Translate this page into:
A systematic review of standard treatment guidelines in India
For correspondence: Dr Yashashri C. Shetty, Department of Pharmacology & Therapeutics, 1st Floor, Main College Building, Seth GS Medical College & KEM Hospital, Parel, Mumbai 400 012, Maharashtra, India e-mail: yashashrirajit@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Standard treatment guidelines (STGs) are the cornerstone to therapeutics. Multiple agencies in India develop STGs. This systematic review was conducted to find out STGs available in India, evaluate if these were as per World Health Organization (WHO) recommendations for STGs and compare these with National Institute for Health and Care Excellence (NICE) guidelines. Information on legal authority and responsibility for formulating STGs was also sought.
Methods:
PRISMA guidelines were followed. Publications from PubMed and Google Scholar were searched for STGs using terms 'Standard Treatment Guidelines AND India'. Data from STGs were compiled in excel as per the WHO and authors' criteria for STGs and compared with NICE guidelines.
Results:
PubMed and Google Scholar search provided 56 publications (out of 1695 search results) mentioning 27 STGs. Google search and replies from authors led us 36 STGs, totalling to 63 STGs. No STG mentioned any specific period of revision, eight STGs were not evidence-based, 55 had some Indian references, 48 STGs were for single disease and the remaining multi-disease, three STGs did not include diagnostic criteria, 16 STGs did not give prescribing information of recommended treatment and 16 STGs provide no referral criteria for patients. Fifty five STGs did not mention level of health care. While NICE is a single legal authority in England and guidelines are as per WHO recommendations for STGs, in India although Acts and rules do not vest authority, National Health Systems Resource Center is generally designated responsible for STGs.
Interpretation & conclusions:
In India, although there are multiple STGs developed by various authorities and professionals for the same conditions, these fulfil WHO recommendations only partially. Authority with statutory duty collaborating with professional organizations, a standard methodology for adopting international guidelines, Indian data for evidence base, attention to local needs will help in developing better STGs and their acceptance.
Keywords
Evidence-based guidelines
rational use of medicines
STGs therapeutic guidelines
treatment guidelines
Providing quality healthcare to all is a key challenge for the Government of India. Standard treatment guidelines (STGs), alternatively known as standard treatment schedules, standard treatment protocols or therapeutic guidelines, are systematically developed statements which are designed to assist practitioners and patients in making informed decisions about suitable healthcare for specific clinical conditions. These include the preferred pharmaceutical and non-pharmaceutical treatments for common health problems came across by people in a specific health system1. These STGs are used worldwide to prevent the misuse of medicines through the improper treatment of common problems and to encourage the economically efficient and therapeutically effective usage of medicines2. The rising cost of healthcare, differences in clinical practice among providers and hospitals, and problems faced by prescribers in keeping themselves up-to-date with fast-growing new scientific evidence, especially in places with limited resources, have increased interest in, and the importance of STGs.
Experience and studies have shown that there could be ineffective, unsafe, or wasteful prescribing, even when the drugs supply is based on an approved formulary or essential medicines list. It is falsely believed that STGs bring constraints to prescribing3. STGs only advise prescribers, who still retain the power and responsibility to make decisions about appropriate treatments for their patients, and define the boundaries between the accepted norms in treating a disease, based on clinical evidence and the practice of relying purely on clinical experience3.
Sharma et al2 have noted that while some fruitful approaches to developing STGs have been well documented in India, multiple clinical practice guidelines continue to be produced by insurers, professional organizations, individuals, and others. While the quality of the STGs produced has not been assessed systematically, these are reported to be of poor quality or content or conflict with each other, failing to inspire confidence in prescribers. The development of STGs is a complex and lengthy process, and has its own risks. Because of their non-binding nature, there is a risk that these guidelines will not be accepted by clinicians24. The creation of effective guidelines needs robust development, strong editorial and project management processes, along with a keen eye for details about various sections of the guidelines2.
The aim of this study was to conduct a systematic review of STGs available in India to evaluate if recommendations for STGs by World Health Organization (WHO) are fulfilled. The secondary objective was to compare the manner in which STGs are framed in India with more established processes in the UK and USA.
Material & Methods
Search strategy: The inclusion criteria were published articles in the English language which mentioned STGs in India. 'Standard Treatment Guidelines AND India' were the keywords used for the search strategy. A systematic literature search was conducted till March 2016, in two major databases namely, PubMed and Google Scholar. The PRISMA guidelines and Cochrane handbook for systematic reviews provided a framework for the reporting structure of this systematic review45. The focus of the search was to retrieve STGs mentioned in scholarly articles and in related Google searches. Google search was also done to find any relevant STGs which might not be found in published journal articles. Non-peer reviewed journals were also included in the search as many of the STGs in India are published on either government websites or non-peer reviewed journals. A manual search was also done in references list of the retrieved publications for other publications that might fulfil the study inclusion criteria. The authors of the shortlisted publications (whose emails were available in the publication or on the internet) were contacted by email to pursue their advice on other publications related to our research question, allowing one-month time for them to reply.
Screening process for inclusion: The results were restricted to studies on human subjects. Only STGs that were available online were included. At each stage, two independent reviewers assessed the publications for the inclusion criteria. Publications about non-Indian STGs were excluded.
Assessment of published reports/studies: The STGs obtained from the refined search were reviewed for analysis and to capture the information on the variables of interest (WHO and authors' criteria) in a spreadsheet. The extraction of data was done and entries were checked. Multiple STGs available from a single source were counted as a single STG and not as multiple.
The WHO criteria16 were: condition for which the STG was developed, STG was for a single disease or multi-diseases, presence of diagnostic criteria, treatment objectives, non-drug treatment, drug/ treatment of choice, 2nd or 3rd line of treatment with indications. Prescribing information, patient referral criteria, patient education regarding the condition and the cost of treatments, especially if alternatives were proposed.
Authors' criteria were: name, year of publication, and edition of the STG, number of years after which STGs were revised, whether management algorithm was given, was the STG evidence-based, development authority of the STG, whether STG was part of a national programme and did the STG have specific guidelines for primary, secondary and tertiary levels.
In the Indian context, since the dose recommended (variation due to genetic and environmental factors, nutrition, and body weight) as well as the drug of choice (due to resistant microbes) may be different, evidence from Indian studies would be important. Hence, references were scrutinized and if all references were Indian studies, it was considered as entirely Indian evidence-based STG, and if references were Indian plus international studies, it was considered partially Indian evidence-based.
