Translate this page into:
Elevated levels of glutathionyl haemoglobin as an oxidative stress marker in patients with major depressive disorder
For correspondence: Dr Amit Kumar Mandal, Division of Molecular Medicine, Clinical Proteomics Unit, St. John's Research Institute, St. John's National Academy of Health Sciences, 100 ft Road, Koramangala, Bengaluru 560 034, Karnataka, India e-mail: amit@sjri.res.in
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Oxidative stress has been implicated in the pathophysiology of major depressive disorder (MDD), but biomarkers to assess oxidative stress in patients with MDD have yielded ambiguous results. Glutathionyl haemoglobin (GS-Hb) has been reported as a stable and potential biomarker for oxidative stress in various clinical conditions. The objective of the study was to evaluate GS-Hb as a potential biomarker of oxidative stress in patients with MDD through its quantification and to compare the levels of GS-Hb in age- and gender-matched healthy controls.
Methods:
The levels of GS-Hb were estimated using liquid chromatography coupled to electrospray ionization mass spectrometry in patients diagnosed with MDD and in a subset of patients after six weeks of treatment with selective serotonin reuptake inhibitors (SSRIs).
Results:
GS-Hb levels in drug-naïve patients with MDD (n=26) were significantly elevated compared to matched healthy controls (n=17). GS-Hb levels were not significantly different between MDD patients with and without co-morbid anxiety disorders. There were no significant differences in GS-Hb levels following six weeks of treatment with SSRIs compared to baseline.
Interpretation & conclusions:
Compared to controls, GS-Hb level in patients with MDD was significantly elevated, suggestive of increased oxidative stress associated with MDD. However, six weeks of antidepressant treatment was not sufficient to modify the alterations in antioxidant/oxidant system. Further studies need to be done with a large sample of MDD patients with a longer duration of antidepressant treatment.
Keywords
Antidepressants
glutathionyl haemoglobin
major depressive disorder
mass spectrometry
oxidative stress
Increased oxidative stress has been implicated in the pathophysiology of major depressive disorder (MDD)1. Major depression is a common psychiatric condition and considered as the fourth leading cause of disability worldwide2. In addition, major depression is associated with chronic medical conditions including coronary artery disease and diabetes mellitus3.
Decreased levels of vitamin C4 and superoxide dismutase (SOD)5 and elevated levels of reactive pro-oxidants have been reported in patients with major depression compared to age- and gender-matched healthy controls. Other studies have reported an increased concentration of lipid peroxidation products such as F2-isoprostanes6, conjugated dienes7 and malondialdehyde (MDA) in patients with MDD8. In addition, patients with recurrent depression showed significantly lower levels of glutathione peroxidise (GPx) and SOD compared to first-episode depression patients9. These observations indicated an increased oxidative stress in patients with MDD. Bilici et al10 have shown a significant reduction in the levels of oxidative stress markers in MDD after treatment with antidepressants while others did not find any significant difference after treatment11.
S-glutathionylation of proteins is an example of oxidative post-translational modification, which is known to protect irreversible thiol oxidation of proteins by reactive oxygen species (ROS). Under the condition of oxidative stress, ROS is destroyed by the oxidation of reduced glutathione, an antioxidant present in the living cells. In addition, the elevated levels of oxidised glutathione might undergo thiol exchange with free and accessible cysteine residues of proteins resulting in glutathionylated proteins (GSSPs)12. Elevated levels of glutathionyl haemoglobin (GS-Hb) have been found in several clinical conditions associated with oxidative stress such as chronic renal failure13, iron deficiency anaemia14, type 2 diabetes mellitus15 and Friedreich's ataxia16.
In the present study, the levels of GS-Hb were measured in drug-naïve patients with MDD and compared with that in healthy controls. Furthermore in a subset of MDD patients, changes in the level of GS-Hb were determined after treatment with antidepressant drugs belonging to selective serotonin reuptake inhibitor (SSRI) class.
