Translate this page into:
Feeder & basic fibroblast growth factor-free culture of human embryonic stem cells: Role of conditioned medium from immortalized human feeders
Reprint requests: Dr Sujata Mohanty, Stem Cell Facility, 1st Floor ORBO Complex, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110 029, India e-mail: drmohantysujata@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Human embryonic stem cell (hESC) lines are commonly maintained on inactivated feeder cells, in the medium supplemented with basic fibroblast growth factor (bFGF). However, limited availability of feeder cells in culture, and the high cost of growth factors limit their use in scalable expansion of hESC cultures for clinical application. Here, we describe an efficient and cost-effective feeder and bFGF-free culture of hESCs using conditioned medium (CM) from immortalized feeder cells.
Methods:
KIND-1 hESC cell line was cultured in CM, collected from primary mouse embryonic fibroblast, human foreskin fibroblast (HFF) and immortalized HFF (I-HFF). Pluripotency of KIND-1 hESC cell line was confirmed by expression of genes, proteins and cell surface markers.
Results:
In culture, these cells retained normal morphology, expressed all cell surface markers, could differentiate to embryoid bodies upon culture in vitro. Furthermore, I-HFF feeder cells without supplementation of bFGF released ample amount of endogenous bFGF to maintain stemness of hESC cells.
Interpretation & conclusions:
The study results described the use of CM from immortalized feeder cells as a consistent source and an efficient, inexpensive feeder-free culture system for the maintenance of hESCs. Moreover, it was possible to maintain hESCs without exogenous supplementation of bFGF. Thus, the study could be extended to scalable expansion of hESC cultures for therapeutic purposes.
Keywords
Embryonic stem cells
fibroblast growth factor
immortalized feeder cells
insulin growth factor-II
transforming growth factor-beta
Human embryonic stem cells (hESCs) are capable of unlimited cell growth, self-renewal and pluripotent development and hold great promise for regenerative medicine1. To generate and maintain hESCs in undifferentiated state, presence of feeder cells is required2. Conventionally, murine-derived feeder cells are used to support undifferentiated growth, renewal capacity and pluripotency of hESCs3. Besides, feeders derived from the human foetus, placenta, postnatal and adult fibroblasts are also being used to maintain hESC culture456. However, in some cases, use of feeder cells remains a challenge7. Preparation of feeder cells is labour intensive and requires extensive culture in large-scale experiments. Moreover, feeder cells can be cultured for limited passages, thus involve preparing fresh batches of feeders on a routine basis leading to batch-to-batch variation. A number of immortalized feeders have also been used to overcome this limitation8910; however, transmission of viral and other cryptic pathogens from allogeneic feeder cells limit applicability of cultured hESCs in therapeutics.
Use of feeder-free culture and conditioned medium (CM) from primary feeder cells has been shown to minimize the transmission of pathogens and to maintain hESCs in undifferentiated state11121314; however, availability of CM for scalable expansion of hESCs remains a limiting factor. CM contains a complex supplement of protein and metabolic factors that maintain hESCs in the undifferentiated and pluripotent state. Many of the proteins including transforming growth factor-beta (TGF-β), basic fibroblast growth factor (bFGF) and insulin-like growth factor (IGF) are present in CM and have been demonstrated to have a crucial role in regulating stemness of pluripotent stem cells1516. In addition, supplementation of bFGF is required to sustain pluripotent characteristics of hESCs1317. The bFGF has been reported to cooperate both TGF-β and IGF-II to maintain hESCs in the feeder-free system, by providing a stem cell niche1216.
In the present study, CM was used from immortalized human foreskin fibroblast (I-HFF) feeder cultures and compared with naive HFF to maintain KIND-1 hESC cultures and to overcome the problems of batch-to-batch variation and limited lifespan in culture. In addition, the cooperation and quantification of TGF-β and IGF-II in association with exogenous bFGF in I-HFF feeder and HFF feeder cultures was also explored.
Material & Methods
This study was conducted from June 2013 to April 2014 in the Stem cell Facility of All India Institute of Medical Sciences, New Delhi. Institutional Human Ethics and Stem Cell Ethics Committees approval was taken before using hESCs for research purpose.
Cell culture: HFF cell line was derived from foreskin samples from patients coming for circumcision in the department of Pediatric Surgery. Explants culture method was followed as described by Park et al18. I-HFF (from Geneva University Hospitals, Switzerland) and KIND-1 hESC (from National Institute for Research in Reproductive Health, Mumbai, India) cell lines were cultured as described, previously919. The propagation medium for feeder cell culture was Dulbecco's modified Eagle's medium (DMEM) containing 10 per cent foetal bovine serum (FBS), 1 per cent glutamine and 1 per cent PenStrep (Gibco/Invitrogen, USA). KIND-1 hESC cell line was maintained, in a feeder-free culture over geltrex-coated 65 mm culture dishes in STEMPRO hESC serum-free medium (Gibco/Invitrogen, USA) and taken as positive control. The basal medium used for inactivated feeder cells and KIND-1 hESC cells was composed of Knockout-DMEM (KO-DMEM), 20 per cent Knockout Serum Replacement (Gibco/Invitrogen, USA), 1 per cent non-essential amino acids (Sigma, USA), 1 per cent penicillin/streptomycin (Sigma, USA) and 0.01 mM β-mercaptoethanol (Sigma, USA). The bFGF concentrations used in the study were 0, 2, 5 and 10 ng/ml and designated as groups I, II, III and IV, respectively.
Collection of conditioned medium (CM): CM collection time and culture harvest time are outlined in Fig. 1. Briefly, feeder cells were seeded on 25 cm2 gelatin-coated tissue culture flask at a density of 1.25×106 in propagation medium. After 24 h, feeders were mitotically inactivated, by treating with 10 μg/ml mitomycin C for 3 h. After washing with phosphate-buffered saline, feeder cells were supplemented with basal medium. CM and feeder cells were harvested at days 2 and 5. CM was either used immediately after filtration with 0.22 μm filters or stored at -20°C for further uses.
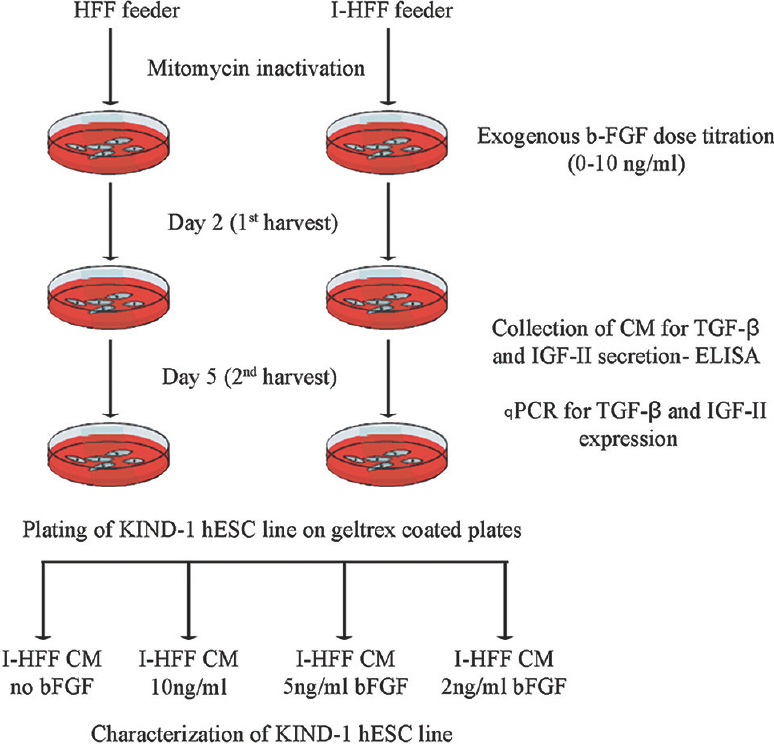
- Schematic diagram for the procedure of human embryonic stem cell culture and time for collection of conditioned medium (CM). HFF, human foreskin fibroblast; I-HFF, immortalized human foreskin fibroblast; bFGF, basic fibroblast growth factor; TGF-β, transforming growth factor-beta; IGF-II, insulin-like growth factor-II.
bFGF secretion in the medium: For the quantification of secreted bFGF, mouse feeder primary mouse embryonic fibroblast (PMEF) and human feeders (I-HFF and HFF) were plated on 0.1 per cent gelatin-coated culture dishes at a density of 3.5×105 cells/cm2 and were mitotically inactivated as described above. The medium was collected on days 1, 3, 5 and 7 from the feeders cultured in the presence of bFGF (10 ng/ml - standard dose) as a positive control or without exogenous bFGF and quantified through enzyme-linked immunosorbent assay (ELISA) detection method using Human FGF basic Quantikine Kit (Quantikine, R&D, USA).