Methodology used for extracting information on the legal authority and responsibility for developing STGs: this information was retrieved by means of a Google search for terms such as 'STGs in India' and 'clinical guidelines in the UK'. In addition, the text of the Indian Clinical Establishments (Registration and Regulation) Act 20107 was scrutinized for its provisions on STGs. The websites of the National Health Mission (NHM) and National Institute for Health and Care Excellence (NICE) were surveyed to find out the authority vested with this responsibility and to understand how STGs in India and the UK are framed and disseminated891011. Lastly, a number of decisions of courts in India and in the UK that were available on case law databases such as Manupatra and Westlaw, or were referred to in the journal articles were searched. The search words used to find these decisions included 'clinical guidelines', 'STGs' and 'medical practitioners are bound'. The type of STGs and the authority that they hold over the conduct of medical practitioners were analysed under these decisions by the courts in India.
Results
The search strategy identified 1695 results through PubMed and Google Scholar search. After removing the duplicates (n=225), the inclusion criteria were applied to 1470 publications that were searched manually. Fifty six full text articles were read from which 27 different STGs were selected (Fig. 1).
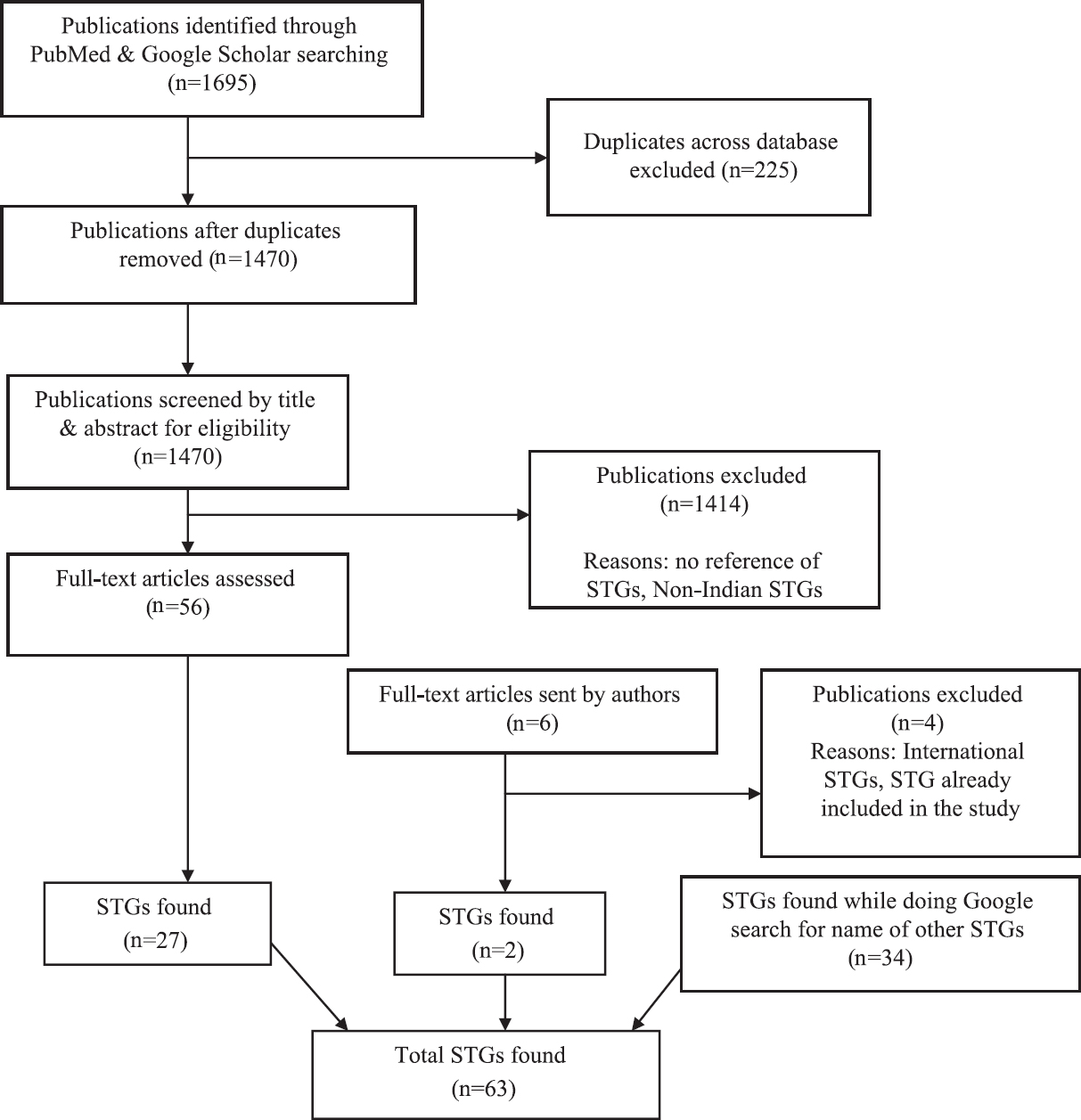
- Search strategy and screening process. STGs, standard treatment guidelines.
A total of 35 emails were sent to the authors, of whom 14 replied. Of these 14, two authors provided further information about six publications, three of which dealt with International STGs, one provided information about an STG already included in the study and two about STGs that were not on our list and were therefore, included in the study. While conducting a Google search for the STGs found in the publications, 34 STGs were found. Finally, 63 different STGs were included. Of these, only three were published in 2015 and 13 in 2014. The remaining 45 guidelines were published in 2012 or earlier. The oldest STG in the list was published in 1999 and was never revised after that. Two STGs did not mention the year of publication.
Forty eight STGs were for a single disease and the rest were for multiple conditions; 55 STGs did not include specific treatment according to levels of healthcare, one each had treatment specific for primary, secondary and tertiary levels of healthcare respectively, and six STGs were for all three levels of healthcare. None of the STGs were updated at regular intervals.
While analysing evidence base it was noted that of the 63 STGs, eight gave no references while 55 provided international and Indian references (partial Indian) (Fig. 2). There was no uniformity in the format regarding STGs in India. This was in contrast with clinical guidelines in the UK developed by NICE, which met all WHO recommendations. One of the reasons for this might be the fact that India has not yet put in place a systematic process for the development of STGs as NICE. The legal framework governing the development of STGs in India is described below, and compared with the process in the UK (Table I).