Material & Methods
The study was conducted in the St. John's Medical College and Hospital, St. John's National Academy of Health Sciences (SJNAHS), Bengaluru, India, during the period of July 2014 to August 2016. The study was approved by the institutional ethical review board. Consecutive patients who were diagnosed with recurrent or first-episode depressive disorder as per the International Classification of Diseases-10 (ICD-10) guidelines17 were recruited into the study after obtaining informed consent (n=26). Patients with comorbid substance dependence syndrome and/or bipolar disorder and coexisting chronic medical conditions were excluded. Healthy controls were recruited among the volunteer staff and students of SJNAHS (n=17). The mental health status of healthy controls was assessed using the Kessler Psychological Distress Scale (K-10)18. Those with no history of any psychiatric illness and use of any psychotropic medication and who scored less than the recommended cut-off score of 20 on K-10 were enrolled in the study as healthy controls.
Psychiatric diagnosis was confirmed using the Mini-International Neuropsychiatric Interview (MINI-Plus)17. The 17-item Hamilton Depression Rating Scale (HDRS)19 was used to assess the severity of depression. Samples were collected from a subset of patients (n=11) after six weeks of treatment with antidepressant to assess changes in GS-Hb levels in response to treatment. All patients in the study were treated with specific serotonin reuptake inhibitor (SSRI) class of antidepressants. Eight patients received sertraline in the dose range of 25-75 mg/day, and three patients were treated with escitalopram (dose range 10-15 mg/day).
Sample collection and processing: All first-episode MDD patients were drug naïve, while patients with recurrent depressive disorder had at least two weeks of washout period for antidepressant medication at the time blood sample was collected. Five millilitres of blood was collected into a vacutainer tube containing EDTA as anticoagulant, the plasma was separated. In a subset of patients, the samples were again collected after the treatment with antidepressant medication for six weeks. The red blood cells were washed with 0.9 per cent NaCl (aqueous) thrice before lysis with eight volumes of ice-cold distilled water. The haemolysate was centrifuged at 13,400 g for 10 min at 4°C to remove the erythrocyte membranes. The haemolysate was immediately dialysed against 10 mM ammonium acetate, pH 7.4 and stored at −120°C until mass spectrometric analysis.
Liquid chromatography (LC) electrospray ionization mass spectrometry (ESI-MS) based relative quantification of glutathionyl haemoglobin: Electrospray ionization mass spectra (ESI-MS) were acquired in positive ion “V” mode using a Q-Tof Synapt HDMS (Waters, UK). The haemolysate (3 μg) was injected into a C18 RP column (Eclipse, 4.6 mm × 150 mm, 5 μm) in a Shimadzu ultra performanceliquid chromatography (LC, Shimadzu, Japan). The different globin chains were separated using a linear gradient of two per cent increase in acetonitrile per minute containing 0.1 per cent acetic acid with a flow rate of 0.2 ml/min. The ESI-MS data were acquired over the mass range of 600-1600 m/z, with capillary voltage of 3 kV using a source temperature of 120°C and desolvation gas temperature of 350°C. The ESI-MS was mass calibrated using sodium iodide. The charge state mass spectra were deconvoluted, and the peak intensity for each globin chain was extracted. The relative percentage of GS-Hb with respect to normal beta globin and its adducts (glutathionyl beta and glycated beta) was calculated from the deconvoluted spectra using the following formula15:

Statistical analysis: The GS-Hb values in patients with MDD were compared with healthy controls using Mann-Whitney U-test. The HDRS score and GS-Hb values measured before and after treatment with antidepressants were compared using the Wilcoxon signed-rank test. Spearman's rank correlation was used to measure the correlation between the change in GS-Hb values and change in HDRS score following six weeks of treatment with antidepressants (OriginPro v 8.0, Origin Lab, USA).
Results & Discussion
Oxidative stress is involved in the pathophysiology of MDD in multiple ways20. Activation of oxidative and nitrosative pathways has been shown to damage membrane lipids and DNA of the neuronal cells altering neuronal membrane fluidity and integrity. This results in activation of the apoptotic pathway leading to cell death21. An increased level of apoptosis has been observed in blood leucocytes and neuronal cells of MDD patients compared to healthy controls22. Furthermore, MDD has also been reported to be associated with chronic inflammation23. Thus, increased oxidative stress in MDD may be a result of chronic inflammation.