ELISA for TGF-β and IGF-II detection: To determine the cooperation of bFGF with TGF-β and IGF-II, I-HFF cells were plated and treated in the presence or absence of bFGF (2, 5 and 10 ng/ml), whereas HFF cells were treated with only concentration of 10 ng/ml bFGF and were taken as standard control20. The medium was collected on days 2 and 5. TGF-β released in the medium was quantified using ELISA (Human TGF-β ELISA set BD OptEIA™, BD Biosciences, USA). For soluble IGF-II detection, the human IGF-II ELISA kit (Diagnostic System Laboratories, Inc., USA) was used as per manufacturer's recommendation.
Blocking of FGF receptor 1 (FGFR1) in cell cultures: FGFR1-specific chemical inhibitor SU5402 (Sigma, USA) was used to check the cooperation of bFGF with TGF-β and IGF-II. I-HFF cultures were incubated with SU5402 at 20 μM concentration for 60 min before addition of bFGF1221. SU5402 stock solution was prepared in dimethyl sulphoxide (DMSO, Sigma, USA). I-HFF cell line treated with bFGF, and the equivalent volume of DMSO was treated and considered as control. The media were harvested after 24 h for quantification of TGF-β and IGF-II using ELISA. The same media after blocking of FGFR1 were also used to culture hESC line, and morphology of embryonic stem cell colonies was assessed.
Alkaline phosphatase (AP) staining: AP staining was performed using AP detection kit following the manufacturer's recommended procedure (Millipore, USA) and observed under the light microscope (Nikon, Japan).
Characterizing the pluripotency of hESCs under feeder-free conditions: The hESC cell line KIND-1 was phenotypically characterized for hESC surface markers i.e., OCT-4, TRA 1-81, TRA 1-60 and SSEA4 (Chemicon, USA), by immunocytochemistry and flow cytometry as previously described14. Briefly, for immunocytochemistry, fixed hESC colonies were permeabilized and blocked with 3 per cent bovine serum albumin (BSA) for 30 min. Colonies were incubated overnight with primary monoclonal antibodies TRA 1-81, OCT4 (1:100) separately at 4°C, and for colocalization primary antibodies TRA 1-60 and SSEA4 (1:100) incubated simultaneously with cells. Conjugated secondary antibodies Alexa Fluor 488- or 568-labelled (Molecular Probes, USA) (1:500) were used for 2 h at room temperature. Cell nuclei were stained with 4’6-diamino-2 phenylindole (DAPI, Thermo Fisher Scientific, USA). Microscopic analysis was performed using Nikon TE 80i (Nikon, Japan) fluorescent microscope, and images were analyzed using NIS-Elements Advanced Research software (Nikon, Japan).
For analyzing the protein distribution by flow cytometry, KIND-1 hESC line cultured in I-HFF-CM from groups I-IV was dissociated with TrypLE Express (Invitrogen, USA) to a single cell suspension. Cells were incubated with anti-TRA 1-60 (1:100) and anti-SSEA4 (1:100) for 2 h at 4°C. Following washing, cells were stained and incubated with Alexa Fluor 488- or 568-labelled secondary antibodies. Isotype controls for secondary antibodies were included in each set of experiments. After labelling, cells were acquired on a Becton Dickinson LSR II flow cytometer (BD Biosciences, USA) and analyzed by BD FACSDiva software version 6.1.2 (BD Biosciences, USA).
Gene expression analysis using reverse transcription-PCR (RT-PCR), qPCR and StellARray analysis
RT-PCR: Total RNA was isolated using Trizol (MRC Inc., USA) reagent and was reverse transcribed using cDNA synthesis kit (Applied Biosystem, USA) according to manufacturer's instructions. Expressions of FGFR1, TGF-β and IGF-II genes were analyzed using RT- PCR. Forward and reverse primer sequences were 5’-GGCTACAAGGTCCGTTATGC-3′ and 5′-TGGTATGTGTGGTTGATGCTG-3′ for FGFR1; 5′-GCGTGCTAATGGTGGAAAC-3′ and 5′-GCTGAGGTATCGCCAGGAAT-3′ for TGF-β1; 5′-TTGCTCTACCCACCCAAGAC-3′ and 5′-GATGGAACCTGATGGAAACG-3′ for IGF-II and 5′-GAGTCAACGGATTTGGTCGT-3′ and 5′-GACAAGCTTCCCGTTCTCAG-3′ for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for both RT and quantitative PCR (qPCR). Primers for GAPDH were designed in our laboratory using online Primer3 program, and for all other genes, previously published primers were used12. All primers were ordered from Genex Life Sciences (India).
qPCR: The relative expression of TGF-β and IGF-II genes in KIND-1 cells cultured in I-HFF-CM from groups I-IV of I-HFF-CM was analyzed and compared with positive control HFF supplemented with 10 ng/ml bFGF. The qPCR analysis was carried out in mastercycler ep realplex4 real-time PCR system (Eppendorf, Germany) using SYBR green (KAPA Biosystems, South Africa) chemistry for the relative quantification of the gene expression. The reaction was performed in triplicates, and endogenous GAPDH gene level was used to normalize the expression of genes. Relative quantification was calculated using the comparative CT (ΔΔCT) method14. The realplex software was used to analyze the data and presented as mean fold change.
StellARray: Quantitative PCR data collected using hESCs 32 StellARray qPCR Array (Lonza, Switzerland) were used to confirm the expression of key ESC related genes. Two groups of hESCs cultured in I-HFF-CM by groups I and III were randomly chosen and compared with positive control. Each StellARray qPCR Array well was loaded with 10 μl of sample-specific, SYBR Green (KAPA Biosystems, South Africa), the master mix containing a chemically modified hot-start Taq polymerase (Applied Biosystems, Inc., USA). The array was heat-sealed and run on a realplex4 real-time PCR system using default cycling parameters for 40 cycles (1 cycle of 50°C for two minutes, 1 cycle of 95°C for 10 min, 40 cycles of 95°C for 15 sec and 60°C for one minute). Fluorescence data were acquired during the 60°C anneal/extension plateau. Array data analysis and statistical significance were calculated using the Global Pattern Recognition™ (GPR) software (Lonza, USA).
Western blot analysis: For protein expression analysis, Western blotting was performed as described earlier22. Lysates with equal amounts (10 μg) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), subsequently transferred to nitrocellulose membrane and blocked with 3 per cent BSA for overnight at 4°C. The membrane was further incubated with primary anti-OCT4 and anti-GAPDH antibody in blocking solution overnight at 4°C. After washing with tris buffered saline-Tween (TBST), the membrane was incubated with respective AP and horse reddish peroxidase (HRP)-coupled secondary antibodies for 2 h at room temperature and washed again with TBST. The bound antibodies on the nitrocellulose membrane (Gibco/Invitrogen, USA) were detected using the enhanced chemiluminescence (ECL) and nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) (Pierce, USA) detection reagents and protein bands were visualized. β-actin was used as a loading control.
Analysis of pluripotency in vitro/embryoid bodies (EB) formation and spontaneous differentiation: In vitro spontaneous differentiation of EBs was induced to confirm the pluripotent status of KIND-1 cells growing in I-HFF-CM in the presence or absence of bFGF supplementation. For this, undifferentiated colonies of KIND-1 cells were transferred to suspension culture dishes and allowed to form cystic EB. The differentiation medium comprised KO-DMEM (Gibco/Invitrogen, USA), 10 per cent FBS (Hyclone, USA), 1 per cent non-essential amino acid (Sigma, USA), 1 per cent L-glutamine (Sigma, USA), 1 per cent penicillin/streptomycin (Sigma) and 0.01 mM β-mercaptoethanol (Sigma, USA), but no bFGF. After 7-21 days, cystic EBs were transferred to gelatin-coated dishes for spontaneous differentiation into various cell lineages.
EB characterization: For characterization of EB in different lineages, EBs were stained for the protein markers of ectodermal lineage anti-beta III tubulin (Tuj1), mesodermal lineage alpha-smooth muscle actin (αSMA) and endodermal lineage: alpha-foetoprotein (AFP) and analyzed by immunofluorescence.