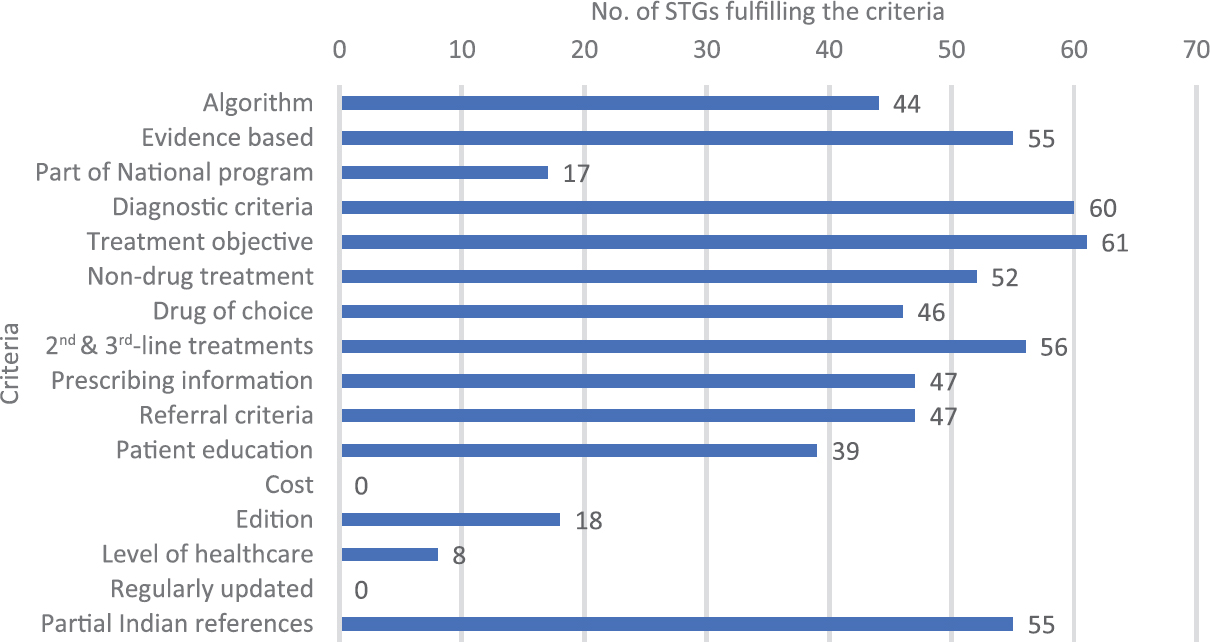
- Number of standard treatment guidelines (STGs) fulfilling World Health Organization and authors' criteria.
| Name of the STG | Year | Edition | Comes under/developed by | Scope of guidelines |
|---|---|---|---|---|
| STGs for single disease | ||||
| Asthma | ||||
| Consensus Guidelines on Management of Childhood Asthma in India12 | 1999 | NM | Expert consensus | Childhood asthma |
| Guidelines for Management of Asthma at Primary and Secondary Levels of Health Care in India (2005)13 | 2005 | NM | WHO and Government of India Collaborative Programme | Bronchial asthma |
| Best Treatment Guidelines For Bronchial Asthma14 | 2007 | NM | Update article by authors | Bronchial asthma |
| Guidelines for diagnosis and management of bronchial asthma: Joint ICS/NCCP (I) recommendations15 | 2015 | NM | Indian Chest Society and National College of Chest Physicians | Bronchial asthma |
| Diabetes | ||||
| Guidelines for Management of Type 2 Diabetes16 | 2005 | 1st | ICMR, WHO | Type 2 diabetes |
| API-ICP Guidelines on Diabetes 200717 | 2007 | NM | API-ICP | Diabetes |
| Guidelines for the Comprehensive Management of Diabetic Retinopathy in India18 | 2008 | NM | Aravind eye care system | Diabetic retinopathy |
| Premix insulin: Initiation and Continuation Guidelines for Management of Diabetes in Primary Care19 | 2009 | 1st | API | Diabetes |
| Type 1 Diabetes Mellitus in Children and Adolescents In India Clinical Practice Guidelines 201120 | 2011 | 1st | ISPAE | Type 1 diabetes mellitus in children and adolescents |
| Special Issue on Consensus Statements on Insulin Therapy21 | 2014 | Special | API | Diabetes |
| National Guidelines for Diagnosis and Management of Gestational Diabetes Mellitus22 | 2014 | NM | MoHFW, UNICEF | GDM |
| Neurological diseases | ||||
| Clinical Practice Guidelines for The Management of Depression23 | 2004 | NM | IPS guideline committee on depression | Depression |
| Clinical Practice Guidelines for the Management of Schizophrenia24 | 2004 | NM | IPS guideline committee on schizophrenia | Schizophrenia |
| Guidelines for The Treatment of Sleep Disorders25 | 2006 | NM | Authors of article | Sleep disorders |
| Clinical Practice Guidelines for Treatment of Depression In Elderly26 | 2007 | NM | Authors of article | Depression in elderly |
| Clinical Practice Guidelines for The Management of Reversible Dementias27 | 2007 | NM | Authors of article | Reversible dementias |
| Clinical Practice Guidelines for Treatment of Vascular Dementia28 | 2007 | NM | Authors of article | Vascular dementia |
| Guidelines for Treatment of Epilepsy29 | 2008 | 1st | Indian Epilepsy Society, Indian Epilepsy Association | Epilepsy |
| Guidelines for Diagnosis and Management of Childhood Epilepsy30 | 2009 | 1st | Indian Academy of Pediatrics | Childhood epilepsy |
| Stroke management31 | 2011 | NM | Authors of article | Stroke |
| Name of the STG | Year | Edition | Comes under/developed by | Scope of guidelines |
| Non-communicable diseases | ||||
| Indian Guidelines on the Management of SLE32 | 2002 | NM | Single author article | SLE |
| Guidelines on the Diagnosis and the Current Management of Headache and Related Disorders33 | 2011 | NM | Drawn up by neurologists with a special interest in headache | Headache |
| Consensus Guidelines on Management of Childhood Convulsive Status Epilepticus34 | 2014 | NM | Multi-disciplinary Consensus Development Workshop on Management of Status Epilepticus in Children in India | Childhood convulsive status epilepticus |
| Guidelines for Pregnancy Care and Management of Common Obstetric Complications by Medical Officers35 | 2005 | NM | MoHFW | Common Obstetric Complications |
| National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Disease and Stroke36 | 2008 | NM | NPCDCS, Government of India - WHO Collaborative Programme’ | Diabetes, hypertension, hypercholesterolemia, CAD, stroke, cancer |
| National Snakebite Management Protocol (India) 37 | 2008 | 1st | Authors | Snakebite |
| AIOS Guidelines to Prevent Intraocular Infection38 | 2009 | 1st | All India Ophthalmological Society and Cipla | Intraocular infection |
| Indian Rheumatology Association Guidelines for the Management of Glucocorticoid-induced Osteoporosis39 | 2011 | NM | Indian Rheumatology Association | Glucocorticoid-induced osteoporosis |
| Standard Treatment Guideline and Essential Medicine List (for pregnant women)40 | 2011 | 1st | NRHM, Health and Family Welfare Department, Government of Odisha | Pregnancy, puerperium and newborn |
| Guidelines for the Management of Cataract in India41 | 2011 | 1st | VISION 2020: The Right to Sight India | Cataract |
| Indian Society of Gastroenterology Consensus on Ulcerative Colitis42 | 2012 | NM | Indian Society of Gastroenterology | Ulcerative colitis |
| Indian Chronic Kidney Disease Guidelines43 | 2013 | 2nd | Indian CKD Guideline Workgroup, Indian Nephrology Society | CKD |
| Indian Guidelines on Hypertension - III44 | 2013 | 3rd | CSI, HSI, ICP, ISN, RSSDI and IAD | Hypertension |
| Consensus and Evidence-Based INOSA Guidelines45 | 2014 | 1st | MoHFW, AIIMS and Multi-speciality disciplines across India- Public and Private Sectors | Obstructive sleep apnoea |
| Management of Neonatal Cholestasis: Consensus Statement of the Pediatric Gastroenterology Chapter of Indian Academy of Pediatrics46 | 2014 | NM | Indian Academy of Pediatrics | Neonatal cholestasis |
| Guidelines for treatment of recurrent or metastatic head and neck cancer47 | 2014 | NM | ICON | Recurrent or metastatic head and neck cancer |
| Guidelines for Diagnosis and Management of Chronic Obstructive Pulmonary Disease48 | 2014 | Special | Indian Chest Society and National College of Chest Physicians (India) | COPD |
| Infectious diseases | ||||
| IAP Guidelines 2006 on Management of Acute Diarrhea49 | 2006 | 1st | The Indian Academy of Pediatrics | Acute diarrhoea |
| Endocrine Society of India Management Guidelines for Patients with Thyroid Nodules: A position statement50 | 2011 | NM | Endocrine Society of India | Thyroid nodules |
| Revised Statement on Management of Urinary Tract Infections51 | 2011 | NM | Indian Society of Pediatric Nephrology | Urinary tract infections |
| Guidelines for Diagnosis and Management of Community and Hospital-Acquired Pneumonia in Adults: Joint ICS/NCCP (I) Recommendations52 | 2012 | NM | ICS and NCCP | Pneumonia |