GSSPs have been reported to act as a protective mechanism against irreversible oxidative modification of important protein thiols24. In the present study, glutathionylated haemoglobin was chosen as a marker of oxidative stress in patients with MDD. The relative level of GS-Hb with respect to normal globin chains and their adducts was calculated from the deconvoluted spectra of ESI-MS analysis (Fig. 1). The levels of normalized GS-Hb were found to be significantly elevated in patients with MDD (n=26), median (Q1, Q3)=8.34 per cent (6.52, 10.16), compared to healthy controls (n=17), median (Q1, Q3)=5.73 per cent (4.62, 6.47) (P=0.01) (Table). The GS-Hb values obtained for patients with MDD having co-morbid anxiety disorders (n=10), median (Q1, Q3)=7.93 per cent (6.72, 11.43), were not statistically significantly different from patients with MDD not having any co-morbid anxiety disorders (n=16), median (Q1, Q3)=8.63 per cent (6.48, 9.75). In a subset of patients (n=11), GS-Hb values were estimated at six-week post-treatment with antidepressant medication. The severity of depression assessed by HDRS showed a significant reduction in post-treatment samples, median (Q1, Q3)=6 (5, 8), compared to pre-treatment samples, median (Q1, Q3)=19 (15, 20) (P=0.001). Although GS-Hb values decreased in post-treatment samples, median (Q1, Q3)=7.68 per cent (5.46, 9.26), compared to pre-treatment samples, median (Q1, Q3)=8.07 per cent (6.54, 10.71), the reduction observed was not significant. However, a significant positive correlation was observed between the changes in GS-Hb levels before and after treatment with changes in the severity of depression on HDRS (correlation coefficient 0.747; P=0.0081). Fig. 2 depicts the scatter plot of the comparison between GS-Hb levels and HDRS scores estimated before and after treatment with antidepressants.
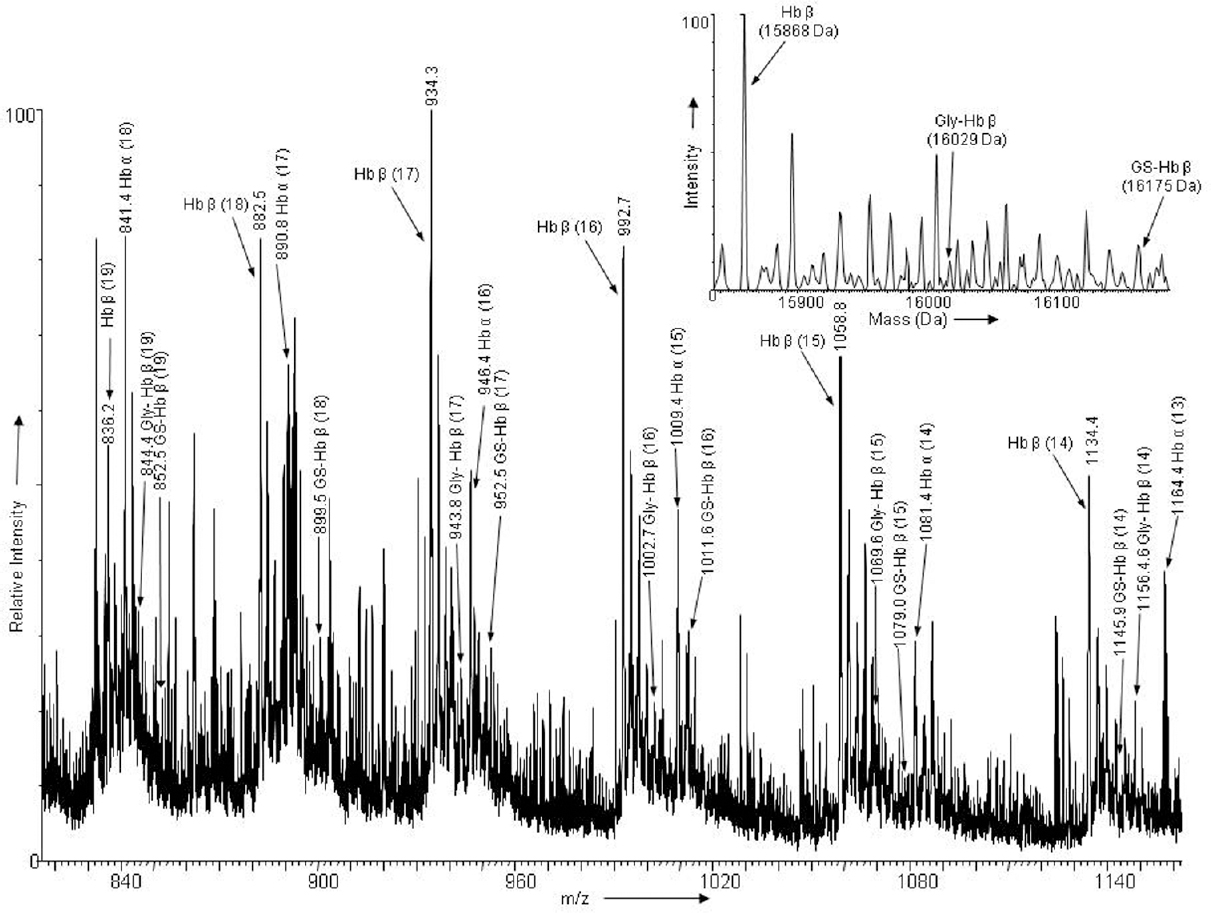
- Electrospray ionization mass spectra of intact alpha and beta globin chains of haemoglobin obtained from a patient with major depressive disorder: The charge states (parenthesis) of Hbα, Hbβ, glutathionylated haemoglobin beta (GS-Hbβ) and glycated haemoglobin beta (Gly-Hbβ) are labelled. The deconvoluted mass spectra of Hbβ, GS-Hbβ and Gly-Hbβ are shown as inset.