Apoptosis analysis: Per cent apoptotic cells, under different groups (I-IV) were analyzed through annexin V staining using Alexa Fluor® 488 annexin V/Dead Cell Apoptosis Kit (Invitrogen, USA) as per manufacturer's instructions. Briefly, KIND-1 hESC cell line growing under different groups of CM was harvested after 24 h, and dissociated with TrypLE Express (Invitrogen, USA). After determining the cell density, cells were diluted in ×1 annexin-binding buffer to 1×106 cells/ml (for all groups), preparing a sufficient volume to have 100 μl per assay. Cells were stained with 5 μl Alexa Fluor 488 annexin V and 1 μl (100 μg/ml) propidium iodide working solution by incubating at room temperature for 15 min. Samples were kept on ice until further analysis. Cells were measured by flow cytometry and analyzed by BD FACSDiva software version 6.1.2.
Statistical analysis: Data were analyzed and plotted using Windows XP Excel (Microsoft, Redmond, USA). Statistical significance was determined using unpaired Student's t test.
Results
Feeder cells derived from human fibroblast, but not mouse fibroblast produce significant bFGF: As shown in Fig. 2, I-HFF cells secreted high concentration of bFGF that reached maximum 582 pg/ml at day 5, and the level was decreased by day 7 without exogenous bFGF addition; in contrast to this, PMEF and HFF cells in the absence of exogenous bFGF had steady basal little secretion of bFGF that did not exceed 19 and 55 ng/ml, respectively. Analysis of bFGF secretion by HFF and PMEF in the presence of bFGF showed enhanced bFGF levels; however, decrease in the level of released bFGF was more rapid by PMEF cell when compared to HFF cells. Therefore, PMEF cell line was not included in further experiments. I-HFF cells, but not HFF cells, showed higher transcriptional expression and more secretion of TGF-β and IGF-II, in the absence of exogenous bFGF.
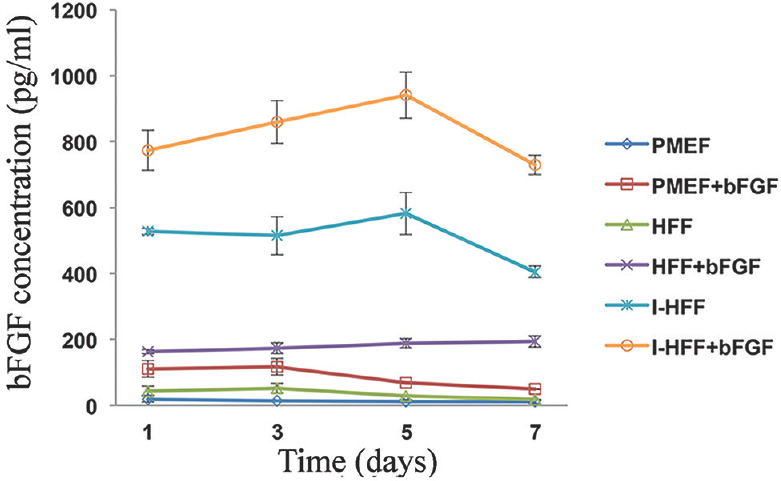
- Quantification of basic fibroblast growth factor (bFGF) secretion in the medium of feeder cells using enzyme-linked immunosorbent assay (ELISA): Time kinetics of bFGF secretion in the medium of cultured primary mouse embryonic fibroblast (PMEF), human foreskin fibroblast (HFF) and immortalized human foreskin fibroblast (I-HFF) feeder cells with or without addition of exogenous bFGF, using ELISA. Results are mean±standard deviation of three independent experiments with triplicate samples each.
To check the mRNA expression and secretion of TGF-β and IGF-II in I-HFF and HFF cells, expression of FGFR1 was analyzed in proliferating I-HFF and HFF cells by RT-PCR (Fig. 3A). Quantitative PCR data showed the increase in mRNA expression of TGF-β (2.66 fold) and IGF-II (2.84 fold) of I-HFF cells when compared to HFF cells (Fig. 3B). Secreted TGF-β and IGF-II were also quantified using ELISA in the collected medium after cell seeding. As shown in qPCR results, a similar trend with a significant (P<0.01) increase in the secretion of TGF-β (1.68 fold) and IGF-II (1.76 fold) in the supernatant was observed in I-HFF, when compared to HFF cells (Fig. 3C). Supplementation of exogenous bFGF is since required for pluripotency and maintenance of stem cells; therefore, we sought to check the mRNA expression and secretion of TGF-β and IGF-II in I-HFF cells, at various concentrations of bFGF, namely, 0, 2, 5 and 10 ng/ml and designated as groups I, II, III and IV, respectively. No significant change was observed in the mRNA expression of TGF-β and IGF-II at all exogenous concentrations of bFGF used using RT-PCR (Fig. 4A). Further, the secreted levels of TGF-β and IGF-II were quantified in the medium of I-HFF cells using ELISA. ELISA assessment revealed no significant change in the secreted level of TGF-β and IGF-II in the medium of I-HFF at any exogenous bFGF concentrations when compared to without bFGF (Fig. 4B). The time course expression and secreted levels of TGF-β and IGF-II, by I-HFF cells in response to different bFGF concentrations were assessed, using qPCR and ELISA, respectively, at days 2 and 5. HFF cell line supplemented with 10 ng/ml bFGF was taken as standard control. The expression and secretion of TGF-β and IGF-II, at all bFGF concentrations were indistinguishable from that of without bFGF by I-HFF cells either on day 2 or day 5; however, the expression and secretion were relatively more at day 5 when compared to day 2. Moreover, the expression and secretion of TGF-β and IGF-II were much higher in I-HFF cells when compared to HFF cells, as assessed by qPCR and ELISA, respectively (Fig. 4C and D).
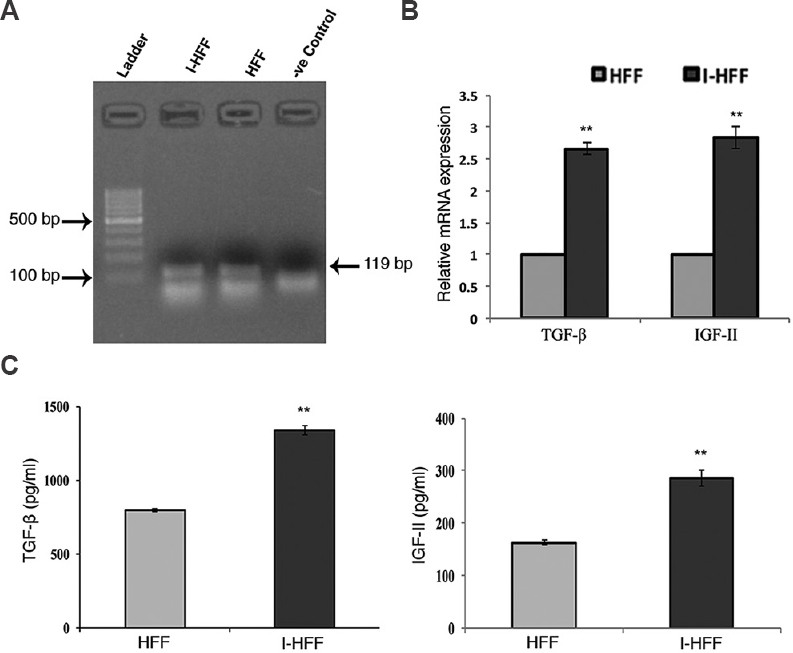
- Gene expression and secretion of transforming growth factor-beta (TGF-β) and insulin-like growth factor-II (IGF-II) in I-HFF and HFF without addition of exogenous basic fibroblast growth factor (bFGF): (A) RT-PCR analysis showing gene expression of fibroblast growth factor receptor 1 (FGFR1) (size=119 bp) in I-HFF and HFF (lanes 2 and 3, respectively); (B) Expression levels of TGF-β and IGF-II in I-HFF relative to HFF using qPCR; and (C) Quantification of secreted TGF-β and IGF-II in the medium of I-HFF and HFF cells in the absence of exogenous bFGF using ELISA. Results are mean±standard deviation of five independent experiments. **P<0.01 compared to HFF.