| Consensus Statement of HCV Task Force of the Indian National Association for Study of the Liver (INASL). Part II: INASL Recommendations for Management of HCV in India53 | 2014 | NM | INASL | HCV infection |
| National Health Programmes | ||||
| National Guidelines on Prevention, Management, and Control of Reproductive Tract Infections including Sexually Transmitted Infections54 | 2007 | NM | MoHFW, NACO, WHO, UNFPA | RTI, STI |
| Guidelines for Filariasis elimination in India55 | 2009 | NM | NVBDCP, MoHFW | Filariasis |
| National Leprosy Eradication Program, Disability Prevention and Medical Rehabilitation - Guidelines for Primary, Secondary and Tertiary Level Care56 | 2012 | NM | MoHFW, NLEP | Leprosy |
| Guidelines for Diagnosis and Treatment of Malaria in India57 | 2013 | NM | NVBDCP, National Institute of Malaria Research, MoHFW | Malaria |
| ART guidelines for HIV-Infected Adults and Adolescents58 | 2013 | NM | MoHFW, NACO | AIDS |
| National Programme for Prevention and Control of Japanese Encephalitis/Acute Encephalitis Syndrome59 | 2014 | NM | NVBDCP, Government of India, Ministry of Health & Family Welfare | JE-AES |
| Operational Guidelines on Kala-azar (visceral leishmaniasis) Elimination in India60 | 2015 | NM | NVBDCP, ‘WHO India, RMRI (ICMR) Patna, NCDC, Patna, BMGF/CARE India/DNDi/KalaCORE/PATH’ | Kala-Azar (Visceral Leishmaniasis) |
| Standards for TB Care in India61 | 2014 | NM | MoHFW, The national TB institutions, RNTCP, WHO | TB |
| National Guidelines for Clinical Management of Dengue Fever62 | 2015 | NM | NVBDCP, WHO, MoHFW | Dengue |
| National Guidelines on Rabies Prophylaxis63 | 2015 | 2nd | National Centre for Disease Control | Rabies |
| Diagnosis and Treatment of Kala-azar64 | NM | NM | MoHFW, NVBDCP | Kala-azar |
| STGs for multiple conditions/speciality | ||||
| Standard Treatment Guidelines65 | NM | NM | MoHFW | Multiple conditions |
| Standard Treatment Guidelines for Medical Officers66 | 2003 | 1st | Government of Chhattisgarh, Department of Health and Family Welfare, State Health Resource Centre, Chhattisgarh | Multiple conditions |
| Standard Treatment Guidelines (medical management and costing of select conditions)67 | 2007 | NM | MoHFW, AFMC, WHO | Multiple conditions |
| Standardisation Initiatives By The FICCI Health Insurance Group68 | 2009 | NM | FICCI | Multiple conditions |
| Standard Treatment Guidelines A Manual for Medical Practitioners69 | 2010 | 1st | TNHSP, Health and Family Welfare Department, Government of Tamil Nadu | Multiple conditions |
| Rajasthan State Standard Treatment Guidelines70 | 2012 | 2nd special | Rajasthan Medical Services Corporation, DSPRUD | Multiple conditions |
| Manual of Standard Treatment Guidelines Haryana71 | 2013 | Special | NRHM, DSPRUD | Multiple conditions |
| ICMR Guidelines72 | 2010 | NM | ICMR | Multiple conditions |
| Standard Treatment Guidelines A Manual for Medical Therapeutics73 | 2013 | 1st | Gujarat Medical Services Corporation Limited, Health and Family Welfare Department, Government of Gujarat | Multiple conditions |
| Standard Treatment Guidelines 201474 | 2014 | 1st | Department of Public Health and Family Welfare, Madhya Pradesh | Multiple conditions |
NM, not mentioned; MoHFW, Ministry of Health & Family Welfare; AFMC, Armed Forces Medical College; TNHSP, Tamil Nadu Health Systems Project; ICMR, Indian Council of Medical Research; IAP, The Indian Academy of Pediatrics; NACO, National AIDS Control Organization; RNTCP, Revised National Tuberculosis Control Programme; API, The Association of Physicians of India; AIIMS, All India Institute of Medical Sciences; NLEP, National Leprosy Eradication Programme; NVBDCP, National Vector Borne Disease Control Programme; UNFPA, United Nations Population Fund; CSI, Cardiological Society of India; HSI, Hypertension Society of India; ICP, Indian College of Physicians; ISN, Indian Society of Nephrology; RSSDI, Research Society for Study of Diabetes in India; IAD, Indian Academy of Diabetes; NRHM, National Rural Health Mission; DSPRUD, Delhi Society for Promotion of Rational Use of Drugs; RMRI, Rajendra Memorial Research Institute of Medical Sciences; BMGF, Bill and Melinda Gates Foundation; DNDi, Drugs for Neglected Diseases initiative; INASL, Indian National Association for Study of the Liver; FICCI, Federation of Indian Chambers of Commerce; NPCDCS, National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Disease and Stroke; ICS, The Indian Chest Society; NCCP, National College of Chest Physicians; UNICEF, The United Nations Children’s Emergency Fund; ICON, Indian Cooperative Oncology Network; ISPAE, Indian Society for Pediatric and Adolescent Endocrinology; AIOS, All India Ophthalmological Society; WHO, World Health Organisation; STGs, standard treatment guidelines; ISPAE, Indian Society for Pediatric and Adolescent Endocrinology; GDM, Gestational Diabetes Mellitus; SLE, Systemic lupus erythematosus; CAD, Coronary artery disease; CKD, Chronic kidney disease; COPD, Chronic Obstructive Pulmonary Disease; RTI, Reproductive Tract Infections; STI, Sexually Transmitted Infections; JE-AES, Japanese Encephalitis - Acute Encephalitis Syndrome; NCDC, National Centre for Disease Control
Legal framework governing the development of STGs in India: The Clinical Establishments (Registration and Regulation) Act 2010 (Act)7 sets out conditions for the registration of clinical establishments. Although the Act itself does not contain a reference to STGs, clause (iii) of Rule 9 of the Clinical Establishments (Central Government) Rules 2012 (Rules)75 requires clinical establishments, as a condition of registration, to ensure compliance with such STGs as may be determined and issued by the Central and State Governments from time to time. Apart from this provision, however, the rules did not prescribe the procedure by which the STGs ought to be framed or the manner in which the power to develop these guidelines ought to be divided between the Central and State Governments. It was important to note that this provision in the Rules only empowered the Central and State Governments to frame STGs and to require compliance with them by clinical establishments as a condition of registration Table II. However, the Clinical Establishments Act does not compel the States to develop STGs as it has been adopted only by a few States and Union Territories7. This was in contrast to the UK, where section 237 of the Health and Social Care Act 201276 read with section 5 of the National Institute for Health and Care Excellence (Constitution and Functions) Regulations 201377 making it a function of NICE to give advice or guidance, provide information or make recommendations regarding any stuff associated with the provision of services under the National Health Service (NHS), public health services as well as social care in England. The Act and regulations additionally require NICE to establish procedures for giving such guidance, including a duty to consult persons if considered appropriate811. In furtherance of these provisions, NICE has developed an established procedure, the key details of which are briefly described below.