| Description | MDD Pre-treatment (n=26) | Healthy controls (n=17) |
|---|---|---|
| Age (yr) | 40.8±13.4 | 38.52±13.93 |
| Gender | Males (n=13) | Males (n=9) |
| Females (n=13) | Females (n=8) | |
| Nature of the episode | First episode (n=18) | Nil |
| RDD (n=8) | ||
| Co-morbidities | Panic (n=4) | Nil |
| Anxiety (n=6) No | ||
| co-morbidity (n=16) | ||
| GS-Hb (%) | Median (Q1, Q3)=8.34 (6.52, 10.16) | Median (Q1, Q3)=5.73 (4.62, 6.47) (P=0.0027) |
GS-Hb, glutathionyl haemoglobin; RDD, recurrent depressive disorder
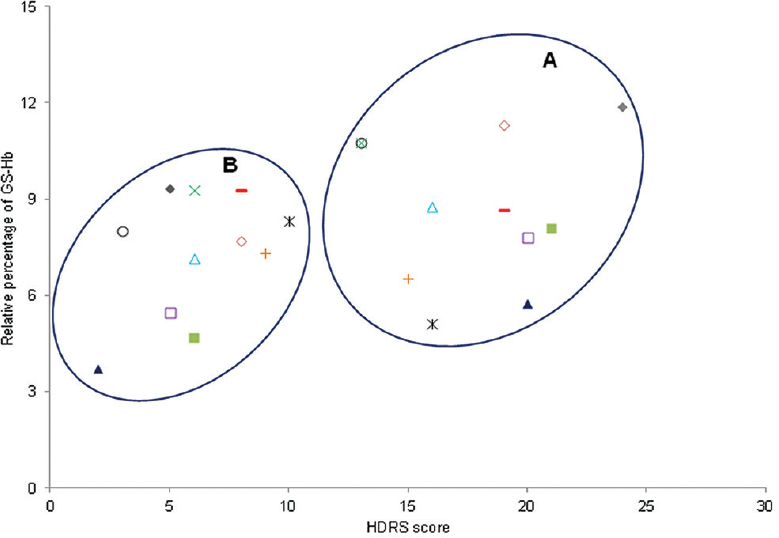
- Scatter plot of Hamilton Depression Rating Scale (HDRS) score against glutathionyl haemoglobin (GS-Hb) values of individual patients (coded with different symbols) measured at pre-treatment and post-treatment conditions: (A) scatter plot of pre-treatment HDRS score versus pre-treatment GS-Hb values. (B) scatter plot of post-treatment HDRS score versus post-treatment GS-Hb values.
The observed increase in GS-Hb levels in drug-naïve MDD patients compared to healthy controls was in agreement with previous reports of elevated oxidative stress in patients with MDD. Bilici et al10 reported a significant decrease in plasmatic GPx and MDA in erythrocyte and plasma after three months of treatment with fluoxetine, fluvoxamine, sertraline and citalopram. Similar results were reported by other investigators with a decrease in oxidative stress following treatment with SSRI group of antidepressant drugs2526. However, some investigators did not find any difference in markers of oxidative stress following treatment1127. These conflicting results may be related to different classes of antidepressants used in different studies. Duration of treatment may also be a factor with negative findings being more common in studies with shorter duration of treatment (6-8 wk)28.