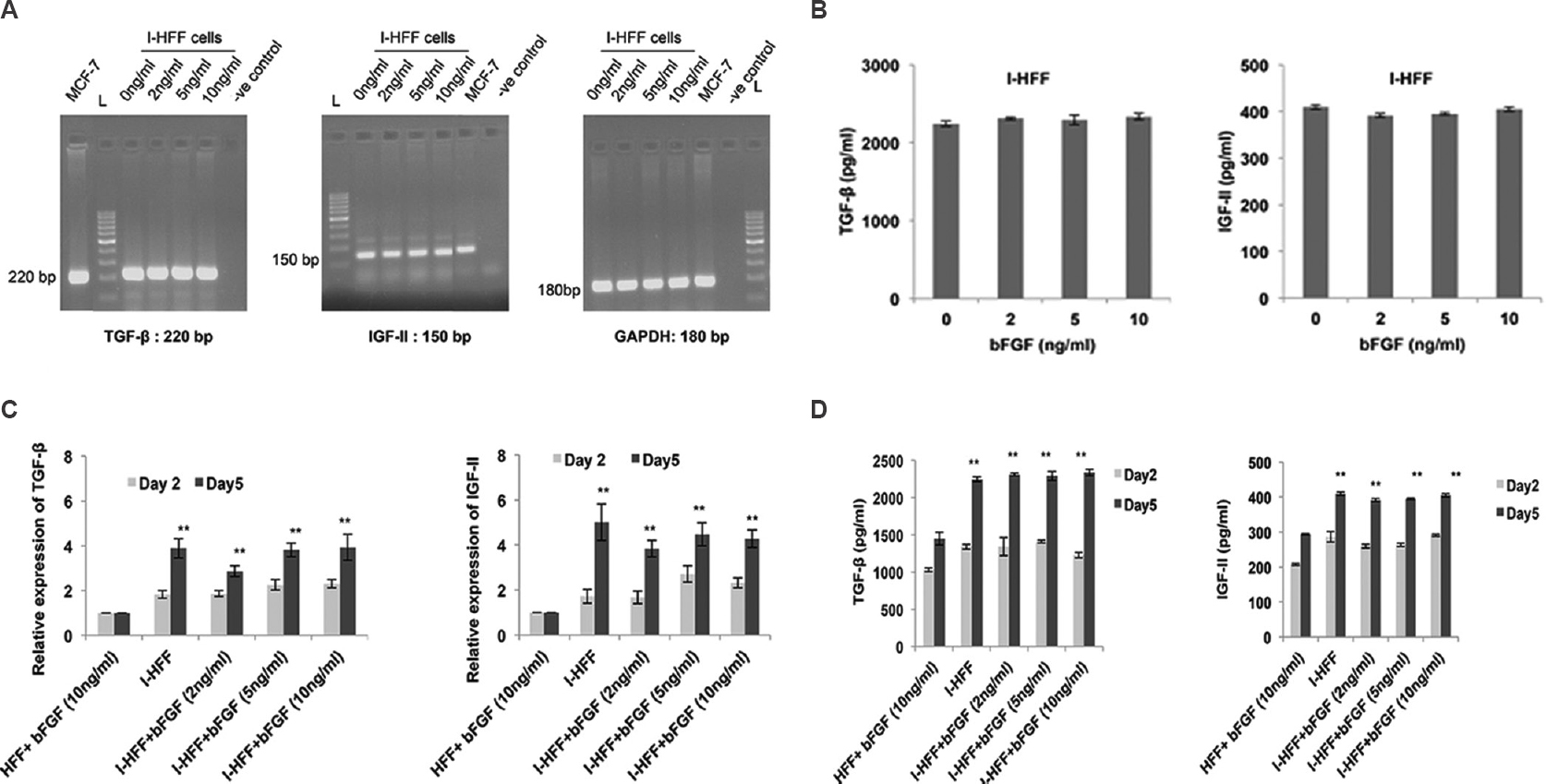
- Gene expression and production of TGF-β and IGF-II in response to bFGF: (A) Gene expression of TGF-β, IGF-II and GAPDH in I-HFF treated with varying concentrations of bFGF, using RT-PCR. MCF-7 cells used as positive control, and GAPDH as internal control; (B) Quantification of secreted TGF-β and IGF-II using ELISA. (C) Quantitative PCR results showing relative gene expression of both TGF-β and IGF-II in all groups, HFF (at bFGF 10 ng/ml) as standard control; and (D) Graph showing the quantification of release of TGF-β and IGF-II in the conditioned medium using ELISA. Results are mean±standard deviation of five independent experiments. **P<0.01, day 2 versus standard control HFF.
CM from inhibitor (SU5402)-treated I-HFF cells, induced differentiation in KIND-1 hESC line: To further confirm the possible role of bFGF in the maintenance of pluripotency in hESCs, the receptor of bFGF (FGFR1) was chemically inhibited with 20 μM of SU5402 in I-HFF cells. The effect of this inhibitor was seen on the secreted level of TGF-β and IGF-II by I-HFF cells in the presence of 10 ng/ml bFGF, using ELISA. The presence of inhibitor significantly reduced the secretion of TGF-β and IGF-II in the supernatant of I-HFF cells by 68 and 60 per cent, respectively, hence suggested a functional cooperation of bFGF with both TGF-β and IGF-II (Fig. 5A). Subsequently, KIND-1 hESC cell line was cultured in CM from inhibitor-treated I-HFF cells (I-HFF-CM). I-HFF-CM from inhibitor-treated I-HFF cells, displayed changes in morphology and induced differentiation of KIND-1 hESC cells (Fig. 5B).
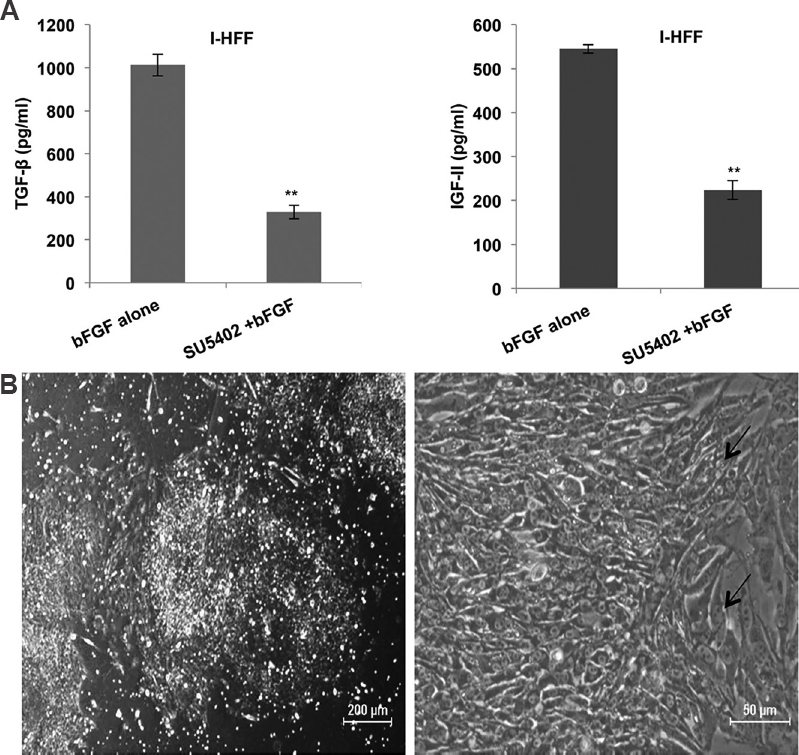
- Demonstration of bFGF role in maintaining stemness of human embryonic stem cell using chemical inhibitor (SU5402) of FGFR1: (A) Effect of inhibitor on secreted amount of TGF-β and IGF-II released by I-HFF in presence of bFGF. Results are mean±standard deviation of three independent experiments. **P<0.01 compared to bFGF alone; and (B) Phase contrast images of KIND-1 human embryonic stem cells showing visible differentiation (black arrow). The cells were cultured in conditioned medium from inhibitor-treated I-HFF.
KIND-1 cells retain in vitro pluripotency in I-HFF-CM in feeder-free system: The hESC line KIND-1 was cultured in the feeder-independent system using CM by I-HFF cells, collected from groups I-IV. Commercially available serum-free medium for the culture of KIND-1 hESC line was considered as a positive control. KIND-1 hESC line propagated at a similar rate in I-HFF-CM from all groups as cultured in the commercially available feeder-free medium. KIND-1 hESC line maintained typical embryonic stem cell-like morphology with no sign of differentiation and no change in growth and morphology pattern, as observed amongst all groups till passage 10 (Fig. 6A). With the time course beyond 10th passage in group I i.e., one without bFGF a sign of paracrine regulation to additionally support hESC culture was observed with the appearance of autologous hESC-derived fibroblast-like cells (hdFs), hence suggested the role of I-HFF-CM in inducing autologous system to maintain stemness of KIND-1 hESC cells without any dependence on exogenous bFGF (Fig. 6B).
