 2010;1:323-34.
- [Google Scholar]
- Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267-85.
- [Google Scholar]
- Possibilities to increase the effectiveness of doxorubicin in cancer cells killing. Drug Metab Rev. 2011;43:540-57.
- [Google Scholar]
- Old drugs, old problems: where do we stand in prediction of rheumatoid arthritis responsiveness to methotrexate and other synthetic DMARDs? BMC Med. 2013;11:17.
- [Google Scholar]
- Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-70.
- [Google Scholar]
- Development of dual responsive nanocomposite for simultaneous delivery of anticancer drugs. J Drug Target. 2014;22:327-42.
- [Google Scholar]
- 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006;42:655-67.
- [Google Scholar]
- Euthanizing agents. In: Adams HR, ed. Veterinary pharmacology and therapeutics. Ames: Iowa State University Press; 2001. p. :397-402.
- [Google Scholar]
- Immunohistochemical detection of Ki67 in breast cancer correlates with transcriptional regulation of genes related to apoptosis and cell death. Mod Pathol. 2005;18:374-81.
- [Google Scholar]
- The effect of dietary and topical celecoxib on 4-nitroquinoline-1-oxide-induced lingual epithelium alternations in rat. J Pak Med Assoc. 2009;59:769-74.
- [Google Scholar]
- Ras gene mutation is not related to tumour invasion during rat tongue carcinogenesis induced by 4-nitroquinoline 1-oxide. J Oral Pathol Med. 2011;40:325-33.
- [Google Scholar]
- The role of the TP53 gene during rat tongue carcinogenesis induced by 4-nitroquinoline 1-oxide. Exp Toxicol Pathol. 2011;63:483-9.
- [Google Scholar]
- 4-NQO carcinogenesis: a model of oral squamous cell carcinoma carcinogenesis. Int J Morphol. 2012;30:309-14.
- [Google Scholar]
- Treatment of glioblastoma with poly(isohexyl cyanoacrylate) nanoparticles. Int J Pharm. 2011;415:244-51.
- [Google Scholar]
- Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials. 2010;31:4995-5006.
- [Google Scholar]
- Cancer chemotherapy with lipid-based nanocarriers. Crit Rev Ther Drug Carrier Syst. 2010;27:371-417.
- [Google Scholar]
- Ki-67 proliferating index and histological grade, type and stage of colorectal carcinoma. J Ayub Med Coll Abbottabad. 2008;20:44-8.
- [Google Scholar]
- Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486.
- [Google Scholar]