The strength of the study was that psychiatric diagnosis of all patients was arrived at using semi-structured psychiatric interview schedule, which also allowed to capture co-morbid psychiatric conditions. Limitations of our study included moderate sample size and relatively short duration of treatment with antidepressant medication. In addition, the presence or absence of significant life stressors in both patients and controls was not considered. The relationship between GS-Hb levels and commonly used inflammatory markers also needs to be explored.
GS-Hb, a marker of oxidative stress, was significantly elevated in patients with MDD compared to healthy controls. The observed lack of significant difference in GS-Hb levels between pre- and post-treatment groups might be due to a relatively shorter duration of intervention with antidepressant medication. Future studies need to explore the association of GS-Hb with MDD in a large sample group and longer duration of treatment with antidepressants.
Acknowledgment
Authors acknowledge all patients with major depression and healthy controls who participated in the study.
Financial support & sponsorship: Authors acknowledge the Department of Science and Technology (DST), New Delhi, India, for supporting the study (SR/SO/HS-131/2007).
Conflicts of Interest: None.
References
- The relationship between potency of oxidative stress and severity of depression. Acta Neuropsychiatr. 2004;16:200-3.
- [Google Scholar]
- The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119-38.
- [Google Scholar]
- Role of antioxidants in generalised anxiety disorder and depression. Indian J Psychiatry. 2012;54:244-7.
- [Google Scholar]
- Lipid peroxidation and antioxidant protection in patients during acute depressive episodes and in remission after fluoxetine treatment. Pharmacol Rep. 2009;61:436-47.
- [Google Scholar]
- Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress – Preliminary findings. PLoS One. 2011;6:e17837.
- [Google Scholar]
- Antioxidative enzymes and increased oxidative stress in depressive women. Clin Biochem. 2009;42:1368-74.
- [Google Scholar]
- A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:102-11.
- [Google Scholar]
- The relevance of oxidative stress status in first episode and recurrent depression. J Affect Disord. 2012;143:34-8.
- [Google Scholar]
- Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J Affect Disord. 2001;64:43-51.
- [Google Scholar]
- Increased oxidative stress in patients with depression and its relationship to treatment. Psychiatry Res. 2013;206:213-6.
- [Google Scholar]
- Protein glutathionylation and oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;855:59-65.
- [Google Scholar]
- Quantitation and characterization of glutathionyl haemoglobin as an oxidative stress marker in chronic renal failure by mass spectrometry. Clin Biochem. 2007;40:986-94.
- [Google Scholar]
- Glutathionyl hemoglobin is elevated in iron deficiency anemia. Acta Haematol. 2012;127:26-30.
- [Google Scholar]
- Increased glutathionylated hemoglobin (HbSSG) in type 2 diabetes subjects with microangiopathy. Clin Biochem. 2005;38:892-9.
- [Google Scholar]
- Glutathione in blood of patients with Friedreich's ataxia. Eur J Clin Invest. 2001;31:1007-11.
- [Google Scholar]
- The Mini International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22-33.
- [Google Scholar]
- Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32:959-76.
- [Google Scholar]
- Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012;36:764-85.
- [Google Scholar]
- The role of oxidative stress in depressive disorders. Curr Pharm Des. 2012;18:5890-9.
- [Google Scholar]
- Accelerated apoptosis of blood leukocytes and oxidative stress in blood of patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:686-94.
- [Google Scholar]
- Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun. 2013;31:143-52.
- [Google Scholar]
- Protein glutathionylation in health and disease. Biochim Biophys Acta. 2013;1830:3165-72.
- [Google Scholar]
- Oxidative damage and major depression: The potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003;8:365-70.
- [Google Scholar]
- Total antioxidant capacity and total oxidant status in patients with major depression: Impact of antidepressant treatment. Psychiatry Clin Neurosci. 2009;63:639-45.
- [Google Scholar]
- Major depressive disorder is accompanied with oxidative stress: Short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22:67-73.
- [Google Scholar]
- Preclinical and clinical evidence of antioxidant effects of antidepressant agents: Implications for the pathophysiology of major depressive disorder. Oxid Med Cell Longev. 2012;2012:609421.
- [Google Scholar]