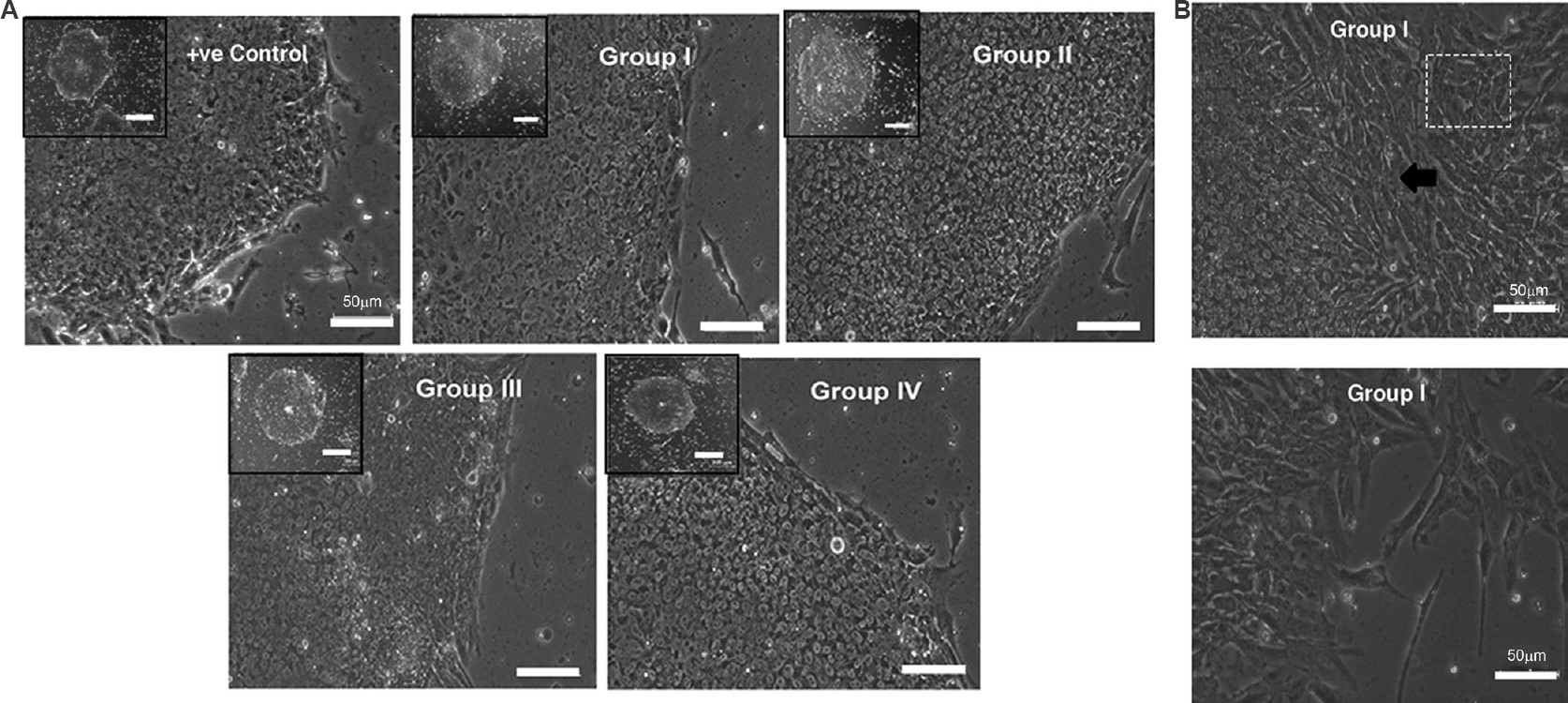
- KIND-1 human embryonic stem cells (hESC) retain in vitro pluripotency in I-HFF conditioned medium lacking exogenous bFGF in feeder-free system: (A) Morphology of hESC colonies cultured under different groups of I-HFF-conditioned medium. Human embryonic stem cell colonies have clear defined borders with high N/C ratio. Scale bar=50 μm and magnification ×10. Inset (Scale bar=200 μm) showing a single colony. (B) Phase contrast images of hESC-derived fibroblast-like cells that appeared in group I of I-HFF conditioned medium. Black arrow and boxed area show the hESC-derived fibroblast-like cells outgrowth from hESC colonies. The lower panel of image shows hESC-derived fibroblast-like cells alone.
To further confirm the undifferentiated pluripotent status of KIND-1 cells colonies, the expression of specific pluripotent markers was analysed. KIND-1 hESCs displayed the presence of AP activity as well as expression and distribution of pluripotency-associated markers, namely, TRA 1-81, OCT4, TRA 1-60 and SSEA4, as assessed by immunocytochemistry and flow cytometry, respectively (Fig. 7A-C). KIND-1 cells cultured in I-HFF-CM from all groups did not reveal any significant difference in activity of AP as well as expression and distribution of stem cell markers either within groups or with the positive control.
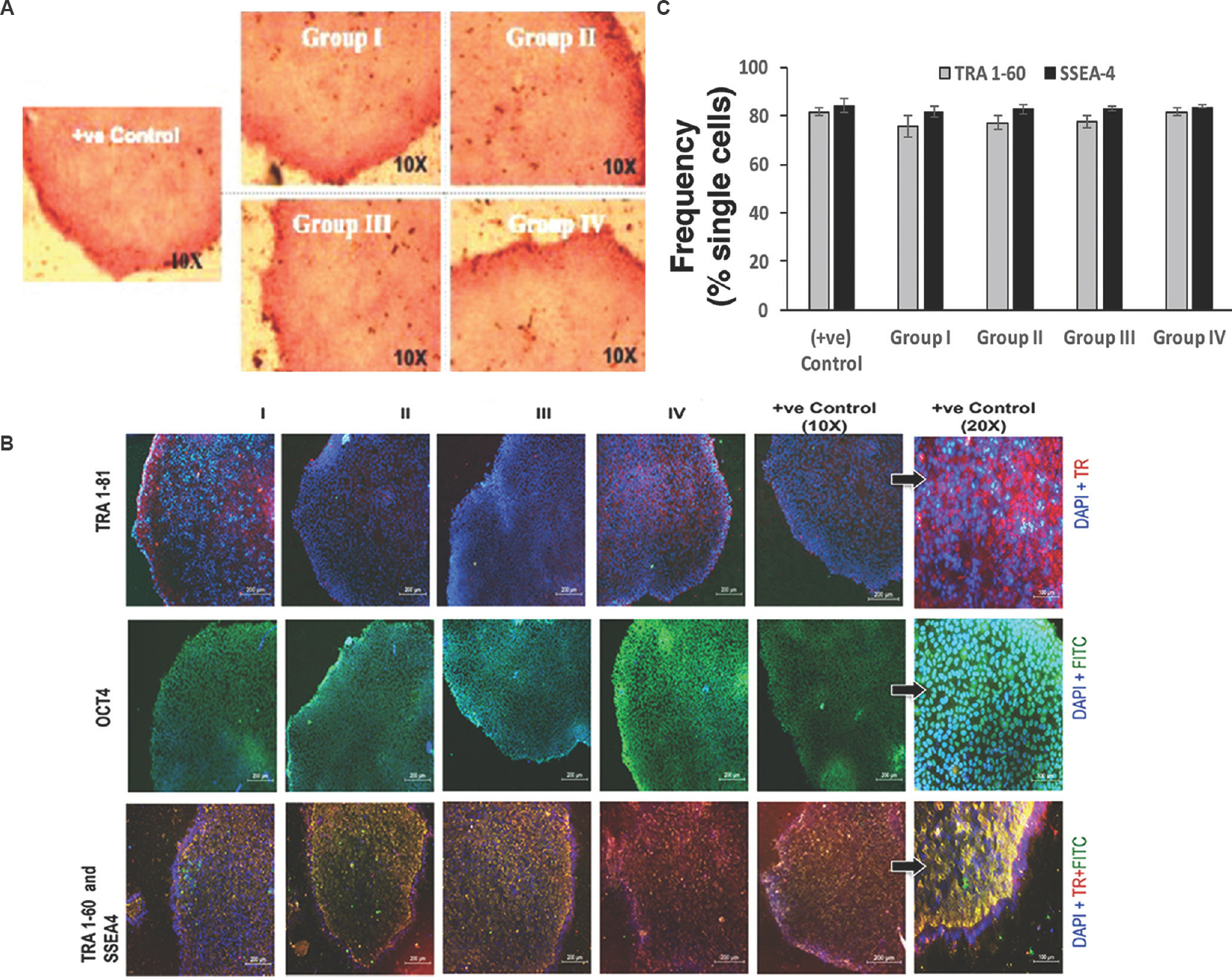
- Characterization of hESCs pluripotency by immunostaining in the presence (Groups II, III and IV) or absence (Group I) of bFGF: (A) Alkaline phosphatase (AP) staining. Scale bar=10 μm, magnification ×10; and (B) Immunofluorescence images of KIND-1 hESC for undifferentiated stem cell surface markers. Texas red (TR) for TRA 1-81 and TRA 1-60 positive cells; green colour FITC for OCT4 and SSEA4 positive cells and blue colour DAPI for cell nuclei staining. Scale bar=200 μm, magnification ×10. KIND-1 hESC (positive control) at higher magnification ×20, scale bar=100 μm. (C) Comparative analysis of expression for pluripotent surface markers for TRA1-60 and SSEA-4 using flow cytometry (n=4), and represented as frequency of single cells.