| Topic | UK | India |
|---|---|---|
| Designated body for STGs | NICE (statutory) | NHSRC, as designated by the NHM (non-statutory body) |
| Other organizations developing STGs | GAIN, Royal Colleges These are not binding and may be used locally | State governments, ICMR, central and State national programmes and professional organizations |
| Is there a binding duty to frame STGs? | Yes, NICE is statutorily required to provide guidance on matters relating to the provision of NHS services, which would include the framing of STGs or clinical guidelines as these are referred to in the UK | No, central and State governments have the power to issue STGs under the Clinical Establishments (Central Government) Rules |
| Are STGs legal binding? | No, but STGs provide evidence of a ‘responsible body of medical opinion’ | Yes, clinical establishments must ensure compliance with STGs as a condition of registration |
| Procedure for framing of STGs | Topic selection committee, Referral of topics from NHS, England and the Department of Health, Specialist centres frame STG with the involvement of stakeholders and after considering other guidelines, Public consultation | Although the NHSRC is the designated statutory body, no procedure has been prescribed, DSPRUD STG development procedure |
NICE, The National Institute for Health and Care Excellence; NHSRC, National Health Systems Resource Center; STGs, standard treatment guidelines; DSPRUD, Delhi Society for Promotion of Rational Use of Drugs; NHM, National Health Mission; ICMR, Indian Council of Medical Research; NHS, National Health Service; GAIN, Guidelines and Audit Implementation Network
Documentation of the development process in the published Indian STGs was not available on Authority websites and in the STGs.
Development of NICE guidelines: At NICE, a Topic Selection-Oversight Group considers topics for guideline development and discusses their findings with the NHS and the Department of Health. Thereafter, the NHS and the Department of Health finalize the topic and make a referral for the development of clinical guidelines on the topic in question to NICE75. After the topic for framing a guideline is identified, NICE commissions one of its specialist centres to frame a draft of the guideline on such topic10. A number of stakeholders, such as manufacturers of medicines or devices related to the guideline topic, providers and commissioners of health services, national organizations representing patients and carers and/or healthcare professionals, statutory organizations, and research organizations that have formed countrywide recognized research connected to the guideline topic, are consulted throughout the development of each guideline78. Subsequently, there is at least one public consultation on the draft of a guideline to gauge the comments of stakeholders registered with NICE. The guideline is then signed off for publication by the Guidance Executive of the NICE, following which steps are taken to communicate, disseminate and promote awareness about the guideline78.
A number of other organizations such as the Guidelines and Audit Implementation Network (GAIN), the Royal Colleges and various professional organizations formulate clinical guidelines in England. While the clinical guidelines framed by NICE are nationally recognized, the ones framed by GAIN, or the Royal Colleges may be recognized and followed locally. There is nothing to suggest that gives precedence to NICE guidelines over those framed by other bodies. In any case, NICE inspects the clinical guidelines framed by other expert bodies as part of the process of developing its own guidelines78. This inspection is helpful in determining the effectiveness of the existing clinical guidelines and practices, as well as in collating the opinion of expert bodies on the standardized treatments for various diseases and conditions.
While the clinical guidelines framed by NICE are not legally binding on medical practitioners and service providers, the courts are inclined to view such guidelines as a responsible body of medical opinion79808182. Consequently, practitioners and service providers, who do not adhere to such guidelines, are required to provide a reasonable explanation (which may be a counter medical opinion backed by credible sources) in order to discharge their burden of observing due diligence while exercising their functions.
STGs: United States of America:STGs or Clinical Practice Guidelines, as these are more commonly known in the USA are framed by a range of groups/organizations, most of which are made publicly available as resources through the website of the National Guideline Clearinghouse (NGC), set up by the Agency for Healthcare Research and Quality (AHRQ)83. The AHRQ, as its website (https://www.ahrq.gov/cpi/about/profile/index.html)83 states, is 'the lead Federal agency charged with improving the safety and quality of America's health care system.' A wide variety of organizations frame clinical practice guidelines in the US. According to the categories listed on the NGC website, these include academic institutions, disease-specific societies, federal government agencies, hospitals, independent expert panels, professional associations, non-profit organizations, as well as non-U.S. State/local government agencies. Some of these guidelines are rated according to the strength of their recommendations. For instance, ratings may vary from 1A to 2C, with a rating of 1A meaning 'strong recommendation, high-quality evidence', while a rating of 2C means 'weak recommendation, low-or-very-low-quality evidence'83.