After analyzing the protein expression of pluripotent markers, gene expression was analysed using StellARray qPCR. Data from GPR analysis revealed that KIND-1 cells cultured in I-HFF-CM from groups I and III expressed genes responsible for pluripotency similar to positive control. The gene expression was not affected by culture conditions in KIND-1 cells employing I-HFF-CM in feeder-free regimen except, a higher fold increase of stem cell marker, undifferentiated embryonic cell transcription factor 1, in both groups I and III by 7.90 and 5.02 folds, respectively; however, the difference was not significant between groups when compared to positive control. KIND-1 cells grown in I-HFF-CM from group I showed upregulated expression of fms-related tyrosine kinase-1 (FLT1), forkhead box A2 (FOXA2) and GATA binding protein-4 (GATA4) genes, involved in early endodermal differentiation, by 2.46, 2.08 and 2.07 fold, respectively, as compared to positive control, while downregulation of these genes was observed in group III. Similarly, the expression of T, brachyury homolog (T), a mesoderm marker was found to be upregulated in group III, as compared to positive control by 1.19 fold. However, all the changes observed were not significant between the groups and positive control (Table & Fig. 8A). In accord with transcriptional studies, analysis of expression of OCT4 protein in KIND-1 cells using Western blot did not reveal any significant difference between the groups and positive control (Fig. 8B). Functionally, hESC lines were able to differentiate in vitro through EB formation into three germ layers along with the appearance of spontaneous beating cardiomyocytes, in the absence of bFGF, as confirmed by the expression of markers, namely, endoderm: anti-beta III tubulin with DAPI (Tuj1+DAPI), mesoderm: αSMA with DAPI (αSMA+DAPI) and ectoderm: AFP with DAPI (AFP+DAPI), thus demonstrating pluripotent features of KIND-1 hESC cells, regardless of their expansion in I-HFF CM lacking exogenous bFGF (Fig. 8C).
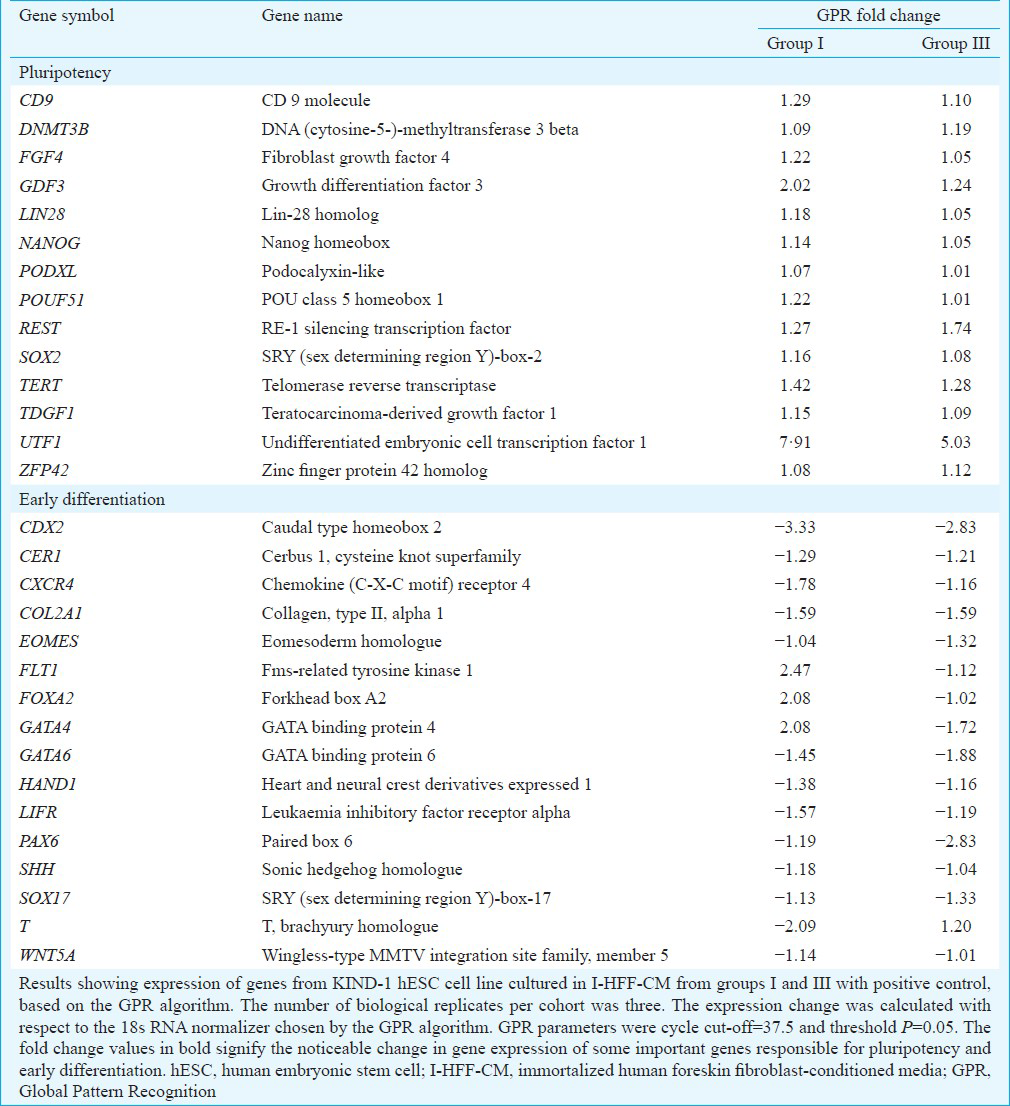
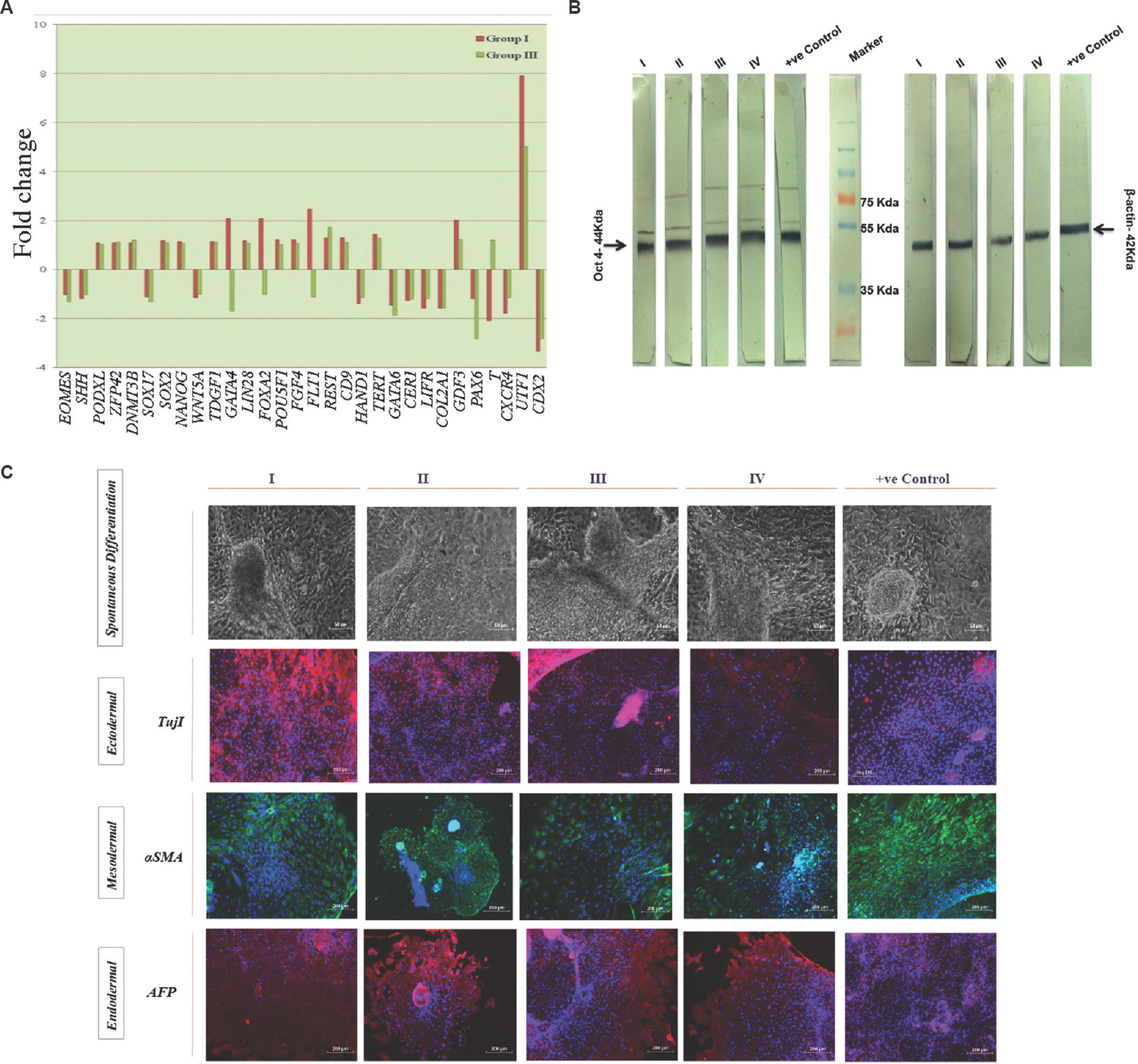
- Gene and protein expression study of KIND-1 hESCs involved in pluripotency maintenance in I-HFF-conditioned medium lacking exogenous bFGF in feeder-free system: (A) Global Pattern Recognition™ Fold Change Values. The top 30 genes involved in pluripotency and early differentiation are shown; (B) Western blot analysis of KIND-hESC cultured under I-HFF-conditioned medium from groups I-IV. Loading control β-actin. Result shows representative membrane of three independent experiments; and (C) Protein expression analysis of lineage markers for three germ layers. Magnification ×10, scale bars=50 μm for first panel (spontaneous differentiation) and 200 μm for ectodermal, mesodermal and endodermal.
The cellular death/apoptosis studies showed a slower culture adaptation in hESCs grown in I-HFF CM without supplementation of exogenous bFGF (Group I) when compared to control, as indicated by increased cell death (Fig. 9).
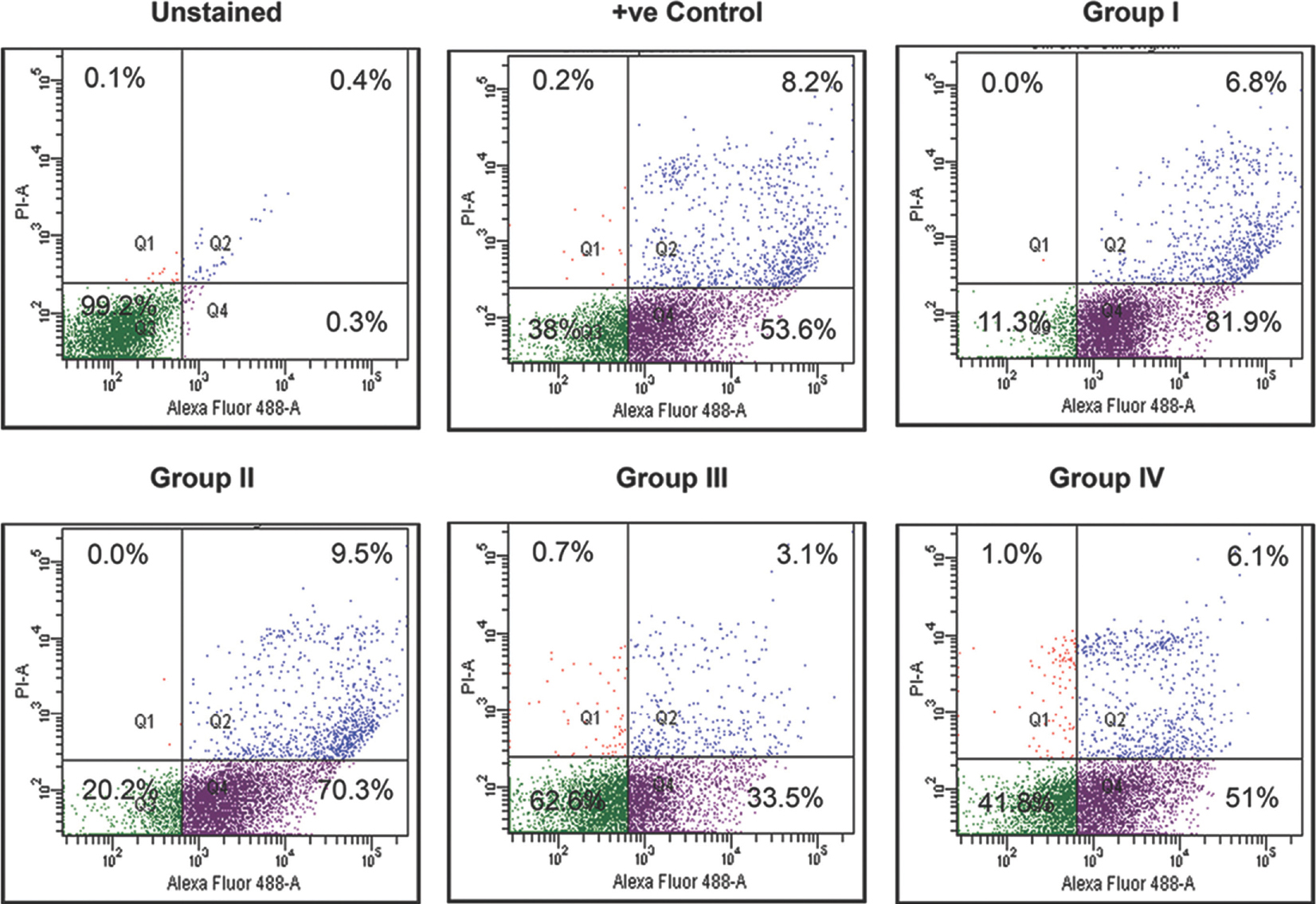
- Cell death analysis of KIND-1 human embryonic stem cell grown in I-HFF conditioned medium by groups I-IV: Frequency of KIND-1 cells undergoing cell death/apoptosis in I-HFF conditioned medium supplemented with different bFGF doses in comparison to commercially available serum-free medium analyzed by flow cytometry after Annexin V and propidium iodide staining. Result showing representative scattered plot of three independent experiments.
Discussion
Feeder cells and feeder cells’ CM to maintain pluripotency of hESCs have been reported previously11232425; however, in some cases, these fail to support hESCs for therapeutic uses. Feeder cells are derived from primary tissues and therefore, attain senescence with culture passages and lead to reducing performance in pluripotent maintenance7. In addition, new feeder cells batches are to be regularly prepared, leading to variations in batches. The additional drawback is cross-transfer of pathogens by feeder cells26. hESCs, in the presence of xenogeneic components have been shown to provoke immune responses in humans2728. In addition, the conventional culture of hESCs is expensive due to the high cost associated with medium supplement such as bFGF but is required for expansion of hESC cultures. Thus, limitation associated with co-culture of feeder cells with hESCs and cost of bFGF necessitates the exploration of a feeder-free and bFGF-free method to maintain stemness of hESCs.