In 2011, a report titled 'Clinical Practice Guidelines we Can Trust' was published by the Institute of Medicine84. This recommended eight standards that clinical practice guidelines ought to adhere to in order to considered trustworthy. The report also recommended that the AHRQ would need the NGC to 'provide a clear indication of the extent to which clinical practice guidelines submitted adhere to the standards for trustworthiness'8586.
Discussion
Development of STGs in India is done by multiple authorities like Central Government, State Government, Hospitals, professional associations and private organizations. This shows involvement and commitment by healthcare professionals, and Indian authorities regarding the improvement of healthcare of the population. Government has been developing STGs through the National Health Systems Resource Centre (NHSRC) and also as part of National Health Programme with the help of experts for fulfilling the need of the population.
Most of the STGs found in our study covered important sections in the STGs but key problems with STGs in India that our analysis revealed were-multiplicity, paucity of Indian evidence for guidelines, failure to periodically revise guidelines, failure to tailor them according to the level of healthcare, and finally, a lack of wide availability and accessibility. There were multiple STGs for the same conditions which were made by many authorities and professionals. For instance, there were six separate guidelines for diabetes. Multiplicity could be due to different purpose of development of the guidelines for insurance reimbursement, level of care and the scope of the guidelines. This duplication of efforts and time is avoidable by collaboration which will also save scarce resources, funds and experts' time.
There is a paucity of Indian research for the development of up to date STGs. Indian studies are particularly needed to answer specific research questions relevant to the Indian setting such as, cost of therapy, variation in dose, antimicrobial resistance. Evidence-based medicine has its own limitations as noted in many research articles but these limitations should not deter the authorities from making evidence-based guidelines87.
None of the STGs mentioned any specific period after which they were revised. Only one STG was updated to a 3rd edition and six were in first or second editions. Utilization of recent systematic reviews and meta-analysis is important to get the highest quality data. Also, a locally produced STGs suing Indian data might improve acceptability than so-called imported/ international guidance2.
Major obstacles reported in several studies in regular revisions of STGs were the availability of local expertise and a dearth of awareness of the concept and also prescribers' firmness on the inclusion of out-of-date practices, or of medications by brand names of untested efficacy. Additionally, it was observed that guidelines review groups often were short of time, interest, resources, and skills to collect and analyse every section of evidence, mainly in relation to editorial responsibilities2.
Many States and authorities in India have been developing different STGs which are not available online. There is a need to ensure that STGs are made freely available online which could help in wider adoption in clinical practice.
Guidelines in India also have a contribution from the Industry, which could be a potential conflict of interest. As noted in a study by Sharma et al88 government and insurance companies using guidelines as a tool of a coercion to limit treatment choices (limiting medicine reimbursement), restraining independence of the practitioner, were perceived as a major barrier in the STG uptake by the physicians. Most respondents in this study did not appreciate the accountability protection offered by the guidelines, which was different from western country reports, where both these aspects (reimbursement limitations and liability perception) act as enablers for STGs.
One of the solutions to the multiplicity of STGs might be to designate a specific authority with the task of developing STGs, preferably by imposing a statutory duty. This duty could be accompanied by enabling rules and regulations describing the procedure for developing STGs, the information that they ought to contain, and methods of dissemination, along the lines of the procedure followed by NICE. This procedure ought to incorporate the requirement of periodic updating. A good quality STG could include points as mentioned in The international Appraisal of Guidelines, Research and Evaluation (AGREE) II checklist89.
A particularly useful approach to adopt from NICE is making available evidence summaries for unlicensed or off-label medicines. These summaries critically review the strengths and weaknesses of such medicines, but do not constitute formal NICE guidance9. STGs that provide physicians with details about the use of off-label medicines would be especially useful for paediatric treatment in India, where there is already widespread off-label use of drugs90.
When Indian STGs are compared to NICE guidelines, they fall short in many points. NICE guidelines are updated many times every few years depending on the condition, year of revision and revised points are mentioned clearly, diagnostic criteria and drug/ treatment of choice are given, and it is evidence-based with appropriate scientific references mentioned. In USA, there are multiple agencies which frame the STGs and these are grounded on evidence which are of high-quality with level of evidence and class of recommendations mentioned in most of the guidelines. But just like NICE guidelines, STGs in the USA do not mention points about the cost of treatment91. Level of healthcare in the STGs is a unique feature to Indian STGs and this does not find a place in NICE guidelines and USA STGs.
Among the major limitations, STGs are often not published in peer-reviewed scientific journals and are often not available online. The process of developing STGs (except the use of evidence base) was not evaluated as this information was not uniformly available.
India demonstrates great regional variation in disease prevalence as well as the kinds of healthcare providers settings and prescribers. The present analysis while appreciating the STGs developed by the government, individuals and professional organizations in India, demonstrated the need for a collaborative and coordinated approach to STG development, with robust consultative mechanisms, sensitivity to local conditions and easy accessibility. An important first step in this regard would be to provide details of well-defined procedures.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Standard Treatment Guidelines: Drug and Therapeutics Committee Training Course. Available from: https://www.who.int/medicines/technical_briefing/tbs/participant-s-guide-all-sessions.pdf
- Barriers and facilitators to development of standard treatment guidelines in India. WHO South East Asia J Public Health. 2015;4:86-91.
- [Google Scholar]
- 2003. Drug and therapeutics committees: A practical guide. Geneva, Switzerland: World Health Organization; Available from: http://apps.who.int/medicinedocs/pdf/s4882e/s4882e.pdf
- Behavior of health workers toward the implementation of clinical guidelines. Health Res Rev. 2016;3:6-10.
- [Google Scholar]
- Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:E1000097.
- [Google Scholar]
- 2008. The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Oxford: Cochrane Collaboration; Available from: http://communityarchive.cochrane.org/sites/default/files/uploads/Handbook 4.2.6Sep2006.pdf
- 2010. Clinical Establishments (Registration & Regulation) Act, 2010. Available from: http://clinicalestablishments.gov.in/cms/Home.aspx
- Understanding the new NHS. Available from: https://www.nhs.uk/nhsengland/thenhs/about/documents/simple-nhs-guide.pdf
- 2019. NICE Advice. Our Programmes, What we do About. NICE. Available from: https://www.nice.org.uk/about/what-wedo/our-programmes/nice-advice
- 2010. National Clinical Guideline Centre – Guidelines. Available from: https://www.rcplondon.ac.uk/about-us/what-we-do/national-guideline-centre-ngc
- 2013. The National Institute for Health and Care Excellence (Constitution and Functions) and the Health and Social Care Information Centre (Functions) Regulations. England: National Health Service; Available from: http://www.legislation.gov.uk/uksi/2013/259/made
- Consensus guidelines on management of childhood asthma in India. Indian Pediatr. 1999;36:157-65.
- [Google Scholar]
- Guidelines for management of asthma at primary and secondary levels of health care in India (2005) Indian J Chest Dis Allied Sci. 2005;47:309-43.