Immortalized I-HFF cells were included as a source of feeders in this study. I-HFF cells in contrast to HFF cells are not only consistent source of feeders but also have shown to secrete ample amount of bFGF to support hESCs9. The CM from immortalized bFGF secreting I-HFF cells (I-HFF-CM) eliminated the additional supplementation of exogenous bFGF. For this, the CM from I-HFF cells was checked regularly for the stability of bFGF secretion up to passage 30. No significant difference was seen in the level of bFGF secretion (data not shown). I-HFF cells were also characterized for genetic stability intermittently up to passage 30 (data not shown). It was also shown that two important signalling molecules i.e., TGF-β and IGF-II in association with bFGF were required in sustaining stem cell niche in the absence of exogenous bFGF with respect to HFFs.
In our study, the basal level of bFGF secretion was initially examined with the time course in the medium conditioned by mouse and human feeders (both naive as well as immortalized). The lesser amount secreted by mouse feeders was in line with previously published report29, HFF cells showed a basal level of bFGF, whereas I-HFFs secreted more bFGF as demonstrated earlier by Unger et al9.
Use of I-HFF cells as a feeder in previous studies and increased secretion of bFGF by these cells encouraged us to consider the use of I-HFF-CM, for the culture of KIND-1 hESCs14. Therefore, the concentration of exogenous bFGF was narrowed down from standard 10 ng/ml to 5, 2 and 0 ng/ml (groups IV-I) in I-HFFs cultures and its association was checked with TGF-β and IGF-II. Since bFGF is reported to instruct hESCs differentiation by balancing renewal signals controlled by TGF-β and IGF-II1230, the effect of bFGF was studied on TGF-β and IGF-II as well as on differentiation of KIND-1 hESCs. Data from chemical inhibition of FGFR1 depicted the functional cooperation of bFGF with both TGF-β and IGF-II and seemed to be specific, as the secretion of TGF-β and IGF-II was reduced, and differentiation was induced in KIND-1 cultures after blocking of FGFR1 receptor. This cooperation of bFGF to TGF-β and IGF-II is required to maintain stemness of feeder-free hESCs system1216. High expression and secretion of TGF-β and IGF-II were observed with time, in b-FGF-secreting I-HFF cells when compared to standard control HFF cells. Use of different concentrations of bFGF did not reveal changes in the expression and secretion of TGF-β and IGF-II within groups. Hence, bFGF was not required for optimal expression and secretion of TGF-β and IGF-II to support undifferentiated growth of KIND-1 cells.
Subsequently, KIND-1 hESCs were cultured in the presence of I-HFF-CM collected from all groups. KIND-1 hESC cell line was gradually adapted to feeder-free culture conditions using I-HFF-CM and could be maintained in the feeder-free system beyond 30 passages in the absence of bFGF. KIND-1 hESCs cultured in I-HFF-CM from group I showed similar kind of propagation, and typical morphology of stem cells when cultured in I-HFF-CM from groups II-IV. In KIND-1 hESCs culture, which was propagated in I-HFF-CM from group I, appearance of some HdFs was observed, which has been shown to support hESCs in undifferentiated state by paracrine regulation31. These cells have also been shown to act like autogenic feeder cell system to efficiently support the growth of undifferentiated hESCs32. Hence, I-HFF-CM from group I not only reduced the cost of growth factor bFGF but also induced paracrine signalling to support the self-renewal ability of hESCs.
The findings were further extended to determine the behaviour of KIND-1 cells in the feeder and bFGF-free system by analyzing their pluripotency and self-renewal properties. KIND-1 ESCs, cultured in I-HFF-CM by all groups, retained the expression of expected cell surface markers and transcription factors associated with pluripotency. Upregulation of some endodermal genes, namely, FLT1, FOXA2 and GATA4 was observed in KIND-1 cells propagated in I-HFF-CM from group I when compared to positive control, but the difference was not significant between groups I and III in respect to positive control. This could be explained by the propensity of KIND-1 cells toward endodermal lineage33. Specific staining for ectodermal marker Tuj1was also confirmed by considering primary control (data not shown).
Deferred culture adaptation was observed in KIND-1 cells grown in I-HFF-CM from group I, which could be explained by increased cell death. This was in line with the previous study34. This study could be helpful in the scalable and economic expansion of hESC cultures for therapeutic purposes.
Acknowledgment
Authors thank Dr. Anis Feki, Geneva University Hospitals, Switzerland and Dr Deepa Bhartiya, National Institute for Research in Reproductive Health, Mumbai, India, for providing I-HFF and KIND-1 hESC cell line, respectively.
Conflicts of Interest: None.
References
- In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129-33.
- [Google Scholar]
- Human embryonic stem cell derivation, maintenance, and differentiation to trophoblast. Methods Mol Biol. 2010;636:1-24.
- [Google Scholar]
- Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-7.
- [Google Scholar]
- Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933-6.
- [Google Scholar]
- Human placenta feeder layers support undifferentiated growth of primate embryonic stem cells. Stem Cells. 2004;22:433-40.
- [Google Scholar]
- Derivation of human embryonic stem cell lines in serum replacement medium using postnatal human fibroblasts as feeder cells. Stem Cells. 2005;23:544-9.
- [Google Scholar]
- Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546-56.
- [Google Scholar]
- Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185-90.
- [Google Scholar]
- Immortalized human skin fibroblast feeder cells support growth and maintenance of both human embryonic and induced pluripotent stem cells. Hum Reprod. 2009;24:2567-81.
- [Google Scholar]
- Human feeder cell line for derivation and culture of hESc/hiPSc. Stem Cell Res. 2011;7:154-62.
- [Google Scholar]
- Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-4.
- [Google Scholar]
- Feeder-free maintenance of hESCs in mesenchymal stem cell-conditioned media: distinct requirements for TGF-beta and IGF-II. Cell Res. 2009;19:698-709.
- [Google Scholar]
- Maintenance of human embryonic stem cells in media conditioned by human mesenchymal stem cells obviates the requirement of exogenous basic fibroblast growth factor supplementation. Tissue Eng Part C Methods. 2012;18:387-96.
- [Google Scholar]
- Enhanced reprogramming efficiency and kinetics of induced pluripotent stem cells derived from human duchenne muscular dystrophy. PLoS Curr 2015 Sept 3: 7
- [Google Scholar]
- An enhanced mass spectrometry approach reveals human embryonic stem cell growth factors in culture. Mol Cell Proteomics. 2009;8:421-32.
- [Google Scholar]
- IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015-21.
- [Google Scholar]
- Production and validation of a good manufacturing practice grade human fibroblast line for supporting human embryonic stem cell derivation and culture. Stem Cell Res Ther. 2012;3:12.
- [Google Scholar]
- Derivation and characterization of two genetically unique human embryonic stem cell lines on in-house-derived human feeders. Stem Cells Dev. 2009;18:435-45.
- [Google Scholar]
- An optimized small molecule inhibitor cocktail supports long-term maintenance of human embryonic stem cells. Nat Commun. 2011;2:167.
- [Google Scholar]
- A complex role for FGF-2 in self-renewal, survival, and adhesion of human embryonic stem cells. Stem Cells. 2009;27:1847-57.
- [Google Scholar]
- Bryostatin-1 causes radiosensitization of BMG-1 malignant glioma cells through differential activation of protein kinase-Cd not evident in the non-malignant AA8 fibroblasts. Mol Cell Biochem. 2015;401:49-59.
- [Google Scholar]
- Properties of four human embryonic stem cell lines maintained in a feeder-free culture system. Dev Dyn. 2004;229:243-58.
- [Google Scholar]
- Virus zoonoses and their potential for contamination of cell cultures. Dev Biol Stand. 1991;75:183-9.
- [Google Scholar]
- Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228-32.
- [Google Scholar]
- Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev. 2012;28:147-75.
- [Google Scholar]
- Comparative study of mouse and human feeder cells for human embryonic stem cells. Int J Dev Biol. 2008;52:353-63.
- [Google Scholar]
- Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell. 2012;10:312-26.
- [Google Scholar]
- Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cell growth. Stem Cells. 2004;22:972-80.
- [Google Scholar]
- An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 2005;23:306-14.
- [Google Scholar]
- Evaluating differentiation propensity of in-house derived human embryonic stem cell lines KIND-1 and KIND-2. In Vitro Cell Dev Biol Anim. 2011;47:406-19.
- [Google Scholar]
- Human embryonic stem cells express elevated levels of multiple pro-apoptotic BCL-2 family members. PLoS One. 2011;6:e28530.
- [Google Scholar]