- [Google Scholar]
- Best treatment guidelines for bronchial asthma. Med J Armed Forces India. 2007;63:264-8.
- [Google Scholar]
- Guidelines for diagnosis and management of bronchial asthma: Joint ICS/NCCP (I) recommendations. Lung India. 2015;32:S3-42.
- [Google Scholar]
- Guidelines for Management of Type 2 Diabetes. Available from: http://icmr.nic.in/guidelines_diabetes/guide_diabetes.htm
- Aravind Eye Care System. Guidelines for the Comprehensive Management of Diabetic Retinopathy in India. VISION 2020. 2008. The Right to Sight INDIA Publication. :1-81. Available from: https://www.iapb.org/sites/iapb.org/files/GuidelinesfortheComprehensiveManagementofDRinIndia.pdf
- [Google Scholar]
- Indian National Consensus Group. Premix insulin: Initiation and continuation guidelines for management of diabetes in primary care. J Assoc Phys India. 2009;57:42-6.
- [Google Scholar]
- Type 1 diabetes mellitus in children and adolescents in India: Clinical Practice Guidelines 2011. Available from: https://www.ispae.org.in/download_docs/Diabetes_guideline_for_Web.pdf
- 2014. Special Issue on Consensus Statements on Insulin Therapy. Mumbai: Shah; Available from: http://www.japi.org/july_2014_special_issue/contents.html
- 2014. National Guidelines for Diagnosis & Management of Gestational Diabetes Mellitus. Ministry of Health and Family Welfare. Available from: http://www.nrhmorissa.gov.in/writereaddata/upload/documents/national%20guidelines%20for%20diagnosis%20&%20management%20of%20gestational%20diabetes%20mellitus.pdf
- Clinical practice guidelines for the management of depression. Available from: http://www.indianjpsychiatry.org/cpg/cpg2004/CPG-PsyInd_13.pdf
- Clinical practice guidelines for the management of schizophrenia. Available from: http://www.indianjpsychiatry.org/cpg/cpg2004/CPG-PsyInd_09.pdf
- Guidelines for the treatment of sleep disorders. Available from: http://www.indianjpsychiatry.org/
- Clinical Practice Guidelines for Treatment of Depression in Elderly. Available from: http://www.indianjpsychiatry.org/cpg/cpg2007/CPGGtiPsy_10.pdf
- Clinical practice guidelines for the management of reversible dementias. Available from: http://www.indianjpsychiatry.org/cpg/cpg2007/CPG-GtiPsy_12.pdf
- Clinical practice guidelines for treatment of vascular dementia. Available from: http://www.indianjpsychiatry.org/cpg/cpg2007/CPG-GtiPsy_11.pdf
- 2008. Guidelines for the Management of Epilepsy in India. Indian Epilepsy Association; Available from: http://www.epilepsyindia.org/ies/GUIDELINES/Gemind_Combine.pdf
- Expert Committee on Pediatric Epilepsy, Indian Academy of Pediatrics. Guidelines for diagnosis and management of childhood epilepsy. Indian Pediatr. 2009;46:681-98.
- [Google Scholar]
- Indian Guidelines on the Management of SLE. Available from: http://medind.nic.in/jaa/t02/i4/jaat02i4p80.pdf
- Guidelines on the diagnosis and the current management of headache and related disorders. Ann Indian Acad Neurol. 2011;14:S40-59.
- [Google Scholar]
- Consensus guidelines on management of childhood convulsive status epilepticus. Indian Pediatr. 2014;51:975-90.
- [Google Scholar]
- Guidelines for Pregnancy Care and Management of Common Obstetric Complications by Medical Officers. Available from: http://www.nrhmorissa.gov.in/writereaddata/upload/documents/normal_delivery_and_management_of_obstetric_complications_.pdf
- 2008. Developed under the Government of India – WHO Collaborative Programme. Available from: http://www.searo.who.int/india/topics/cardiovascular_diseases/NCD_Resources_COMBINED_MANUAL_for_medical_officer.pdf
- National snakebite management protocol (India), 2008 (Shortened version) Indian J Emerg Pediatr. 2008;1:63-81.
- [Google Scholar]
- 2009. AIOS Guidelines to Prevent Intraocular Infection. India: All India Ophthalmological Society, & Cipla; Available from: http://www.aios.org/guidelinesendoph.pdf
- Indian rheumatology association guidelines for management of glucocorticoid-induced osteoporosis. Endorsed by: Endocrine Society of India and Indian Society for Bone & Mineral Research. Indian J Rheumatol. 2011;6:68-75.
- [Google Scholar]
- Standard Treatment Guideline & Essential Medicine List. Available from: http://osmcl.nic.in/sites/default/files/guidelines/standard%20treatment%20guideline%20-%20pregnancy%20%26%20sick%20newborn.pdf
- Guidelines for the Management of Cataract in India. 2011. Vision 2020. Right to Sight India Publication. Royal Commonwealth Society for the Blind; Available from: https://www.sightsaversindia.in/wp-content/uploads/2014/06/16480_Cataract_Manual_VISION2020.pdf
- [Google Scholar]
- Indian society of gastroenterology consensus on ulcerative colitis. Indian J Gastroenterol. 2012;31:307-23.
- [Google Scholar]
- 2013. Indian Chronic Kidney Disease Guidelines. Available from: http://isn-india.org/images/CKD_1.pdf
- Special issue on indian guidelines on hypertension (I.G.H.)-III. J Assoc Phys India. 2013;61:6-36.
- [Google Scholar]
- 2014. Consensus & evidencebased INOSA guidelines (Indian initiative on Obstructive Sleep Apnea Guidelines). (1st ed). New Delhi: All India Institute of Medical Sciences; Available from: https://www.icmr.nic.in/sites/default/files/guidelines/consensus%20and%20evidence%20based%20inosa%20guidelines%202014.pdf
- Management of neonatal cholestasis: Consensus statement of the pediatric gastroenterology chapter of Indian academy of pediatrics. Indian Pediatr. 2014;51:203-10.
- [Google Scholar]
- Guidelines for treatment of recurrent or metastatic head and neck cancer. Indian J Cancer. 2014;51:89-94.
- [Google Scholar]
- Guidelines for diagnosis and management of chronic obstructive pulmonary disease: Joint Recommendations of Indian Chest Society and National College of Chest Physicians (India) Indian J Chest Dis Allied Sci. 2014;56:5-54.
- [Google Scholar]
- IAP guidelines 2006 on management of acute diarrhea. Indian Pediatr. 2007;44:380-9.
- [Google Scholar]
- Endocrine society of india management guidelines for patients with thyroid nodules: A position statement. Indian J Endocrinol Metab. 2011;15:2-8.
- [Google Scholar]
- Revised Statement on Management of Urinary Tract Infections. Available from: http://medind.nic.in/ibv/t11/i9/ibvt11i9p709.pdf
- Guidelines for diagnosis and management of community- and hospital-acquired pneumonia in adults: Joint ICS/NCCP(I) recommendations. Lung India. 2012;29:S27-62.
- [Google Scholar]
- Consensus statement of HCV task force of the Indian national association for study of the liver (INASL). Part II: INASL recommendations for management of HCV in India. J Clin Exp Hepatol. 2014;4:117-40.
- [Google Scholar]
- National guidelines on prevention, management, and control of reproductive tract infections including sexually transmitted infections. Available from: http://naco.gov.in/sites/default/files/national_guidelines_on_pmc_of_rti_including_sti%201.pdf
- Guidelines for Filariasis Elimination in India. Available from: https://nvbdcp.gov.in/writereaddata/l892s/43461824631532409675.pdf
- 2012. General of Health Services, Ministry of Health & Family Welfare, Government of India Nirman Bhavan, New Delhi Guidelines for Primary, Secondary and Tertiary Level Care Disability Prevention & Medical Rehabilitation. Available from: http://nlep.nic.in/pdf/guidelines%20for%20primary,%20secondary%20and%20tlc%20(atul%20shah%2024.7.2012.pdf
- Diagnosis and Treatment of Malaria. Available from: http://nvbdcp.gov.in/Doc/Diagnosis-Treatment-Malaria-2013.pdf
- ART Guidelines for HIV-Infected Adults and Adolescents. Available from: http://www.naco.gov.in/upload/Policies&Guidelines/AntiretroviralTherapyGuidelinesforHIV-InfectedAdultsandAdolescents.pdf
- National Programme for Prevention and Control of Japanese Encephalitis/Acute Encephalitis Syndrome. Available from: http://www.epilepsyindia.org/ies/GUIDELINES/Gemind_Combine.pdf
- 2015. Operational Guidelines on Kala-Azar (Visceral Leishmaniasis) Elimination In India. Available from: http://nvbdcp.gov.in/Doc/opertional-guideline-KA-2015.pdf
- Standards for TB Care in India: Ministry of Health and Family Welfare. Available from: http://www.tbcindia.nic.in/showfile.php?lid=3061
- National Guidelines for Clinical Management of Dengue Fever. Available from: http://pbhealth.gov.in/denguenational-guidelines-2014%20compressed.pdf
- 2015. National Guidelines on Rabies Prophylaxis. National Rabies Control Program; Available from: http://pbhealth.gov.in/guideline%20for%20rabies%20prophylasix.pdf
- Diagnosis & Treatment of Kala-Azar. Available from: http://nvbdcp.gov.in/Doc/Guidelines-Diagnosis-Treatment-KA.pdf
- Standard Treatment Guidelines. Available from: http://www.clinicalestablishments.nic.in/En/1068-standard-treatmentguidelines.aspx
- 2003. State Heal Resour Cent. Government of Chhattisgarh, Department of Health & Family Welfare. Available from: http://www.cspc.in/dic/pdf/cg_stg.pdf
- 2007. Standard treatment guidelines: Medical management and costing of select conditions. Pune: WHO Country Office; Available from: http://opac.tiss.edu/cgi-bin/koha/opac-detail.pl?biblionumber=247509
- 2009. Standardisation Initiatives By The FICCI Health Insurance Group. Available from: http://ficci.in/SEdocument/20106/health-insuracne-brochure.pdf
- Standard Treatment Guidelines: A Manual for Medical Practitioners. Available from: http://www.tnhsp.org/files/StandardtreatmentGuidelines.pdf
- 2012. Rajasthan State Standard Treatment Guidelines 2012. Available from: http://rmsc.health.rajasthan.gov.in/content/dam/doitassets/medical-and-health-portal/rajasthan-medical-corporation/pdf/standard%20treatment%20guidelines/prelims-2012.indd.pdf
- 2013. Standard Treatment Guidelines Haryana. Available from: http://hshrc.gov.in/wp-content/uploads/STG-PDF.pdf
- Indian Council of Medical Research. ICMR Guidelines. Available from: http://icmr.nic.in/About_Us/Guidelines.html
- 2013. Standard Treatment Guidelines A Manual for Medical Therapeutics Health & Family Welfare Department Government of Gujarat. (1st ed). Available from: https://gmscl.gujarat.gov.in/Images/pdf/standard-treatment-guidelines.pdf
- 2014. Standard Treatment Guidelines. Available from: http://www.health.mp.gov.in/sites/default/files/documents/STG-2014_0.pdf
- 2012. Clinical Establishments Rules 2012. New Delhi: Central Government; :5-10. Available from: http://clinicalestablishments.gov.in/WriteReadData/386.pdf
- Health and Social Care Act 2012 [Internet]. Queen's Printer of Acts of Parliament; 2012 p. Section 237. Available from: http://www.legislation.gov.uk/ukpga/2012/7/section/237/enacted
- The National Institute for Health and Care Excellence (Constitution and Functions) and the Health and Social Care Information Centre (Functions) Regulations 2013 (SI 2013/259). Available from: http://www.legislation.gov.uk/uksi/2013/259/regulation/5/made
- Legal considerations of clinical guidelines: Will NICE make a difference? J R Soc Med. 2003;96:133-8.
- [Google Scholar]
- Legal and political considerations of clinical practice guidelines. BMJ. 1999;318:661-4.
- [Google Scholar]
- How does evidence based guidance influence determinations of medical negligence? BMJ. 2004;329:1024-8.
- [Google Scholar]
- Status of national guidelines in dictating individual clinical practice and defining negligence. Br J Anaesth. 2012;108:557-61.
- [Google Scholar]
- Agency for Healthcare Research and Quality: A Profile. Available from: https://www.ahrq.gov/cpi/about/profile/index.html
- Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E, eds. Clinical practice guidelines we can trust. Washington (DC): National Academies Press; 2011.
- Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. 2011. Clinical Practice Guidelines We Can Trust. Washington (DC): National Academies Press (US); Available from: http://www.ncbi.nlm.nih.gov/pubmed/24983061
- [Google Scholar]
- 2016. Determining extent adherence to the IOM standards in AHRQ's NGC. National Guideline Clearinghouse. Available from: https://www.guideline.gov/help-and-about/summaries/determining-extentadherence-to-the-iom-standards-in-ngc
- Potential facilitators and barriers to adopting standard treatment guidelines in clinical practice. Int J Health Care Qual Assur. 2017;30:285-98.
- [Google Scholar]
- AGREE Next Steps Consortium. The AGREE reporting checklist: A tool to improve reporting of clinical practice guidelines. BMJ. 2016;352:I1152.
- [Google Scholar]
- Cost-Effectiveness and Clinical Practice Guidelines: Have We Reached a Tipping Point?-An Overview. Value Health. 2016;19:512-5.
- [Google Scholar]






